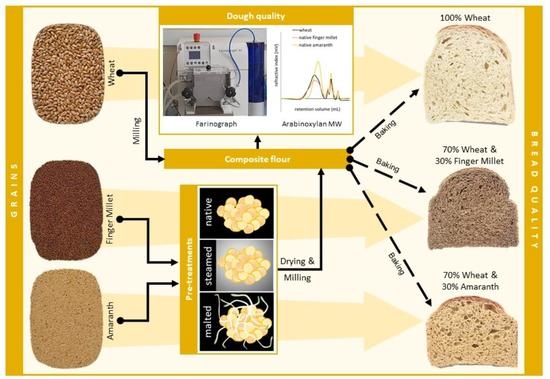
Utilisation of Amaranth and Finger Millet as Ingredients in Wheat Dough and Bread for Increased Agro-Food Biodiversity
Abstract
:1. Introduction
2. Materials and Method
2.1. Modification of Finger Millet
2.2. Characterization of Flours
2.3. Properties of Flours and Doughs
2.4. Bread Making and Evaluation of Physical and Textural Properties of Breads
2.5. Nutrient Qualities of Breads
2.6. Experimental Design and Statistical Data Analysis
3. Results and Discussion
3.1. Characterization of Flours
3.2. Properties of Flours and Doughs
3.3. Physical and Textural Properties of Breads
3.4. Nutrient Qualities of Bread
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Onyango, C.; Luvitaa, S.K.; Unbehend, G.; Haase, N. Physico-chemical properties of flour, dough and bread from wheat and hydrothermally-treated finger millet. J. Cereal Sci. 2020, 93, 102954. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Suitability of Oat, Millet and Sorghum in Breadmaking. Food Bioprocess Technol. 2013, 6, 1486–1493. [Google Scholar] [CrossRef]
- Angioloni, A.; Collar, C. Effects of pressure treatment of hydrated oat, finger millet and sorghum flours on the quality and nutritional properties of composite wheat breads. J. Cereal Sci. 2012, 56, 713–719. [Google Scholar] [CrossRef]
- Zhou, Y.; Dhital, S.; Zhao, C.; Ye, F.; Chen, J.; Zhao, G. Dietary fiber-gluten protein interaction in wheat flour dough: Analysis, consequences and proposed mechanisms. Food Hydrocoll. 2020, 111, 106203. [Google Scholar] [CrossRef]
- Koletta, P.; Irakli, M.; Papageorgiou, M.; Skendi, A. Physicochemical and technological properties of highly enriched wheat breads with wholegrain non wheat flours. J. Cereal Sci. 2014, 60, 561–568. [Google Scholar] [CrossRef]
- Skendi, A.; Biliaderis, C.; Papageorgiou, M.; Izydorczyk, M.S. Effects of two barley β-glucan isolates on wheat flour dough and bread properties. Food Chem. 2010, 119, 1159–1167. [Google Scholar] [CrossRef]
- Bhol, S.; Bosco, S.J.D. Influence of malted finger millet and red kidney bean flour on quality characteristics of developed bread. LWT Food Sci. Technol. 2014, 55, 294–300. [Google Scholar] [CrossRef]
- Sanz-Penella, J.; Wronkowska, M.; Soral-Smietana, M.; Haros, M. Effect of whole amaranth flour on bread properties and nutritive value. LWT Food Sci. Technol. 2013, 50, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Ferrero, C. Hydrocolloids in wheat breadmaking: A concise review. Food Hydrocoll. 2017, 68, 15–22. [Google Scholar] [CrossRef]
- Schoenlechner, R.; Szatmari, M.; Bagdi, A.; Tömösközi, S. Optimisation of bread quality produced from wheat and proso millet (Panicum miliaceum L.) by adding emulsifiers, transglutaminase and xylanase. LWT Food Sci. Technol. 2013, 51, 361–366. [Google Scholar] [CrossRef]
- Oszvald, M.; Tamás, C.; Rakszegi, M.; Tömösközi, S.; Békés, F.; Tamás, L. Effects of incorporated amaranth albumins on the functional properties of wheat dough. J. Sci. Food Agric. 2009, 89, 882–889. [Google Scholar] [CrossRef]
- Silva-Sánchez, C.; González-Castañeda, J.; Rodriguez, A.D.L.; De La Rosa, A.B. Functional and Rheological Properties of Amaranth Albumins Extracted From Two Mexican Varieties. Plant Foods Hum. Nutr. 2004, 59, 169–174. [Google Scholar] [CrossRef]
- Guardianelli, L.M.; Salinas, M.V.; Puppo, M.C. Hydration and rheological properties of amaranth-wheat flour dough: Influence of germination of amaranth seeds. Food Hydrocoll. 2019, 97, 105242. [Google Scholar] [CrossRef]
- Mlakar, S.G.; Bavec, M.; Turinek, M.; Bavec, F. Rheological properties of dough made from grain amaranth-cereal composite flours based on wheat and spelt. Czech J. Food Sci. 2009, 27, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Tosi, E.A.; Ré, E.D.; Masciarelli, R.; Sánchez, H.; Osella, C.; de la Torre, M.A. Whole and Defatted Hyperproteic Amaranth Flours Tested as Wheat Flour Supplementation in Mold Breads. LWT Food Sci. Technol. 2002, 35, 472–475. [Google Scholar] [CrossRef]
- Patil, S.S.; Rudra, S.G.; Varghese, E.; Kaur, C. Effect of extruded finger millet (Eleusine coracan L.) on textural properties and sensory acceptability of composite bread. Food Biosci. 2016, 14, 62–69. [Google Scholar] [CrossRef]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; Tungtrakul, P. Germination conditions affect selected quality of composite wheat-germinated brown rice flour and bread formulations. J. Food Sci. 2010, 75, S312–S318. [Google Scholar] [CrossRef]
- Hugo, L.F.; Rooney, L.W.; Taylor, J.R.N. Fermented Sorghum as a Functional Ingredient in Composite Breads. Cereal Chem. 2003, 80, 495–499. [Google Scholar] [CrossRef]
- Hugo, L.F.; Rooney, L.W.; Taylor, J.R.N. Malted Sorghum as a Functional Ingredient in Composite Bread. Cereal Chem. 2000, 77, 428–432. [Google Scholar] [CrossRef]
- ICC Standards. No. 114/1; Method for Using Brabender Extensograph. International Association for Cereal Science and Technology: Vienna, Austria, 2008.
- ICC Standards. No. 115/1; Method for Using Brabender Farinograph. International Association for Cereal Science and Technology: Vienna, Austria, 1992.
- Schmidt, M.; Sciurba, E. Determination of FODMAP contents of common wheat and rye breads and the effects of processing on the final contents. Eur. Food Res. Technol. 2021, 247, 395–410. [Google Scholar] [CrossRef]
- Houben, R.; De Ruijter, C.; Brunt, K. Determination of the Pentosan Content of Wheat Products by Hydrolysis, Glucose Oxidase Treatment and Analysis by HPAEC/PAD. J. Cereal Sci. 1997, 26, 37–46. [Google Scholar] [CrossRef]
- Dharmaraj, U.; Malleshi, N.G. Changes in carbohydrates, proteins and lipids of finger millet after hydrothermal processing. LWT Food Sci. Technol. 2011, 44, 1636–1642. [Google Scholar] [CrossRef]
- Becker, R.; Wheeler, E.L.; Lorenz, K.; Stafford, A.E.; Grosjean, O.K.; Betschart, A.A.; Saunders, R.M. A Compositional Study of Amaranth Grain. J. Food Sci. 1981, 46, 1175–1180. [Google Scholar] [CrossRef]
- Qi, X.; Tester, R.F. Effect of native starch granule size on susceptibility to amylase hydrolysis. Starch Staerke 2016, 68, 807–810. [Google Scholar] [CrossRef]
- Rao, M.V.S.S.T.S.; Muralikrishna, G. Non-starch polysaccharides and bound phenolic acids from native and malted finger millet (ragi, E. coracana, Indaf-15). Food Chem. 2001, 72, 187–192. [Google Scholar]
- Olawoye, B.T.; Gbadamosi, S.O. Effect of different treatments on in vitro protein digestibility, antinutrients, antioxidant prop-erties and mineral composition of Amaranthus viridis seed. Cogent Food Agric. 2017, 3, 1296402. [Google Scholar] [CrossRef]
- Hejazi, S.N.; Orsat, V. Malting process optimization for protein digestibility enhancement in finger millet grain. J. Food Sci. Technol. 2016, 53, 1929–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udeh, H.O.; Duodu, K.G.; Jideani, A.I.O. Effect of malting period on physicochemical properties, minerals, and phytic acid of finger millet (Eleusine coracana) flour varieties. Food Sci. Nutr. 2018, 6, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Qvirist, L.; Carlsson, N.G.; Andlid, T. Assessing phytase activity—Methods, definitions and pitfalls. J. Biol. Meth. 2015, 2, E16. [Google Scholar] [CrossRef] [Green Version]
- Sapirstein, H.; Wu, Y.; Koksel, F.; Graf, R. A study of factors influencing the water absorption capacity of Canadian hard red winter wheat flour. J. Cereal Sci. 2018, 81, 52–59. [Google Scholar] [CrossRef]
- Gomez, M.; Ronda, F.; Blanco, C.A.; Caballero, P.A.; Apesteguía, A. Effect of dietary fibre on dough rheology and bread quality. Eur. Food Res. Technol. 2003, 216, 51–56. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.S.; Courtin, C.M.; Gebruers, K.; Delcour, J.A. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Noort, M.W.; van Haaster, D.; Hemery, Y.; Schols, H.A.; Hamer, R.J. The effect of particle size of wheat bran fractions on bread quality—Evidence for fibre–protein interactions. J. Cereal Sci. 2010, 52, 59–64. [Google Scholar] [CrossRef]
- Gamel, T.H.; Linssen, J.P.; Mesallem, A.S.; Damir, A.A.; Shekib, L.A. Effect of seed treatments on the chemical composition and properties of two amaranth species: Starch and protein. J. Sci. Food Agric. 2005, 85, 319–327. [Google Scholar] [CrossRef]
- Mollakhalili-Meybodi, N.; Khorshidian, N.; Nematollahi, A.; Arab, M. Acrylamide in bread: A review on formation, health risk assessment, and determination by analytical techniques. Environ. Sci. Pollut. Res. 2021, 28, 15627–15645. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Saeed, F.; Pasha, I.; Anjum, F.M.; Sultan, M.T. Arabinoxylans and Arabinogalactans: A Comprehensive Treatise. Crit. Rev. Food Sci. Nutr. 2011, 51, 467–476. [Google Scholar] [CrossRef]

| Nutrient | Wheat | Finger Millet | Amaranth | ||||
|---|---|---|---|---|---|---|---|
| Native | Steamed | Malted | Native | Steamed | Malted | ||
| Monosaccharides (mg/100 g) * | 53 ± 1 g | 77 ± 0 e | 219 ± 1 d | 1257 ± 0 a | 65 ± 0 f | 282 ± 1 c | 1030 ± 0 b |
| Disaccharides (mg/100 g) ** | 710 ± 0 c | 692 ± 1 d | 460 ± 0 f | 54 ± 0 g | 784 ± 0 b | 593 ± 1 e | 817 ± 0 a |
| Trisaccharides (mg/100 g) *** | 104 ± 1 d | 82 ± 1 e | 71 ± 1 f | nd | 760 ± 0 a | 451 ± 0 b | 226 ± 0 c |
| Sugar alcohols (mg/100 g) **** | 12 ± 0 f | 30 ± 0 b | 15 ± 0 d | 7 ± 0 g | 14 ± 0 e | 18 ± 0 c | 63 ± 1 a |
| Total starch (g/100 g) | 73 ± 0 bc | 82 ± 0 a | 83 ± 1 a | 79 ± 1 ab | 69 ± 1 c | 61 ± 5 d | 58 ± 0 d |
| Digestible starch (% of total starch) | 88 ± 2 b | 84 ± 1 b | 86 ± 3 b | 88 ± 1 b | 97 ± 1 a | 97 ± 0 a | 98 ± 2 a |
| Insoluble dietary fibre (g/100 g) | 2.3 ± 0.2 d | 12 ± 0 a | 12 ± 0 ab | 11 ± 0 ab | 7.6 ± 0.2 c | 8.5 ± 0.9 c | 10 ± 2 b |
| Soluble dietary fibre (g/100 g) | 1.3 ± 0.3 a | 0.6 ± 0.2 b | 0.6 ± 0.5 b | 0.8 ± 0.3 b | 0.7 ± 0.3 b | 1.0 ± 0.1 ab | 1.0 ± 0.0 ab |
| Total dietary fibre (g/100 g) | 3.6 ± 0.3 c | 13 ± 0 a | 12 ± 1 a | 12 ± 0 a | 8.3 ± 0.4 b | 9.5 ± 0.9 b | 11 ± 2 a |
| Arabinoxylan (mg/100 g) | 1312 ± 0 d | 1555 ± 2 b | 1455 ± 1 c | 2017 ± 3 a | 1061 ± 2 g | 1184 ± 0 f | 1228 ± 1 e |
| Arabinoxylan molecular weight (kDa) | 195 ± 25 a | 177 ± 16 a | 189 ± 17 a | 166 ± 8 a | 99 ± 4 b | 139 ± 15 ab | 93 ± 10 b |
| Total protein (g/100 g) | 15 ± 1 a | 11 ± 0 bc | 9 ± 0 c | 9 ± 1 c | 14 ± 2 ab | 16 ± 1 a | 18 ± 2 a |
| In vitro protein digestibility (% of total protein) | 80 ± 4 b | 88 ± 2 ab | 79 ± 2 b | 80 ± 1 b | 87 ± 4 ab | 92 ± 0 a | 93 ± 1 a |
| Lipid (g/100 g) | 1.8 ± 0.1 c | 1.3 ± 0.0 d | 1.6 ± 0.1 cd | 1.4 ± 0.3 cd | 8.0 ± 0.1 ab | 7.6 ± 0.3 b | 8.3 ± 0.1 a |
| Ash (g/100 g) | 0.8 ± 0.0 c | 3.9 ± 0.0 a | 3.4 ± 0.5 ab | 3.1 ± 0.0 b | 2.9 ± 0.1 b | 2.8 ± 0.0 b | 3.2 ± 0.0 b |
| Phytate (mg/100 g) | 621 ± 69 c | 1260 ± 133 c | 1087 ± 37 c | 1144 ± 311 c | 1366 ± 310 bc | 2062 ± 107 ab | 2209 ± 176 a |
| Total phenolic content (mg GAE/100 g) | 103 ± 5 c | 162 ± 2 a | 162 ± 2 a | 142 ± 6 b | 39 ± 0 f | 68 ± 4 e | 85 ± 0 d |
| Flour | α-Amylase Activity (CU/g) | WAC (%) | DDT (min) | Stability (min) | DS (FU) | FQN (mm) |
|---|---|---|---|---|---|---|
| WHE | 0.6 ± 0.1 | 59 ± 0.2 e | 1.9 ± 0.2 b | 3.6 ± 0.3 c | 76 ± 4 d | 40 ± 3 d |
| WHE-NFM | 0.6 ± 0.0 | 58 ± 0.1 e | 1.8 ± 0.4 b | 5.5 ± 0.1 a | 122 ± 2 c | 55 ± 1 c |
| WHE-SFM | 0.4 ± 0.2 | 60 ± 0.0 d | 1.5 ± 0.1 b | 5.2 ± 0.3 ab | 113 ± 7 c | 56 ± 1 c |
| WHE-MFM | 0.7 ± 0.0 | 61 ± 0.2 bc | 1.4 ± 0.3 b | 2.5 ± 0.0 d | 168 ± 11 b | 32 ± 1 e |
| WHE-NAM | 0.5 ± 0.1 | 61 ± 0.1 c | 3.8 ± 0.4 a | 5.0 ± 0.7 ab | 114 ± 6 c | 70 ± 1 a |
| WHE-SAM | 0.4 ± 0.0 | 63 ± 0.3 a | 3.8 ± 0.3 a | 4.1 ± 0.1 bc | 132 ± 8 c | 66 ± 4 ab |
| WHE-MAM | 0.6 ± 0.2 | 62 ± 0.1 b | 4.2 ± 0.1 a | 4.2 ± 0.1 bc | 193 ± 1 a | 61 ± 1 bc |
| Dough | 45 min | 90 min | 135 min | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Energy (cm2) | E (mm) | MR (EU) | MR/E | Energy (cm2) | E (mm) | MR (EU) | MR/E | Energy (cm2) | E (mm) | MR (EU) | MR/E | |
| WHE | 127 ± 11 a | 167 ± 10 a | 626 ± 62 a | 3.9 ± 0.5 a | 102 ± 6 a | 139 ± 3 a | 661 ± 33 a | 4.8 ± 0.2 b | 70 ± 8 a | 122 ± 10 a | 523 ± 22 a | 4.3 ± 0.3 ab |
| WHE-NFM | 54 ± 6 b | 93 ± 1 b | 446 ± 43 b | 4.8 ± 0.4 a | 38 ± 1 b | 74 ± 3 c | 432 ± 18 b | 5.9 ± 0.1 a | 16 ± 2 b | 68 ± 7 b | 189 ± 14 d | 2.8 ± 0.1 cd |
| WHE-SFM | 45 ± 0 bc | 85 ± 0 b | 405 ± 8 b | 4.8 ± 0.1 a | 35 ± 1 bc | 70 ± 1 c | 432 ± 17 b | 6.2 ± 0.3 a | 25 ± 3 b | 66 ± 5 b | 321 ± 16 b | 4.9 ± 0.1 a |
| WHE-MFM | 44 ± 3 bc | 88 ± 2 b | 383 ± 36 b | 4.4 ± 0.6 a | 29 ± 6 bc | 72 ± 4 c | 330 ± 46 cd | 4.6 ± 0.4 b | 17 ± 5 b | 63 ± 7 b | 204 ± 52 cd | 3.2 ± 0.4 c |
| WHE-NAM | 43 ± 3 bc | 89 ± 4 b | 379 ± 12 b | 4.3 ± 0.0 a | 39 ± 1 b | 79 ± 5 bc | 410 ± 18 bc | 5.3 ± 0.5 ab | 32 ± 3 b | 76 ± 1 b | 352 ± 33 b | 4.7 ± 0.5 a |
| WHE-SAM | 27 ± 3 c | 99 ± 8 b | 198 ± 4 c | 2.0 ± 0.1 b | 22 ± 0 c | 89 ± 1 b | 183 ± 3 e | 2.1 ± 0.1 c | 20 ± 1 b | 84 ± 2 b | 178 ± 4 d | 2.1 ± 0.0 d |
| WHE-MAM | 28 ± 1 c | 104 ± 0 b | 199 ± 9 c | 1.9 ± 0.1 b | 29 ± 2 bc | 88 ± 2 b | 252 ± 7 de | 2.9 ± 0.0 c | 31 ± 2 b | 84 ± 3 b | 285 ± 6 bc | 3.4 ± 0.0 bc |
| Bread | Weight (g) | Volume (cm3) | Specific Volume (cm3/g) | Firmness (N) | Cohesiveness ** | Resilience ** | Springiness (%) | Chewiness (N) | ΔL* |
|---|---|---|---|---|---|---|---|---|---|
| WHE | 341 ± 2 ab | 1448 ± 58 a | 4.3 ± 0.2 a | 3.0 ± 0.5 d | 0.74 ± 0.02 a | 0.31 ± 0.02 a | 91 ± 1 a | 2.0 ± 0.3 e | - |
| WHE-NFM | 335 ± 2 c | 1135 ± 30 d | 3.4 ± 0.1 cd | 7.2 ± 1.0 bc | 0.56 ± 0.02 d | 0.22 ± 0.01 de | 88 ± 1 b | 3.5 ± 0.5 bc | −20 ± 1 c |
| WHE-SFM | 340 ± 1 ab | 1135 ± 70 d | 3.3 ± 0.1 cd | 8.8 ± 0.8 b | 0.57 ± 0.04 d | 0.23 ± 0.02 cde | 86 ± 1 bc | 4.3 ± 0.6 b | −19 ± 1 c |
| WHE-MFM | 344 ± 1 a | 1070 ± 26 d | 3.1 ± 0.1 d | 6.6 ± 0.5 c | 0.55 ± 0.01 d | 0.22 ± 0.01 e | 86 ± 2 bc | 3.1 ± 0.3 cd | −21 ± 1 c |
| WHE-NAM | 339 ± 2 b | 1240 ± 28 c | 3.7 ± 0.1 bc | 4.2 ± 0.4 d | 0.67 ± 0.01 bc | 0.27 ± 0.01 b | 86 ± 1 bc | 2.4 ± 0.2 de | −8 ± 3 a |
| WHE-SAM | 344 ± 2 a | 1110 ± 42 d | 3.2 ± 0.2 d | 10.7 ± 1.4 a | 0.63 ± 0.03 c | 0.25 ± 0.01 bcd | 86 ± 1 bc | 5.8 ± 0.6 a | −10 ± 4 ab |
| WHE-MAM | 344 ± 2 a | 1350 ± 26 b | 3.9 ± 0.1 b | 2.7 ± 0.2 d | 0.68 ± 0.02 b | 0.26 ± 0.01 bc | 85 ± 1 c | 1.6 ± 0.1 e | −13 ± 2 b |
| Nutrient | WHE | WHE-NFM | WHE-SFM | WHE-MFM | WHE-NAM | WHE-SAM | WHE-MAM |
|---|---|---|---|---|---|---|---|
| Monosaccharides (mg/100 g) * | 1047 ± 1 g | 1347 ± 0 f | 1532 ± 1 e | 1715 ± 2 d | 3275 ± 1 b | 3253 ± 2 c | 4085 ± 1 a |
| Disaccharides (mg/100 g) ** | 2877 ± 2 a | 1577 ± 0 d | 1628 ± 1 c | 1951 ± 1 b | 587 ± 0 f | 509 ± 0 g | 714 ± 1 e |
| Sugar alcohols (mg/100 g) *** | 23 ± 0 b | 25 ± 0 a | 19 ± 0 c | 15 ± 0 e | 11 ± 0 g | 13 ± 0 f | 17 ± 0 d |
| Total starch (g/100 g) | 78 ± 1 | 77 ± 1 | 78 ± 3 | 78 ± 0 | 76 ± 0 | 76 ± 0 | 76 ± 0 |
| Digestible starch (% of total starch) | 96 ± 4 | 96 ± 3 | 95 ± 2 | 95 ± 2 | 98 ± 2 | 98 ± 1 | 98 ± 1 |
| Insoluble dietary fibre (g/100 g) | 2.7 ± 0.4 c | 5.4 ± 0.2 a | 5.5 ± 0.1 a | 5.5 ± 0.2 a | 4.4 ± 0.4 b | 4.1 ± 0.3 b | 4.5 ± 0.3 b |
| Soluble dietary fibre (g/100 g) | 1.8 ± 0.2 a | 1.7 ± 0.3 ab | 1.3 ± 0.1 b | 1.4 ± 0.2 b | 1.5 ± 0.2 ab | 1.6 ± 0.1 ab | 1.5 ± 0.2 ab |
| Total dietary fibre (g/100 g) | 4.5 ± 0.4 c | 7.1 ± 0.3 a | 6.8 ± 0.2 a | 6.9 ± 0.2 a | 6.0 ± 0.5 b | 5.7 ± 0.2 b | 6.0 ± 0.1 b |
| Arabinoxylan (mg/100 g) | 1363 ± 0 g | 1375 ± 0 f | 1413 ± 1 d | 1480 ± 1 b | 1497 ± 0 a | 1410 ± 0 e | 1441 ± 1 c |
| Arabinoxylan molecular weight (kDa) | 153 ± 5 a | 120 ± 16 bc | 134 ± 10 ab | 119 ± 6 bc | 91 ± 7 cd | 90 ± 0 cd | 85 ± 6 d |
| Total protein (g/100 g) | 14 ± 1 | 12 ± 0 | 12 ± 0 | 15 ± 2 | 15 ± 1 | 14 ± 2 | 15 ± 1 |
| In vitro protein digestibility (% of total protein) | 91 ± 3 | 89 ± 1 | 83 ± 5 | 87 ± 1 | 89 ± 2 | 84 ± 1 | 86 ± 2 |
| Lipid (g/100 g) | 2.1 ± 0.5 ab | 1.4 ± 0.3 b | 1.2 ± 0.2 b | 1.4 ± 0.2 b | 2.7 ± 0.3 a | 2.9 ± 0.0 a | 2.9 ± 0.2 a |
| Ash (g/100 g) | 1.6 ± 0.2 c | 2.5 ± 0.1 a | 2.4 ± 0.0 a | 2.2 ± 0.0 ab | 2.2 ± 0.0 ab | 2.2 ± 0.0 ab | 1.9 ± 0.1 bc |
| Phytate (mg/100 g) | 170 ± 36 b | 609 ± 141 a | 551 ± 7 a | 598 ± 8 a | 668 ± 15 a | 698 ± 16 a | 669 ± 44 a |
| Total phenolic content (mg GAE/100 g) | 59 ± 3 d | 88 ± 7 bc | 84 ± 9 bc | 94 ± 11 b | 60 ± 3 d | 74 ± 9 cd | 122 ± 10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onyango, C.; Luvitaa, S.K.; Lagat, K.; Hüsken, A.; Smit, I.; Schmidt, M. Utilisation of Amaranth and Finger Millet as Ingredients in Wheat Dough and Bread for Increased Agro-Food Biodiversity. Foods 2022, 11, 911. https://doi.org/10.3390/foods11070911
Onyango C, Luvitaa SK, Lagat K, Hüsken A, Smit I, Schmidt M. Utilisation of Amaranth and Finger Millet as Ingredients in Wheat Dough and Bread for Increased Agro-Food Biodiversity. Foods. 2022; 11(7):911. https://doi.org/10.3390/foods11070911
Chicago/Turabian StyleOnyango, Calvin, Susan Karenya Luvitaa, Kibet Lagat, Alexandra Hüsken, Inga Smit, and Marcus Schmidt. 2022. "Utilisation of Amaranth and Finger Millet as Ingredients in Wheat Dough and Bread for Increased Agro-Food Biodiversity" Foods 11, no. 7: 911. https://doi.org/10.3390/foods11070911
APA StyleOnyango, C., Luvitaa, S. K., Lagat, K., Hüsken, A., Smit, I., & Schmidt, M. (2022). Utilisation of Amaranth and Finger Millet as Ingredients in Wheat Dough and Bread for Increased Agro-Food Biodiversity. Foods, 11(7), 911. https://doi.org/10.3390/foods11070911