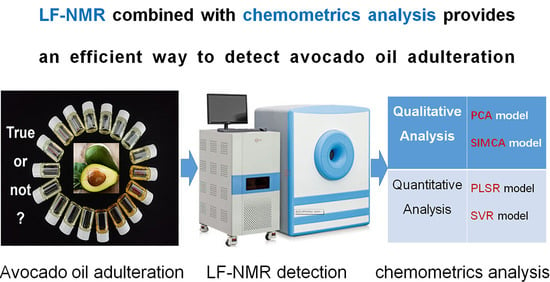
Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Oil Samples
2.2. Acquisition of LF-NMR Signals
2.3. Viscosity Measurement
2.4. Analysis of Fatty Acid Composition
2.5. Statistics Analysis
3. Results and Discussion
3.1. Comparison of T2 Parameters between Pure and Adulterated AO
3.2. Classification of Pure and Adulterated AO by PCA and SIMCA Analyses
3.3. Quantitative Analysis of Adulteration Levels by PLSR and SVR
3.4. Comparison of Viscosity and Fatty Acid Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rydlewski, A.A.; Pizzo, J.S.; Manin, L.P.; Galuch, M.B.; Santos, P.D.S.; Zapiello, C.; Santos, O.O.; Visentainer, J.V. Evaluation of possible fraud in avocado oil-based products from the composition of fatty acids by GC-FID and lipid profile by ESI-MS. Chem. Pap. 2020, 74, 2799–2812. [Google Scholar] [CrossRef]
- Berasategi, I.; Barriuso, B.; Ansorena, D.; Astiasarán, I. Stability of avocado oil during heating: Comparative study to olive oil. Food Chem. 2012, 132, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Ramírez, C.A.; de la Paz, J.L.H.; Ortiz-Avila, O.; Raya-Farias, A.; González-Hernández, J.C.; Rodríguez-Orozco, A.R.; Salgado-Garciglia, R.; Saavedra-Molia, A.; Godinez-Hernandez, D. Comparative effects of avocado oil and losartan on blood pressure, renal vascular function, and mitochondrial oxidative stress in hypertensive rats. Nutrition 2018, 54, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X. Virgin avocado oil: An emerging source of functional fruit oil. J. Funct. Food. 2019, 54, 381–392. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Franco, E.D.S.; Barreto, R.R.; Cordeiro, D.P.; Melo, R.G.; Aquino, C.M.F.; Silva, A.A.R.E.; Medeiros, P.L.; Silva, T.G.; Goes, A.J.D. Effect of semisolid formulation of persea Americana mill (avocado) oil on wound healing in rats. Evid.-Based Complement. Altern. Med. 2013, 2013, 472382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forero-Doría, O.; Garcia, M.F.; Vergara, C.E.; Guzman, L. Thermal analysis and antioxidant activity of oil extracted from pulp of ripe avocados. J. Therm. Anal. Calorim. 2017, 130, 959–966. [Google Scholar] [CrossRef]
- Unlu, N.Z.; Bohn, T.; Clinton, S.K.; Schwartz, S.J. Carotenoid absorption from salad and salsa by humans is enhanced by the addition of avocado or avocado oil. J. Nutr. 2004, 135, 431–436. [Google Scholar] [CrossRef]
- Ashton, O.B.O.; Wong, M.; McGhie, T.K.; Vather, R.; Wang, Y.; Requejo-Jackman, C.; Ramankutty, P.; Woolf, A.B. Pigments in avocado tissue and oil. J. Agric. Food Chem. 2006, 54, 10151–10158. [Google Scholar] [CrossRef]
- Green, H.S.; Wang, S.C. First report on quality and purity evaluations of avocado oil sold in the us. Food Control 2020, 116, 107328. [Google Scholar] [CrossRef]
- Quiñones-Islas, N.; Meza-Márquez, O.G.; Osorio-Revilla, G.; Gallardo-Velazquez, T. Detection of adulterants in avocado oil by mid-FTIR spectroscopy and multivariate analysis. Food Res. Int. 2013, 51, 148–154. [Google Scholar] [CrossRef]
- Espinosa-Alonso, L.G.; Paredes-López, O.; Valdez-Morales, M.; Oomah, B.D. Avocado oil characteristics of mexican creole genotypes. Eur. J. Lipid Sci. Technol. 2017, 119, 1600406. [Google Scholar] [CrossRef]
- Martín-Torres, S.; Jiménez-Carvelo, A.M.; González-Casado, A.; Cuadros-Rodríguez, L. Authentication of the geographical origin and the botanical variety of avocados using liquid chromatography fingerprinting and deep learning methods. Chemom. Intell. Lab. Syst. 2020, 199, 103960. [Google Scholar] [CrossRef]
- Ruiz-Samblás, C.; Marini, F.; Cuadros-Rodríguez, L.; González-Casado, A. Quantification of blending of olive oils and edible vegetable oils by triacylglycerol fingerprint gas chromatography and chemometric tools. J. Chromatogr. B 2012, 910, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ferro, M.D.; Cavaco, I.; Liang, Y. Detection and identification of extra virgin olive oil adulteration by GC-MS combined with chemometrics. J. Agric. Food Chem. 2013, 61, 3693–3702. [Google Scholar] [CrossRef]
- Shi, T.; Wu, G.C.; Jin, Q.Z.; Wang, X.G. Detection of camellia oil adulteration using chemometrics based on fatty acids GC fingerprints and phytosterols GC-MS fingerprints. Food Chem. 2021, 352, 129422. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shen, D.; Mo, R.; Zhong, D.; Tang, F. Simultaneous determination of 15 multiresidue organophosphorous pesticides in camellia oil by MSPD-GC-MS. Bull. Environ. Contam. Toxicol. 2013, 90, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Salghi, R.; Armbruster, W.; Schwack, W. Detection of argan oil adulteration with vegetable oils by high-performance liquid chromatography-evaporative light scattering detection. Food Chem. 2014, 153, 387–392. [Google Scholar] [CrossRef] [PubMed]
- De la Mata-Espinosa, P.; Bosque-Sendra, J.M.; Bro, R.; Cuadros-Rodríguez, L. Olive oil quantification of edible vegetable oil blends using triacylglycerols chromato-graphic fingerprints and chemometric tools. Talanta 2011, 85, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, A.; Jamwal, R.; Kumari, S.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid detection of pure coconut oil adulteration with fried coconut oil using ATR-FTIR spectroscopy coupled with multivariate regression modelling. LWT-Food Sci. Technol. 2020, 125, 109250. [Google Scholar] [CrossRef]
- Rohman, A.; Windarsih, A.; Riyanto, S.; Sudjadi; Ahmad, S.A.S.; Rosman, A.S.; Yusoff, F.M. Fourier transform infrared spectroscopy combined with multivariate calibrations for the authentication of avocado oil. Int. J. Food Prop. 2016, 19, 680–687. [Google Scholar] [CrossRef]
- Huang, Z.M.; Xin, J.X.; Sun, S.S.; Li, Y.; Wei, D.X.; Zhu, J.; Wang, X.L.; Wang, J.C.; Yao, Y.F. Rapid identification of adulteration in edible vegetable oils based on low-field nuclear magnetic resonance relaxation fingerprints. Foods 2021, 10, 3068. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Caravaca, A.M.; Maggio, R.M.; Cerretani, L. Chemometric applications to assess quality and critical parameters of virgin and extra-virgin olive oil. A review. Anal. Chim. Acta 2016, 913, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.W.; Wang, G.L.; Wang, X.; Ge, X.M.; Fan, Y.R.; Nie, S.D. Convolutional neural network based approach for classification of edible oils using low-field nuclear magnetic resonance. J. Food Compos. Anal. 2020, 92, 103566. [Google Scholar] [CrossRef]
- Toledo, R.M.; Steinberg, M.P.; Nelson, A.I. Quantitative determination of bound water by NMR. J. Food Sci. 2010, 33, 315–317. [Google Scholar] [CrossRef]
- Galvan, D.; Tanamati, A.A.C.; Casanova, F.; Danieli, E.; Bona, E.; Killner, M.H.M. Compact low-field NMR spectroscopy and chemometrics applied to the analysis of edible oils. Food Chem. 2021, 365, 130476. [Google Scholar] [CrossRef]
- Sun, Y.N.; Zhang, M.; Fan, D.C. Effect of ultrasonic on deterioration of oil in microwave vacuum frying and prediction of frying oil quality based on low field nuclear magnetic resonance (LF-NMR). Ultrason. Sonochem. 2019, 51, 77–89. [Google Scholar] [CrossRef]
- Jamwal, A.; Jamwal, R.; Kumari, S.; Dhaulaniya, A.S.; Balan, B.; Singh, D.K. Application of ATR-FTIR spectroscopy along with regression modelling for the detection of adulteration of virgin coconut oil with paraffin oil. LWT-Food Sci. Technol. 2020, 118, 108754. [Google Scholar] [CrossRef]
- Ogegbo, O.L.; Eyob, S.; Parmar, S.; Wang, Z.T.; Bligh, S.W.A. Metabolomics of four TCM herbal products: Application of HPTLC analysis. Anal. Methods 2012, 4, 2522–2527. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Trygg, J.; Wuttge, D.M.; Sturfelt, G.; Theander, E.; Donten, M.; Moritz, T.; Sennbro, C.J.; Torell, F.; Lood, C.; et al. Metabolic profiling of systemic lupus erythematosus and comparison with primary sjgren’s syndrome and systemic sclerosis. PLoS ONE 2016, 11, e0159384. [Google Scholar] [CrossRef]
- Fiamegos, Y.; Dumitrascu, C.; Papoci, S.; de la Calle, M.B. Authentication of PDO paprika powder (pimentón de la vera) by multivariate analysis of the elemental fingerprint determined by ED-XRF. A feasibility study. Food Control 2020, 120, 107496. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Tu, D.W. Application of Fourier Transform Infrared (FT-IR) Spectroscopy combined with chemometrics for analysis of rapeseed oil adulterated with refining and purificating waste cooking oil. Food Anal. Methods 2015, 8, 2581–2587. [Google Scholar] [CrossRef]
- Rohman, A.; Che Man, Y.B.; Ismail, A.; Hashim, P. FTIR spectroscopy coupled with chemometrics of multivariate calibration and discriminant analysis for authentication of extra virgin olive oil. Int. J. Food Prop. 2017, 20, S1173–S1181. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Rapid and non-destructive detection of chicken adulteration in minced beef using visible near-infrared hyperspectral imaging and machine learning. J. Food Eng. 2016, 170, 8–15. [Google Scholar] [CrossRef]
- Olivieri, A.C. Practical guidelines for reporting results in single- and multi-component analytical calibration: A tutorial. Anal. Chim. Acta 2015, 868, 10–22. [Google Scholar] [CrossRef]
- Nikolskaya, E.; Hiltunen, Y. Determination of carbon chain lengths of fatty acid mixtures by time domain NMR. Appl. Magn. Reson. 2018, 49, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.H.; Lai, G.Y.; Lin, J.Z.; Xia, F.; Ding, Z.N.; Feng, J.H.; Xu, J.J.; Shen, G.P. Rapid detection of adulteration in extra virgin olive oil by low-field nuclear magnetic resonance combined with pattern recognition. Food Anal. Methods 2021, 14, 1322–1335. [Google Scholar] [CrossRef]
- Lu, R.; Zhou, X.; Wu, W.; Zhang, Y.; Ni, Z. Development of the miniature NMR apparatus for edible oil quality control. Appl. Magn. Reson. 2014, 45, 461–469. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.; Chen, L. Rapid detection of peanut oil adulteration using low-field nuclear magnetic resonance and chemometrics. Food Chem. 2017, 216, 268–274. [Google Scholar] [CrossRef]
- Gribnau, M.C.M. Determination of solid/liquid ratios of fats and oils by low-resolution pulsed NMR. Trends Food Sci. Technol. 1992, 3, 186–190. [Google Scholar] [CrossRef]





| Model | Number | PCs | R2X | Q2 | AUC |
|---|---|---|---|---|---|
| PCA | 141 | 6 | 0.995 | 0.981 | - |
| SIMCA (AO) | 15 | 7 | 0.996 | 0.886 | 1.000 |
| SIMCA (AO-SO) | 42 | 6 | 0.994 | 0.975 | 0.993 |
| SIMCA (AO-CO) | 42 | 5 | 0.993 | 0.974 | 0.963 |
| SIMCA (AO-RO) | 42 | 5 | 0.998 | 0.945 | 0.997 |
| Parameters | Calibration | Validation | ||||||
|---|---|---|---|---|---|---|---|---|
| AO | AO-SO | AO-CO | AO-RO | AO | AO-SO | AO-CO | AO-RO | |
| True positive rate (TPR) | 0.93 | 0.98 | 1.00 | 0.98 | 0.6 | 1.00 | 1.00 | 0.89 |
| False negative rate (FNR) | 0.07 | 0.02 | 0.00 | 0.02 | 0.4 | 0.00 | 0.00 | 0.11 |
| Positive predictive value (PPV) | 1.00 | 1.00 | 0.95 | 0.98 | 1.00 | 1.00 | 0.90 | 0.89 |
| False discovery rate (FDR) | 0.00 | 0.00 | 0.05 | 0.02 | 0.00 | 0.00 | 0.10 | 0.11 |
| Accuracy | 0.98 | 0.93 | ||||||
| Samples | Avocado Oil | Soybean Oil | Corn Oil | Rapeseed Oil | |||
|---|---|---|---|---|---|---|---|
| Place of Origin | France | France | New Zealand | Mexico | China | China | China |
| Viscosity (mPa s) | 61.56 ± 1.33 | 61.50 ± 0.70 | 61.22 ± 0.80 | 60.12 ± 0.61 | 48.20 ± 0.74 | 50.96 ± 0.24 | 56.56 ± 0.28 |
| Fatty acids (%) | |||||||
| Myristic acid (C14:0) | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.07 ± 0.00 | 0.04 ± 0.00 | 0.06 ± 0.00 |
| Palmitic acid (C16:0) | 11.84 ± 0.03 | 10.66 ± 0.01 | 11.47 ± 0.01 | 12.76 ± 0.01 | 10.66 ± 0.03 | 11.21 ± 0.01 | 4.67 ± 0.02 |
| Palmitoleic acid (C16:1) | 1.69 ± 0.00 | 1.82 ± 0.01 | 1.93 ± 0.00 | 2.53 ± 0.00 | 0.07 ± 0.00 | 0.09 ± 0.00 | 0.22 ± 0.00 |
| Stearic acid (C18:0) | 2.44 ± 0.02 | 2.31 ± 0.02 | 2.33 ± 0.01 | 2.25 ± 0.01 | 4.19 ± 0.01 | 2.06 ± 0.01 | 1.85 ± 0.03 |
| Oleic acid (C18:1n9c) | 69.2 ± 0.05 | 70.91 ± 0.01 | 71.03 ± 0.03 | 64.49 ± 0.04 | 24.09 ± 0.04 | 28.19 ± 0.02 | 58.14 ± 0.04 |
| Linoleic acid (C18:2n6c) | 13.24 ± 0.02 | 12.76 ± 0.02 | 11.77 ± 0.02 | 16.25 ± 0.02 | 53.39 ± 0.03 | 56.84 ± 0.02 | 20.24 ± 0.02 |
| Arachidic acid (C20:0) | 0.35 ± 0.00 | 0.32 ± 0.00 | 0.34 ± 0.00 | 0.32 ± 0.00 | 0.37 ± 0.00 | 0.34 ± 0.00 | 0.62 ± 0.00 |
| γ-Linolenic acid (C18:3n6) | ND | ND | ND | ND | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.59 ± 0.00 |
| cis-11-Eicosadienoic acid (C20:1) | 0.27 ± 0.00 | 0.26 ± 0.00 | 0.27 ± 0.00 | 0.26 ± 0.00 | 0.20 ± 0.00 | 0.25 ± 0.00 | ND |
| α-Linolenic acid (C18:3n3) | 0.65 ± 0.00 | 0.52 ± 0.00 | 0.59 ± 0.00 | 0.93 ± 0.00 | 6.37 ± 0.01 | 0.58 ± 0.00 | 7.78 ± 0.03 |
| cis-11,14-Eicosadienoic acid (C20:2) | ND | ND | ND | ND | ND | ND | 0.12 ± 0.00 |
| Behenic acid (C22:0) | 0.22 ± 0.00 | 0.28 ± 0.00 | 0.18 ± 0.00 | 0.17 ± 0.00 | 0.40 ± 0.00 | 0.22 ± 0.00 | 0.35 ± 0.00 |
| cis-11,14,17-Eicosatrienoic acid (C20:3n3) | ND | ND | ND | ND | ND | ND | 5.09 ± 0.02 |
| cis-5,8,11,14,17-Eicosapentaenoic acid (C20:5n3) | 0.09 ± 0.00 | 0.12 ± 0.01 | 0.08 ± 0.00 | ND | 0.13 ± 0.00 | 0.14 ± 0.00 | ND |
| Nervonic acid (C24:1n9) | ND | ND | ND | ND | ND | ND | 0.28 ± 0.00 |
| Saturated fatty acids (SFA) | 14.87 ± 0.05 | 13.62 ± 0.03 | 14.36 ± 0.03 | 15.54 ± 0.02 | 15.69 ± 0.03 | 13.87 ± 0.02 | 7.55 ± 0.06 |
| Monounsaturated fatty acids (MUFA) | 71.16 ± 0.05 | 72.99 ± 0.02 | 73.22 ± 0.03 | 67.28 ± 0.04 | 24.36 ± 0.04 | 24.36 ± 0.02 | 58.65 ± 0.04 |
| Polyunsaturated fatty acids (PUFA) | 13.97 ± 0.02 | 13.39 ± 0.02 | 12.43 ± 0.02 | 17.18 ± 0.03 | 59.95 ± 0.04 | 57.60 ± 0.02 | 33.81 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, H.; Wang, Y.; Lv, B.; Zhang, K.; Zhu, Z.; Zhao, D.; Li, C. Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance. Foods 2022, 11, 1134. https://doi.org/10.3390/foods11081134
Jin H, Wang Y, Lv B, Zhang K, Zhu Z, Zhao D, Li C. Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance. Foods. 2022; 11(8):1134. https://doi.org/10.3390/foods11081134
Chicago/Turabian StyleJin, Haoquan, Yuxuan Wang, Bowen Lv, Kexin Zhang, Zhe Zhu, Di Zhao, and Chunbao Li. 2022. "Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance" Foods 11, no. 8: 1134. https://doi.org/10.3390/foods11081134
APA StyleJin, H., Wang, Y., Lv, B., Zhang, K., Zhu, Z., Zhao, D., & Li, C. (2022). Rapid Detection of Avocado Oil Adulteration Using Low-Field Nuclear Magnetic Resonance. Foods, 11(8), 1134. https://doi.org/10.3390/foods11081134