Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Colloid
2.2. Standard Solution Preparation
2.3. Sample Preparation
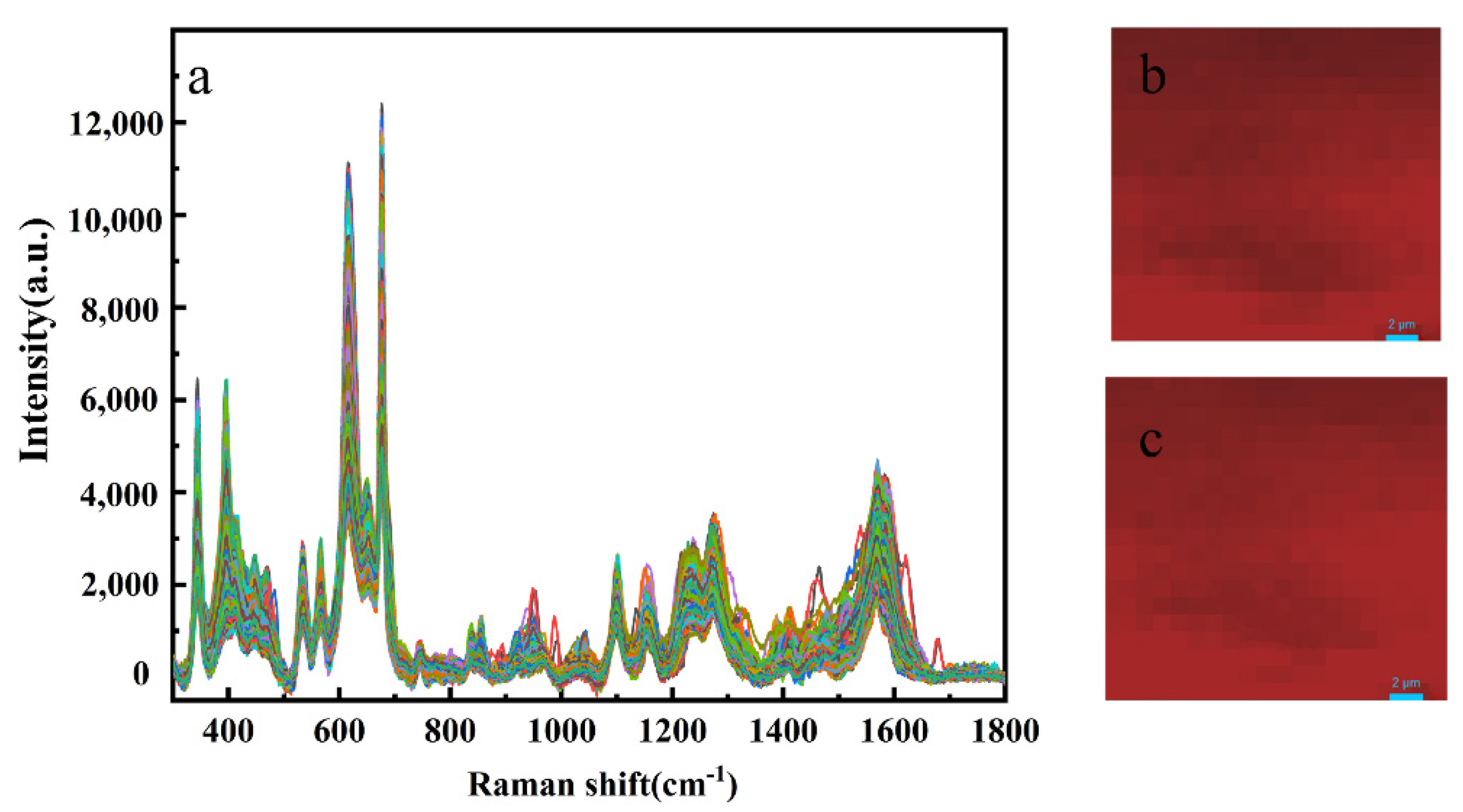
2.4. SERS Spectral Collection and Data Analysis
3. Results and Discussion
3.1. Spectral Features of CPF and 2,4-D Solid Sample
3.2. SERS Measurement and Analysis of CPF and 2,4-D Standard Solutions
3.3. SERS Measurements and Analysis of Apple Surface Samples Treated with CPF and 2,4-D
3.4. Reproducibility of the SERS Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hassan, M.M.; Li, H.; Ahmad, W.; Zareef, M.; Wang, J.; Xie, S.; Wang, P.; Ouyang, Q.; Wang, S.; Chen, Q. Au@Ag nanostructure based SERS substrate for simultaneous determination of pesticides residue in tea via solid phase extraction coupled multivariate calibration. Lebensm. Wiss. Und-Technol./Food Sci. Technol. 2019, 105, 290–297. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Anjum, F.M.; Ahmed, A.; Randhawa, M.S. Field incurred chlorpyrifos and 3,5,6-trichloro-2-pyridinol residues in fresh and processed vegetables. Food Chem. 2007, 103, 1016–1023. [Google Scholar] [CrossRef]
- Garcia, S.J.; Seidler, F.J.; Slotkin, T.A. Developmental neurotoxicity of chlorpyrifos: Targeting glial cells. Environ. Toxicol. Pharmacol. 2005, 19, 455–461. [Google Scholar] [CrossRef]
- Eaton, D.L.; Daroff, R.B.; Autrup, H.; Bridges, J.; Buffler, P.; Costa, L.G.; Coyle, J.; McKhann, G.; Mobley, W.C.; Nadel, L.; et al. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit. Rev. Toxicol. 2008, 38 (Suppl. 2), 1–125. [Google Scholar] [CrossRef] [PubMed]
- Meeker, J.D.; Ryan, L.; Barr, D.B.; Herrick, R.F.; Bennett, D.H.; Bravo, R.; Hauser, R. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environ. Health. Perspect. 2004, 112, 1665–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rauh, V.A.; Garfinkel, R.; Perera, F.P.; Andrews, H.F.; Hoepner, L.; Barr, D.B.; Whitehead, R.; Tang, D.; Whyatt, R.W. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics 2006, 118, e1845–e1859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bus, J.S.; Hammond, L.E. Regulatory progress, toxicology, and public concerns with 2,4-D: Where do we stand after two decades? Crop Prot. 2007, 26, 266–269. [Google Scholar] [CrossRef]
- Garabrant, H.D.; Philbert, M.A. Review of 2,4-Dichlorophenoxyacetic Acid (2,4-D) Epidemiology and Toxicology. Crit. Rev. Toxicol. 2002, 32, 233–257. [Google Scholar] [CrossRef]
- Cimino, A.M.; Boyles, A.L.; Thayer, K.A.; Perry, M.J. Effects of Neonicotinoid Pesticide Exposure on Human Health: A Systematic Review. Environ. Health Perspect. 2017, 125, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Delnat, V.; Tran, T.T.; Janssens, L.; Stoks, R. Resistance to a chemical pesticide increases vulnerability to a biopesticide: Effects on direct mortality and mortality by predation. Aquat. Toxicol. 2019, 216, 105310. [Google Scholar] [CrossRef]
- Tzatzarakis, M.; Kokkinakis, M.; Renieri, E.; Goumenou, M.; Kavvalakis, M.; Vakonaki, E.; Chatzinikolaou, A.; Stivaktakis, P.; Tsakiris, I.; Rizos, A.; et al. Multiresidue analysis of insecticides and fungicides in apples from the Greek market. Applying an alternative approach for risk assessment. Food Chem. Toxicol. 2020, 140, 111262. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control. 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Weichselbaum, E.; Wyness, L.; Stanner, S. Apple polyphenols and cardiovascular disease—A review of the evidence. Nutr. Bull. 2010, 35, 92–101. [Google Scholar] [CrossRef]
- Al-Nasir, F.M.; Jiries, A.G.; Al-Rabadi, G.J.; Alu’datt, M.H.; Tranchant, C.C.; Al-Dalain, S.A.; Alrabadi, N.; Madanat, O.Y.; Al-Dmour, R.S. Determination of pesticide residues in selected citrus fruits and vegetables cultivated in the Jordan Valley. LWT 2020, 123, 109005. [Google Scholar] [CrossRef]
- Li, G.; Yu, W.; Xiao, Z.; Long, M.; Tong, L.; Qiu, Y. A modified QuEChERS/GC–MS for simultaneous determination of 16 pesticide adjuvant residues in fruits and vegetables. SN Appl. Sci. 2019, 2, 35. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Liang, P.; Ye, J.; Xia, J.; Zhou, Y.; Huang, J.; Ni, D.; Tang, L.; Jin, S.; Yu, Z. Detection of systemic pesticide residues in tea products at trace level based on SERS and verified by GC-MS. Anal. Bioanal. Chem. 2019, 411, 7187–7196. [Google Scholar] [CrossRef]
- Pang, G.; Chang, Q.; Bai, R.; Fan, C.; Zhang, Z.; Yan, H.; Wu, X. Simultaneous Screening of 733 Pesticide Residues in Fruits and Vegetables by a GC/LC-Q-TOFMS Combination Technique. Engineering 2020, 6, 432–441. [Google Scholar] [CrossRef]
- Valera-Tarifa, N.M.; Santiago-Valverde, R.; Hernandez-Torres, E.; Martinez-Vidal, J.L.; Garrido-Frenich, A. Development and full validation of a multiresidue method for the analysis of a wide range of pesticides in processed fruit by UHPLC-MS/MS. Food Chem. 2020, 315, 126304. [Google Scholar] [CrossRef]
- Cheng, S.-C.; Lee, R.-H.; Jeng, J.-Y.; Lee, C.-W.; Shiea, J. Fast screening of trace multiresidue pesticides on fruit and vegetable surfaces using ambient ionization tandem mass spectrometry. Anal. Chim. Acta 2020, 1102, 63–71. [Google Scholar] [CrossRef]
- Armenta, S.; Quintas, G.; Garrigues, S.; Delaguardia, M. Mid-infrared and Raman spectrometry for quality control of pesticide formulations. TrAC Trends Anal. Chem. 2005, 24, 772–781. [Google Scholar] [CrossRef]
- Stiles, P.L.; Dieringer, J.A.; Shah, N.C.; Van Duyne, R.P. Surface-Enhanced Raman Spectroscopy. Annu. Rev. Anal. Chem. 2008, 1, 601–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Hu, Y.; Gao, J.; Zhong, L. Highly sensitive detection of clenbuterol using competitive surface-enhanced Raman scattering immunoassay. Anal. Chim. Acta 2011, 697, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; He, L. Surface-Enhanced Raman Spectroscopy for the Chemical Analysis of Food. Compr. Rev. Food Sci. Food Saf. 2014, 13, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.Y. Shell thickness-dependent Raman enhancement for rapid identification and detection of pesticide residues at fruit peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Silva, S.; Guedes, C.; Rodrigues, S.; Teixeira, A. Non-Destructive Imaging and Spectroscopic Techniques for Assessment of Carcass and Meat Quality in Sheep and Goats: A Review. Foods 2020, 9, 1074. [Google Scholar] [CrossRef]
- Czaja, T.; Sobota, A.; Szostak, R. Quantification of Ash and Moisture in Wheat Flour by Raman Spectroscopy. Foods 2020, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; You, T.; Yang, N.; Gao, Y.; Yin, P. Flexible SERS substrate based on Ag nanodendrite-coated carbon fiber cloth: Simultaneous detection for multiple pesticides in liquid droplet. Anal. Bioanal. Chem. 2020, 412, 1159–1167. [Google Scholar] [CrossRef]
- Ren, Z.; Zhou, X.; Gao, X.; Tan, Y.; Chen, H.; Tan, S.; Liu, W.; Tong, Y.; Chen, C. Rapid detection of carbamate pesticide residues using microchip electrophoresis combining amperometric detection. Anal. Bioanal. Chem. 2021, 413, 3017–3026. [Google Scholar] [CrossRef]
- Huang, D.; Zhao, J.; Wang, M.; Zhu, S. Snowflake-like gold nanoparticles as SERS substrates for the sensitive detection of organophosphorus pesticide residues. Food Control. 2020, 108, 106835. [Google Scholar] [CrossRef]
- Zhu, C.; Zhao, Q.; Meng, G.; Wang, X.; Hu, X.; Han, F.; Lei, Y. Silver nanoparticle-assembled micro-bowl arrays for sensitive SERS detection of pesticide residue. Nanotechnology 2020, 31, 205303. [Google Scholar] [CrossRef]
- Ma, P.; Wang, L.; Xu, L.; Li, J.; Zhang, X.; Chen, H. Rapid quantitative determination of chlorpyrifos pesticide residues in tomatoes by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2019, 246, 239–251. [Google Scholar] [CrossRef]
- Hu, B.; Sun, D.W.; Pu, H.; Wei, Q. Rapid nondestructive detection of mixed pesticides residues on fruit surface using SERS combined with self-modeling mixture analysis method. Talanta 2020, 217, 120998. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Huang, M.; Chen, J.; Lin, M. Fabrication of sensitive silver-decorated cotton swabs for SERS quantitative detection of mixed pesticide residues in bitter gourds. New J. Chem. 2020, 44, 12779–12784. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, X.; Zhang, S.J.; Liu, L.; Zhao, Y.M.; Xu, H.J. Ultrasensitive and quantitative detection of paraquat on fruits skins via surface-enhanced Raman spectroscopy. Sens. Actuators B Chem. 2015, 213, 452–456. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, L.; Zhang, Y.; Kang, Y.; Hu, H.; Tang, H.; Du, Y. Production of stable and sensitive SERS substrate based on commercialized porous material of silanized support. Talanta 2017, 174, 301–306. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.; Ma, P.; Li, J.; Zhang, Z.; Shi, H.; Zhang, X. In situ synthesis of graphene oxide/gold nanocomposites as ultrasensitive surface-enhanced Raman scattering substrates for clenbuterol detection. Anal. Bioanal. Chem. 2019, 412, 193–201. [Google Scholar] [CrossRef]
- Sacré, P.Y.; Bleye, C.D.; Chavez, P.F.; Netchacovitch, L.; Hubert, P.; Ziemons, E. Data processing of vibrational chemical imaging for pharmaceutical applications. J. Pharm. Biomed. Anal. 2014, 101, 123–140. [Google Scholar] [CrossRef]
- Bussian, B.; Härdle, W. Robust Smoothing Applied to White Noise and Single Outlier Contaminated Raman Spectra. Appl. Spectrosc. 1984, 38, 309–313. [Google Scholar] [CrossRef]
- Vo-Dinh, T.; Wu, W.; Lieberman, R.A.; Hu, M.; Ou, F.S.; Gauglitz, G.; Williams, R.S.; Li, Z. Rational engineering of highly sensitive SERS substrate based on nanocone structures. Chem. Phys. 2010, 7673, 76730O. [Google Scholar]
- Zhu, Y.; Wu, L.; Yan, H.; Lu, Z.; Yin, W.; Han, H. Enzyme induced molecularly imprinted polymer on SERS substrate for ultrasensitive detection of patulin. Anal. Chim. Acta 2020, 1101, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Rindzevicius, T.; Schmidt, M.S.; Thilsted, A.H.; Boisen, A. Optimizing silver-capped silicon nanopillars to simultaneously realize macroscopic, practical-level SERS signal reproducibility and high enhancement at low costs. J. Raman Spectrosc. 2017, 48, 1808–1818. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Li, Y.; Shi, W.; Shen, L.; Chen, Q.; Huang, L. Highly sensitive, reproducible and stable SERS substrate based on reduced graphene oxide/silver nanoparticles coated weighing paper. Appl. Surf. Sci. 2017, 404, 334–341. [Google Scholar] [CrossRef]
- Chen, N.; Yuan, Y.; Lu, P.; Wang, L.; Zhang, X.; Chen, H.; Ma, P. Detection of carbamazepine in saliva based on surface-enhanced Raman spectroscopy. Biomed. Opt. Express 2021, 12, 7673–7688. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Wang, H.; He, B. Quantitative Detection of Pesticides Based on SERS and Gold Colloid; HAL: Lyon, France, 2015. [Google Scholar]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.; Ahmad, W.; Li, H.; Chen, Q. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.M.; Li, H.; Chen, Q. Synthesized Au NPs@silica composite as surface-enhanced Raman spectroscopy (SERS) substrate for fast sensing trace contaminant in milk. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 206, 405–412. [Google Scholar] [CrossRef]
- Chen, H.; Luo, C.; Xing, L.; Guo, H.; Ma, P.; Zhang, X.; Zeng, L.; Sui, M. Simultaneous and ultra-sensitive SERS detection of SLPI and IL-18 for the assessment of donor kidney quality using black phosphorus/gold nanohybrids. Opt. Express 2022, 30, 1452–1465. [Google Scholar] [CrossRef]
- Jing, C.; Fang, Y. Experimental (SERS) and theoretical (DFT) studies on the adsorption behaviors of l-cysteine on gold/silver nanoparticles. Chem. Phys. 2007, 332, 27–32. [Google Scholar] [CrossRef]
- Tang, J.; Chen, W.; Ju, H. Rapid detection of pesticide residues using a silver nanoparticles coated glass bead as nonplanar substrate for SERS sensing. Sens. Actuators B Chem. 2019, 287, 576–583. [Google Scholar] [CrossRef]
- Ibanez, D.; Gonzalez-Garcia, M.B.; Hernandez-Santos, D.; Fanjul-Bolado, P. Detection of dithiocarbamate, chloronicotinyl and organophosphate pesticides by electrochemical activation of SERS features of screen-printed electrodes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 248, 119174. [Google Scholar] [CrossRef]
- Xu, Y.; Hassan, M.M.; Ali, S.; Li, H.; Chen, Q. SERS-based rapid detection of 2,4-dichlorophenoxyacetic acid in food matrices using molecularly imprinted magnetic polymers. Mikrochim. Acta 2020, 187, 454. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-K.; Kang, M.-A.; Kim, H.-J.; Park, Y.-S.; Kim, S.-H.; Kang, J.-S. Development of a LC-MS/MS for Quantification of Venlafaxine in Human Plasma and Application to Bioequivalence Study in healthy Korean Subjects. Transl. Clin. Pharmacol. 2014, 22, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.M.; Zhu, J.; Li, H.; Chen, Q. Functionalized hollow Au@Ag nanoflower SERS matrix for pesticide sensing in food. Sens. Actuators Chem. 2020, 324, 128718. [Google Scholar] [CrossRef]


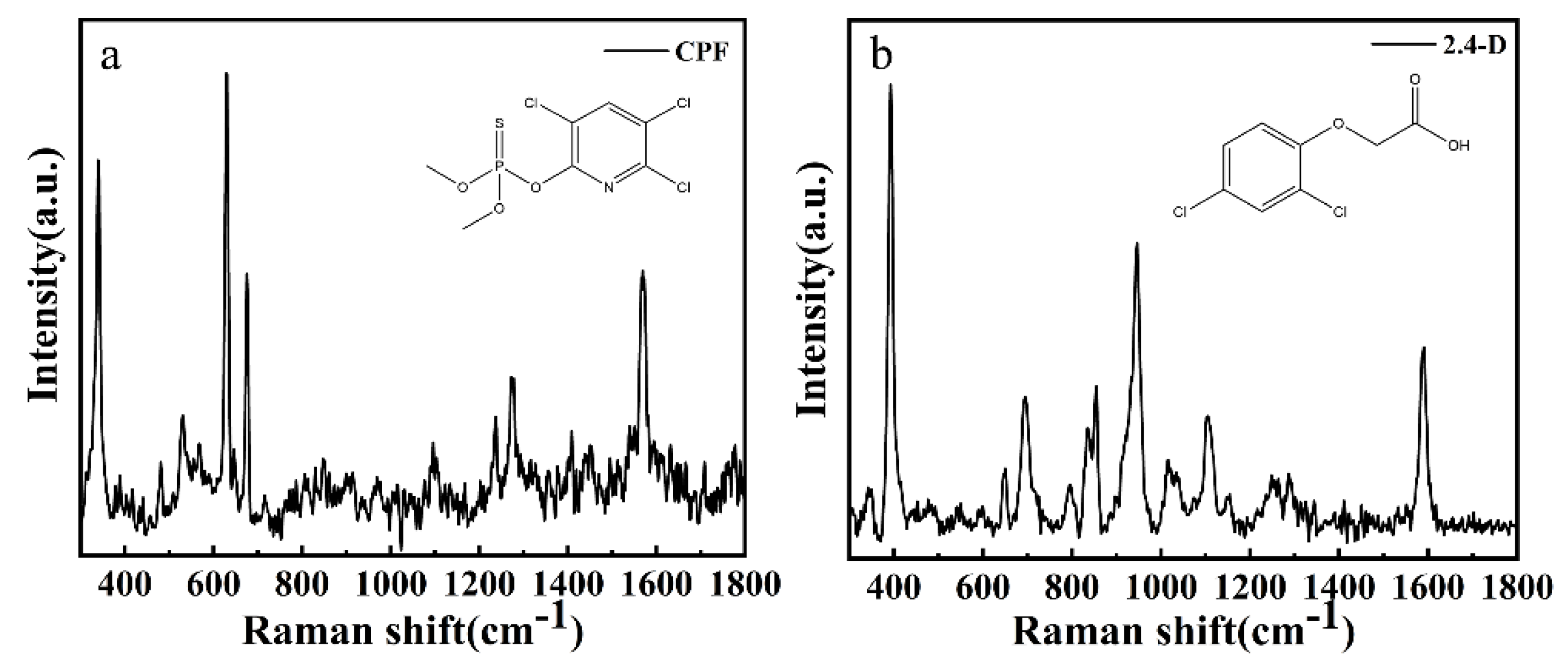
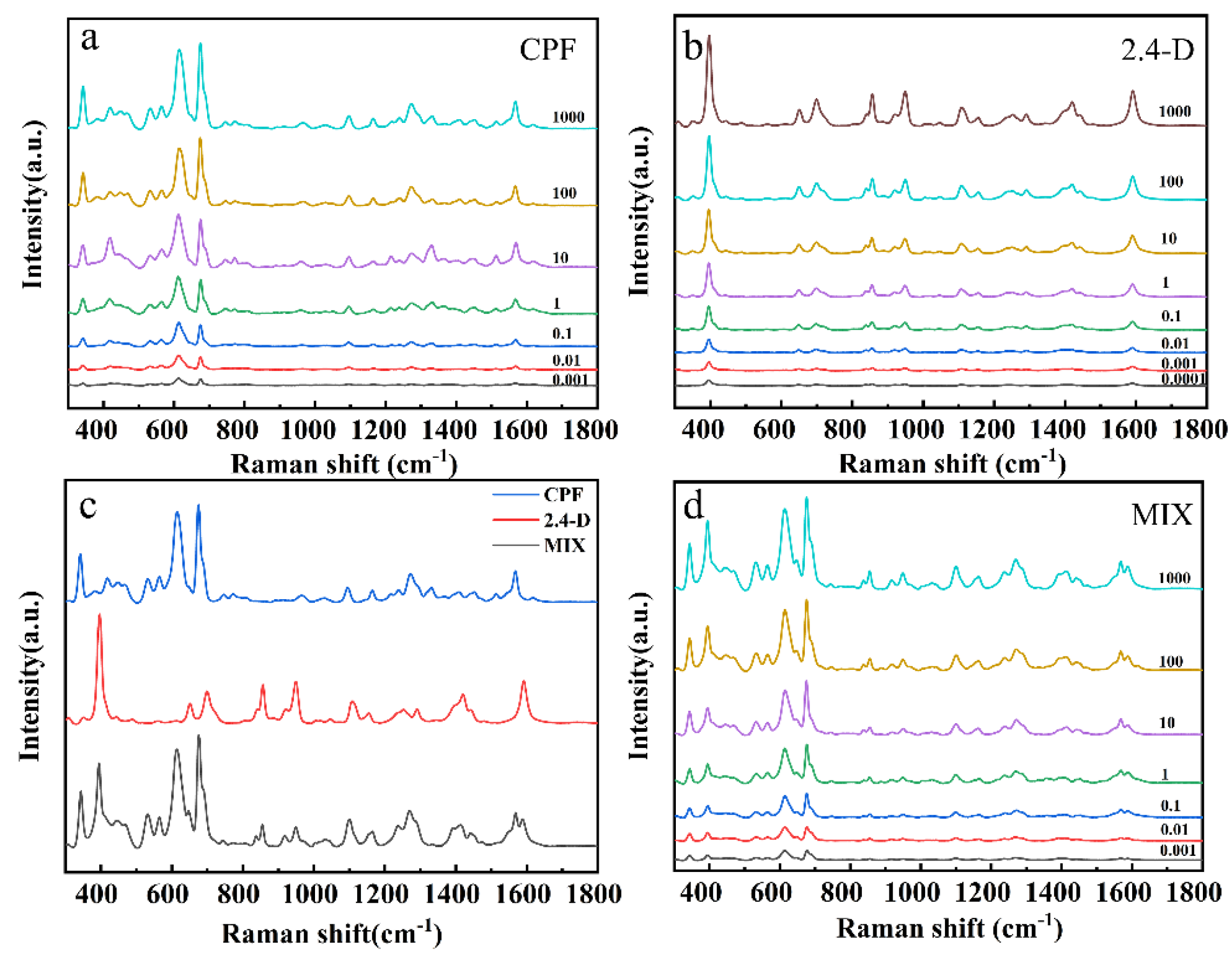



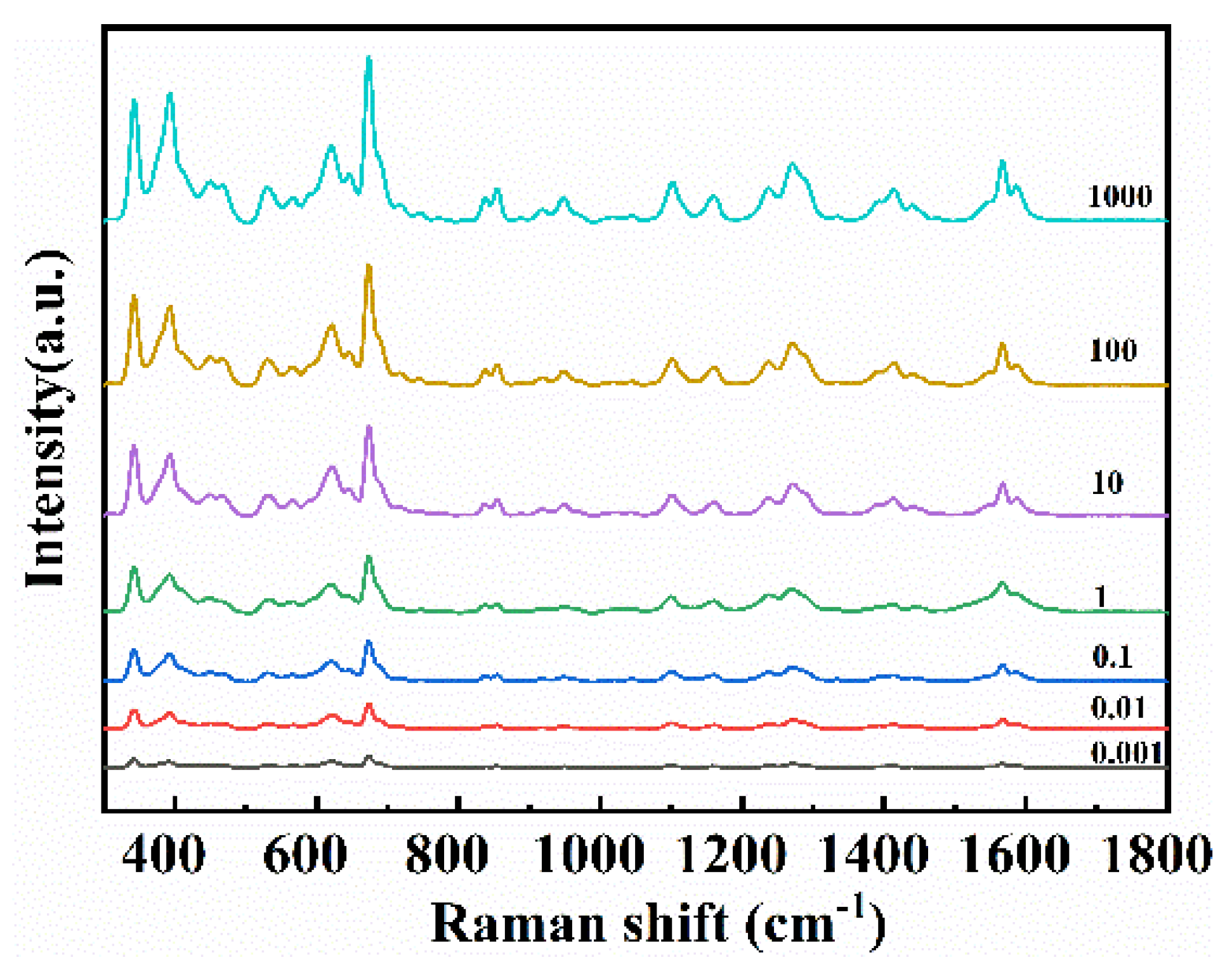
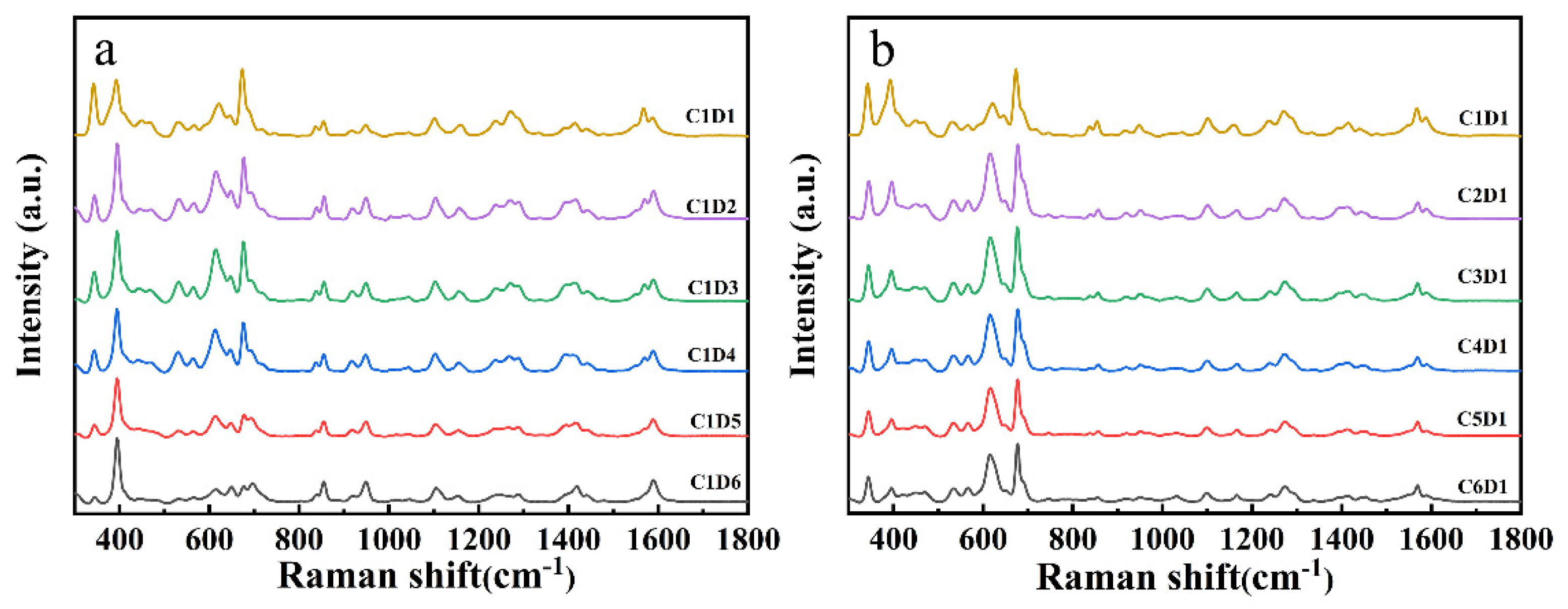

| Analyte | Raman/cm−1 | SERS/cm−1 | Assignment |
|---|---|---|---|
| CPF | 340 | 341 | N-cyclopropyl bending vibration |
| 411 | 395 | P–O–C stretch | |
| 631 | 613 | P=S | |
| 678 | 675 | P=S | |
| 970 | 962 | Cl-ring wagging | |
| 1103 | 1094 | P–O–C stretch | |
| 1240 | 1164 | Cl-ring, δ(C_H) | |
| 1278 | 1269 | Cl-ring, δ(C_H), νas(C=C) | |
| 1409 | 1406 | Cl-ring, ν(C_N), δ(C_H) | |
| 1455 | 1448 | Cl-ring, ν(C=C) | |
| 1573 | 1567 | Ring stretching | |
| 2,4-D | 386 | 392 | δ(COC) + δ(CCl) |
| 662 | 646 | υ(CC)ring + υ(C-Cl) | |
| 709 | 696 | δ(COO−) + υ(CC)ring | |
| 860 | 855 | υ(CC) ring + υ(C-O) + υ(C-Cl) | |
| 956 | 945 | υ(C- COO−) | |
| 1092 | 1101 | υ(CC)ring + υ(CO) + δ(CH)ring | |
| 1428 | 1415 | υs (COO−) + υ(CC) + ω(CH2) | |
| 1590 | 1590 | υas (COO−) + υ(CC)ring |
| Analyte | Peaks (cm−1) | Linear Equation | R2 |
|---|---|---|---|
| CPF | 342 | y = 0.215∗x + 3.70 | 0.96 |
| 613 | y = 0.141∗x + 4.15 | 0.95 | |
| 675 | y = 0.184∗x + 4.08 | 0.97 | |
| 2,4-D | 392 | y = 0.185∗x + 4.09 | 0.98 |
| 852 | y = 0.189∗x + 3.76 | 0.95 | |
| 1587 | y = 0.167∗x + 3.68 | 0.96 |
| Analyte | Peaks (cm−1) | Linear Equation | R2 |
|---|---|---|---|
| CPF | 341 | y = 0.159∗x + 3.91 | 0.96 |
| 621 | y = 0.158∗x + 3.88 | 0.96 | |
| 675 | y = 0.155∗x + 3.97 | 0.98 | |
| 2,4-D | 392 | y = 0.175∗x + 4.01 | 0.98 |
| 852 | y = 0.174∗x + 3.57 | 0.96 | |
| 1587 | y = 0.165∗x + 3.61 | 0.97 |
| Analyte | Added Concentration (mg L−1) | Detected Concentration (mg L−1) | Recovery Rate (%) |
|---|---|---|---|
| CPF | 100 | 96.210 | 96.21 |
| 1 | 0.8930 | 89.30 | |
| 0.01 | 0.0093 | 93.35 | |
| 2,4-D | 100 | 97.060 | 97.06 |
| 1 | 0.8797 | 87.97 | |
| 0.01 | 0.0089 | 89.45 |
| Peaks (cm−1) | Linear Equation | R2 |
|---|---|---|
| 392 | y = −373.664∗x + 23792.31 | 0.98 |
| 675 | y = −365.346∗x + 33583.38 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Ma, P.; Chen, H.; Chang, M.; Lu, P.; Chen, N.; Yuan, Y.; Chen, N.; Zhang, X. Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy. Foods 2022, 11, 1089. https://doi.org/10.3390/foods11081089
Wang L, Ma P, Chen H, Chang M, Lu P, Chen N, Yuan Y, Chen N, Zhang X. Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy. Foods. 2022; 11(8):1089. https://doi.org/10.3390/foods11081089
Chicago/Turabian StyleWang, Luyao, Pei Ma, Hui Chen, Min Chang, Ping Lu, Ning Chen, Yanbing Yuan, Nan Chen, and Xuedian Zhang. 2022. "Rapid Determination of Mixed Pesticide Residues on Apple Surfaces by Surface-Enhanced Raman Spectroscopy" Foods 11, no. 8: 1089. https://doi.org/10.3390/foods11081089







