Study on the Pasting Properties of Indica and Japonica Waxy Rice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Waxy Rice Flour
2.3. Pasting Properties
2.4. Physicochemical Analysis of Waxy Rice Flour
2.5. X-ray Diffraction
2.6. Determination of Structural Features of Isolated Starches
2.6.1. Isolation of Rice Starch
2.6.2. Molecular Weight and Radius of Rotation Analysis
2.6.3. Fine Structure of Amylopectin
2.7. Removal of Lipid, and Lipid and Protein from Rice Flours
2.7.1. Removal of Lipid
2.7.2. Removal of Lipid and Protein
2.8. Pasting Properties of the Isolated Rice Starch with Protein Addition
2.9. Extraction of Waxy Rice Protein
2.10. Pasting Properties of the Isolated Rice Starch with Globulin and Glutelin Addition
2.11. Statistical Analysis
3. Results and Discussion
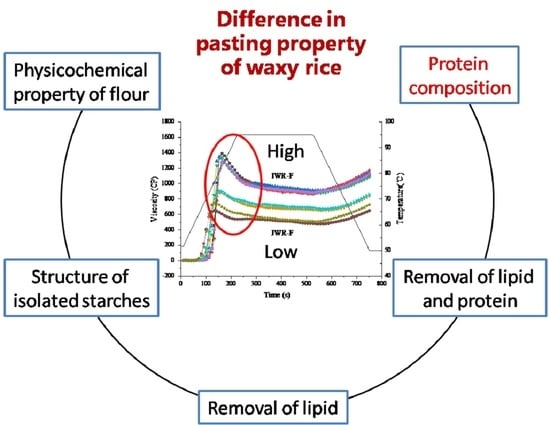
3.1. Pasting Properties of Waxy Rice Flours
3.2. Physicochemical Properties of Waxy Rice Flour
3.3. Structural Information of Isolated Starches
3.4. Effect of Lipid and Protein Removal on the Pasting Properties of Waxy Rice Flours
3.5. Effect of Protein Addition on the Pasting Properties of Isolated Starch
3.6. Analysis of Protein Composition in Waxy Rice Flour
3.7. Effect of Glutelin and Globulin Addition on the Pasting Properties of Isolated Starch
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, J.; Kaur, L.; Ogawa, Y. Importance of chemistry, nutrition and technology in rice processing. Food Chem. 2016, 191, 1. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.-H.; Lee, S.-J.; Hong, J.-Y.; Chung, H.-J.; Lee, Y.-T.; Kang, B.-S.; Lim, S.-T. Correlation between physicochemical properties of japonica and indica rice starches. LWT 2016, 66, 530–537. [Google Scholar] [CrossRef]
- Guo, L.; Li, J.; Gui, Y.; Zhu, Y.; Cui, B. Improving waxy rice starch functionality through branching enzyme and glu-coamylase: Role of amylose as a viable substrate. Carbohydr. Polym. 2019, 230, 115712. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Cho, J.H.; Kang, H.W.; Koh, B.K. Rice varieties in relation to rice bread quality. J. Sci. Food Agric. 2012, 92, 1462–1467. [Google Scholar] [CrossRef] [PubMed]
- Hasjim, J.; Li, E.; Dhital, S. Milling of rice grains: Effects of starch/flour structures on gelatinization and pasting properties. Carbohydr. Polym. 2013, 92, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Umemoto, T.; Hamada, S.; Suzuki, K.; Suzuki, Y. The Amylose Content and Amylopectin Structure Affect The Shape and Hardness of Rice Bread. J. Appl. Glycosci. 2012, 59, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Roman, L.; Reguilon, M.P.; Gomez, M.; Martinez, M.M. Intermediate length amylose increases the crumb hardness of rice flour gluten-free breads. Food Hydrocoll. 2020, 100, 105451. [Google Scholar] [CrossRef]
- Fredriksson, H.; Silverio, J.; Andersson, R.; Eliasson, A.-C.; Åman, P. The influence of amylose and amylopectin charac-teristics on gelatinization and retrogradation properties of different starches. Carbohydr. Polym. 1998, 35, 119–134. [Google Scholar] [CrossRef]
- Lin, J.-H.; Singh, H.; Chang, Y.-T.; Chang, Y.-H. Factor analysis of the functional properties of rice flours from mutant genotypes. Food Chem. 2011, 126, 1108–1114. [Google Scholar] [CrossRef]
- Sasaki, T.; Kohyama, K.; Suzuki, Y.; Okamoto, K.; Noel, T.R.; Ring, S.G. Physicochemical characteristics of waxy rice starch influencing the in vitro digestibility of a starch gel. Food Chem. 2009, 116, 137–142. [Google Scholar] [CrossRef]
- Baxter, G.; Blanchard, C.; Zhao, J. Effects of glutelin and globulin on the physicochemical properties of rice starch and flour. J. Cereal Sci. 2014, 60, 414–420. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, B.; Li, M.-N.; Xie, Y.; Chen, H.-Q. Effects of glutenin and gliadin modified by protein-glutaminase on pasting, rheological properties and microstructure of potato starch. Food Chem. 2018, 253, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, N.; Duan, B.; Zhu, Z.; Liao, X. Impact of proteins on pasting and cooking properties of waxy and non-waxy rice. J. Cereal Sci. 2008, 47, 372–379. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Chen, Z.; Yu, Q.; Feng, W. Impact of protein content on processing and texture properties of waxy rice flour and glutinous dumpling. J. Cereal Sci. 2018, 81, 30–36. [Google Scholar] [CrossRef]
- Martin, M.; Fitzgerald, M. Proteins in Rice Grains Influence Cooking Properties! J. Cereal Sci. 2002, 36, 285–294. [Google Scholar] [CrossRef]
- Leewatchararongjaroen, J.; Anuntagool, J. Effects of Dry-Milling and Wet-Milling on Chemical, Physical and Gelatinization Properties of Rice Flour. Rice Sci. 2016, 23, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Park, I.-M.; Ibáñez, A.M.; Zhong, F.; Shoemaker, C.F. Gelatinization and Pasting Properties of Waxy and Non-waxy Rice Starches. Starch-Stärke 2007, 59, 388–396. [Google Scholar] [CrossRef]
- Zhu, L.-J.; Liu, Q.-Q.; Sang, Y.; Gu, M.-H.; Shi, Y.-C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2010, 120, 94–100. [Google Scholar] [CrossRef]
- Lu, X.; Chang, R.; Lu, H.; Ma, R.; Qiu, L.; Tian, Y. Effect of amino acids composing rice protein on rice starch digestibility. LWT 2021, 146, 111417. [Google Scholar] [CrossRef]
- De Souza, D.; Sbardelotto, A.F.; Ziegler, D.R.; Marczak, L.D.F.; Tessaro, I.C. Characterization of rice starch and protein obtained by a fast alkaline extraction method. Food Chem. 2016, 191, 36–44. [Google Scholar] [CrossRef]
- Liu, H.; Rong, L.; Antoniou, J.; Fei, L.; Shoemaker, C.F.; Li, Y.; Fang, Z. The effect of high moisture heat-acid treatment on the structure and digestion property of normal maize starch. Food Chem. 2014, 159, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Han, X.-Z.; Hamaker, B.R. Amylopectin Fine Structure and Rice Starch Paste Breakdown. J. Cereal Sci. 2001, 34, 279–284. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Chen, Z.; Zhong, Q. Amylopectin-Sodium Palmitate Complexes as Sustainable Nanohydrogels with Tunable Size and Fractal Dimensions. J. Agric. Food Chem. 2020, 68, 3796–3805. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Luo, S.; McClements, D.J.; Liang, L.; Liu, C. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018, 106, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiao, A.; Zhao, S.; Liu, Q.; Fu, X.; Jin, Z. Effect of removal of endogenous non-starch components on the structural, physicochemical properties, and in vitro digestibility of highland barley starch. Food Hydrocoll. 2021, 117, 106698. [Google Scholar] [CrossRef]
- Yi, C.; Gao, W.; Zhong, C.; He, Y. Effect of Alkaline-Soluble Proteins on Pasting Properties of Nonwaxy Rice Flour. Cereal Chem. 2014, 91, 502–507. [Google Scholar] [CrossRef]
- Ju, Z.; Hettiarachchy, N.; Rath, N. Extraction, denaturation and hydrophobic Properties of Rice Flour Proteins. J. Food Sci. 2001, 66, 229–232. [Google Scholar] [CrossRef]
- Vega-Rojas, L.J.; Londoño-Restrepo, S.M.; Rodriguez-García, M.E. Study of morphological, structural, thermal, and pasting properties of flour and isolated starch from unripe plantain (Musa paradisiaca). Int. J. Biol. Macromol. 2021, 183, 1723–1731. [Google Scholar] [CrossRef]
- Kumar, L.; Brennan, M.; Brennan, C.; Zheng, H. Thermal, pasting and structural studies of oat starch-caseinate interactions. Food Chem. 2022, 373, 131433. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, Y.; Wu, G.; Qi, X.; Dag, D.; Kong, F.; Zhang, H. Characteristics of pasting properties and morphology changes of rice starch and flour under different heating modes. Int. J. Biol. Macromol. 2020, 149, 246–255. [Google Scholar] [CrossRef]
- Hu, W.-X.; Chen, J.; Xu, F.; Chen, L.; Zhao, J.-W. Study on crystalline, gelatinization and rheological properties of japonica rice flour as affected by starch fine structure. Int. J. Biol. Macromol. 2020, 148, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Asmeda, R.; Noorlaila, A.; Norziah, M. Relationships of damaged starch granules and particle size distribution with pasting and thermal profiles of milled MR263 rice flour. Food Chem. 2016, 191, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Prakash, S.; Nicholson, T.M.; Fitzgerald, M.A.; Gilbert, R.G. The importance of amylose and amylopectin fine structure for textural properties of cooked rice grains. Food Chem. 2016, 196, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Keeratipibul, S.; Luangsakul, N.; Lertsatchayarn, T. The effect of Thai glutinous rice cultivars, grain length and cultivating locations on the quality of rice cracker (arare). LWT 2008, 41, 1934–1943. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, Z.; Zhu, L.; Hong, Y. Comparative study on the interaction between native corn starch and different hy-drocolloids during gelatinization. Int. J. Biol. Macromol. 2018, 116, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G.N.; Bustos, M.C.; Iturriaga, L.; Flores, S.; León, A.E.; Ribotta, P. Effect of damaged starch on the rheological properties of wheat starch suspensions. J. Food Eng. 2013, 116, 233–239. [Google Scholar] [CrossRef]
- You, S.-Y.; Lim, S.-T.; Lee, J.H.; Chung, H.-J. Impact of molecular and crystalline structures on in vitro digestibility of waxy rice starches. Carbohydr. Polym. 2014, 112, 729–735. [Google Scholar] [CrossRef]
- Zhang, T.; Li, X.; Chen, L.; Situ, W. Digestibility and structural changes of waxy rice starch during the fermentation process for waxy rice vinasse. Food Hydrocoll. 2016, 57, 38–45. [Google Scholar] [CrossRef]
- Xla, C.; Rui, X.; Jza, B.; Long, C.C.; Zja, C.; Yta, C. Pasting, rheology, and fine structure of starch for waxy rice powder with high-temperature baking. Int. J. Biol. Macromol. 2020, 146, 620–626. [Google Scholar]
- Tappiban, P.; Ying, Y.; Pang, Y.; Sraphet, S.; Srisawad, N.; Smith, D.R.; Wu, P.; Triwitayakorn, K.; Bao, J. Gelatinization, pasting and retrogradation properties and molecular fine structure of starches from seven cassava cultivars. Int. J. Biol. Macromol. 2020, 150, 831–838. [Google Scholar] [CrossRef]
- Ding, Y.; Cheng, J.; Lin, Q.; Wang, Q.; Wang, J.; Yu, G. Effects of endogenous proteins and lipids on structural, thermal, rheological, and pasting properties and digestibility of adlay seed (Coix lacryma-jobi L.) starch. Food Hydrocoll. 2021, 111, 106254. [Google Scholar] [CrossRef]
- Han, X.-Z.; Benmoussa, M.; Gray, J.A.; BeMiller, J.N.; Hamaker, B.R. Detection of Proteins in Starch Granule Channels. Cereal Chem. 2005, 82, 351–355. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Lai, H.-M. Characteristics of the starch fine structure and pasting properties of waxy rice during storage. Food Chem. 2014, 152, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Kawamura-Konishi, Y.; Shoda, K.; Koga, H.; Honda, Y. Improvement in gluten-free rice bread quality by protease treatment. J. Cereal Sci. 2013, 58, 45–50. [Google Scholar] [CrossRef]








| Sample | Protein (%) | Fat (%) | Total Starch (%) | AMYLOSE (%) | Ash (%) | Starch Damage (%) | Average Particle Size (μm) |
|---|---|---|---|---|---|---|---|
| IWR-1-F | 8.83 ± 0.17 d | 0.53 ± 0.01 e | 89.77 ± 0.00 b | 4.58 ± 0.09 d | 0.29 ± 0.01 b | 9.40 ± 0.11 bc | 164.33 ± 0.88 e |
| IWR-2-F | 7.27 ± 0.14 a | 0.58 ± 0.02 f | 92.86 ± 0.14 d | 4.44 ± 0.05 d | 0.26 ± 0.00 a | 8.26 ± 0.21 a | 163.33 ± 1.89 e |
| IWR-3-F | 8.30 ± 0.00 c | 0.56 ± 0.02 ef | 91.05 ± 1.40 c | 4.13 ± 0.09 c | 0.35 ± 0.01 c | 11.42 ± 0.07 f | 76.65 ± 1.96 a |
| IWR-4-F | 7.46 ± 0.26 ab | 0.44 ± 0.015 d | 91.58 ± 0.62 c | 4.13 ± 0.09 c | 0.35 ± 0.01 c | 9.61 ± 0.10 cd | 140.17 ± 2.14 d |
| IWR-5-F | 7.90 ± 0.10 bc | 0.41 ± 0.02 d | 90.83 ± 1.23 bc | 4.08 ± 0.05 c | 0.36 ± 0.01 c | 10.43 ± 0.10 e | 164.83 ± 7.41 e |
| JWR-1-F | 7.67 ± 0.11 ab | 0.28 ± 0.01 b | 89.90 ± 0.60 b | 3.68 ± 0.00 b | 0.34 ± 0.01 c | 12.03 ± 0.14 gh | 98.47 ± 1.94 b |
| JWR-2-F | 8.13 ± 0.10 c | 0.24 ± 0.005 a | 91.69 ± 0.57 c | 3.54 ± 0.05 b | 0.37 ± 0.01 c | 12.48 ± 0.31 hi | 113.28 ± 5.90 c |
| JWR-3-F | 9.41 ± 0.18 e | 0.69 ± 0.00 g | 87.76 ± 2.14 a | 3.27 ± 0.05 a | 0.44 ± 0.00 d | 11.62 ± 0.14 fg | 86.73 ± 0.53 a |
| JWR-4-F | 10.50 ± 0.02 f | 0.36 ± 0.00 c | 89.95 ± 1.70 b | 3.18 ± 0.05 a | 0.42 ± 0.00 d | 11.99 ± 0.23 gh | 81.87 ± 1.76 a |
| Sample | Mw/ × 10 7 g/mol | Rz/nm | Average DP/% | A Chain/% | B1 Chain/% | B2 Chain/% | B3 Chain/% |
|---|---|---|---|---|---|---|---|
| IWR-1-S | 3.91 ± 0.00 f | 133.5 ± 0.1 f | 16.46 ± 0.00 e | 34.18 ± 0.00 c | 54.17 ± 0.00 g | 9.23 ± 0.01 f | 2.42 ± 0.00 b |
| IWR-2-S | 4.57 ± 0.00 h | 128.8 ± 0.2 e | 16.46 ± 0.00 e | 34.02 ± 0.01 b | 54.09 ± 0.01 e | 9.77 ± 0.00 h | 2.12 ± 0.01 a |
| IWR-3-S | 3.06 ± 0.01 c | 126.8 ± 0.2 d | 16.47 ± 0.00 e | 34.44 ± 0.01 d | 53.91 ± 0.01 d | 8.94 ± 0.00 d | 2.71 ± 0.00 h |
| IWR-4-S | 3.94 ± 0.01 g | 135.1 ± 0.1 g | 16.48 ± 0.01 f | 34.52 ± 0.01 e | 53.51 ± 0.01 a | 9.50 ± 0.01 g | 2.47 ± 0.01 d |
| IWR-5-S | 3.28 ± 0.03 e | 126.6 ± 0.1 d | 16.61 ± 0.01 g | 33.25 ± 0.01 a | 55.10 ± 0.01 h | 9.03 ± 0.01 e | 2.61 ± 0.01 f |
| JWR-1-S | 2.75 ± 0.01 a | 121.6 ± 0.2 a | 16.32 ± 0.01 d | 34.80 ± 0.00 f | 54.11 ± 0.00 f | 8.43 ± 0.01 c | 2.66 ± 0.01 g |
| JWR-2-S | 3.20 ± 0.02 d | 124.2 ± 0.2 b | 16.28 ± 0.00 c | 34.94 ± 0.01 g | 54.12 ± 0.01 f | 8.35 ± 0.00 b | 2.59 ± 0.01 e |
| JWR-3-S | 3.19 ± 0.09 d | 125.3 ± 0.3 c | 16.19 ± 0.01 b | 35.52 ± 0.01 h | 53.71 ± 0.01 c | 8.35 ± 0.00 b | 2.42 ± 0.01 b |
| JWR-4-S | 3.04 ± 0.01 b | 124.2 ± 0.2 b | 16.17 ± 0.00 a | 35.59 ± 0.01 i | 53.65 ± 0.01 b | 8.32 ± 0.01 a | 2.43 ± 0.00 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, S.; Chen, C.; Yao, Y.; Nsor-Atindana, J.; Liu, F.; Chen, M.; Zhong, F. Study on the Pasting Properties of Indica and Japonica Waxy Rice. Foods 2022, 11, 1132. https://doi.org/10.3390/foods11081132
Fang S, Chen C, Yao Y, Nsor-Atindana J, Liu F, Chen M, Zhong F. Study on the Pasting Properties of Indica and Japonica Waxy Rice. Foods. 2022; 11(8):1132. https://doi.org/10.3390/foods11081132
Chicago/Turabian StyleFang, Sicong, Cheng Chen, Yuan Yao, John Nsor-Atindana, Fei Liu, Maoshen Chen, and Fang Zhong. 2022. "Study on the Pasting Properties of Indica and Japonica Waxy Rice" Foods 11, no. 8: 1132. https://doi.org/10.3390/foods11081132
APA StyleFang, S., Chen, C., Yao, Y., Nsor-Atindana, J., Liu, F., Chen, M., & Zhong, F. (2022). Study on the Pasting Properties of Indica and Japonica Waxy Rice. Foods, 11(8), 1132. https://doi.org/10.3390/foods11081132