Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil
Abstract
1. Introduction
2. Literature Search and Analysis
3. Characteristics of Microalgal Polyunsaturated Fatty Acids
3.1. Microalgal Production of n-3 PUFA
3.1.1. Production of n-3 PUFA in Wild-Type Microalgae
3.1.2. Production of PUFA in Recombinant Microalgae
3.2. Synthetic Pathways of PUFA in Microalgae
4. Commercial Production of PUFA Using Microalgae
4.1. Environmental Factors Influencing Microalgal PUFA Production
4.1.1. Light
4.1.2. Temperature
4.1.3. Salinity
4.1.4. Carbon
4.1.5. Nitrogen
4.1.6. Phosphorus
4.1.7. Other Minerals
4.2. Commercial Cultivation Systems for Microalgal PUFA Production
4.2.1. Photoautotrophic Cultivation
4.2.2. Heterotrophic Fermentation
4.3. Harvesting and Drying of Microalgae
4.4. Pretreatment of Microalgae by Cell Wall Disruption
4.5. Extraction of Microalgae Oil
4.6. Concentration and Purification of Microalgae Oil
5. Protection of PUFA via Microencapsulation
5.1. Spray Drying
5.2. Spray Cooling/Chilling (or Prilling)
5.3. Freeze Drying
5.4. Complex Coacervation
5.5. Nanoemulsions and Self-Emulsifying Emulsions
5.6. Liposome
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, Q.; Li, H.; Xiao, Y.; Liu, H. A state-of-the-art review on the synthetic mechanisms, production technologies, and practical application of polyunsaturated fatty acids from microalgae. Algal Res. 2021, 55, 102281. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Cvejic, J.H.; Langellotti, A.L.; Bonnefond, H.; Verardo, V.; Bernard, O. Microalgae as a source of edible oils. In Lipids and Edible Oils: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 175–210. [Google Scholar]
- Guiry, M. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef]
- Wolkers, H.; Barbosa, M.J.; Kleinegris, D.; Bosma, R.; Wijffels, R.; Harmsen, P. Microalgae: The Green Gold of the Future? Large-Scale Sustainable Cultivation of Microalgae for the Production of Bulk Commodities; Wageningen UR-Food & Biobased Research: Wageningen, The Netherlands, 2011. [Google Scholar]
- Buono, S.; Langellotti, A.L.; Martello, A.; Rinna, F.; Fogliano, V. Functional ingredients from microalgae. Food Funct. 2014, 5, 1669–1685. [Google Scholar] [CrossRef]
- Blackburn, S.; Volkman, J. Microalgae: A Renewable Source of Bioproducts; Wiley-Blackwell: New York, NY, USA, 2012; pp. 221–241. [Google Scholar]
- Wang, Y.; Tibbetts, S.; McGinn, P. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. The potential of microalgae and their biopolymers as structuring ingredients in food: A review. Biotechnol. Adv. 2019, 37, 107419. [Google Scholar] [CrossRef]
- Remize, M.; Brunel, Y.; Silva, J.; Berthon, J.-Y.; Filaire, E. Microalgae n-3 PUFAs Production and Use in Food and Feed Industries. Mar. Drugs 2021, 19, 113. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Mimouni, V. Lipids From Microalgae. In Microalgae in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2018; pp. 109–131. [Google Scholar]
- Udayan, A.; Arumugam, M.; Pandey, A. Nutraceuticals From Algae and Cyanobacteria. In Algal Green Chemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 65–89. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef]
- Lafourcade, M.; Larrieu, T.; Mato, S.; Duffaud, A.; Sepers, M.; Matias, I.; Smedt-Peyrusse, V.D.; Labrousse, V.F.; Bretillon, L.; Matute, C. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat. Neurosci. 2011, 14, 345–350. [Google Scholar] [CrossRef]
- Yurko-Mauro, K.; Mccarthy, D.; Rom, D.; Nelson, E.B.; Ryan, A.S.; Blackwell, A.; Salem, N.; Stedman, M. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimers Dement. 2010, 6, 456–464. [Google Scholar] [CrossRef]
- Cottin, S.C.; Sanders, T.A.; Hall, W.L. The differential effects of EPA and DHA on cardiovascular risk factors. Proc. Nutr. Soc. 2011, 70, 215–231. [Google Scholar] [CrossRef]
- Khan, M.I.; Jin, H.S.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Uma, V.S.; Dineshbabu, G. Biobased Fats and Oils from Microalgae; Elsevier: Amsterdam, The Netherlands, 2020; pp. 273–298. [Google Scholar]
- Chen, Z.; Wang, L.; Qiu, S.; Ge, S. Determination of Microalgal Lipid Content and Fatty Acid for Biofuel Production. Biomed. Res. Int. 2018, 2018, 1503126. [Google Scholar] [CrossRef]
- Sathasivam, R.; Radhakrishnan, R.; Hashem, A.; Abd_Allah, E.F. Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 2019, 26, 709–722. [Google Scholar] [CrossRef]
- Singh, D.V.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Health benefits of bioactive compounds from microalgae. Phytomedicine 2021, 291–319. [Google Scholar] [CrossRef]
- Viso, A.-C.; Marty, J.-C. Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Kainz, M.; Telmer, K.; Mazumder, A. Bioaccumulation patterns of methyl mercury and essential fatty acids in lacustrine planktonic food webs and fish. Sci. Total Environ. 2006, 368, 271–282. [Google Scholar] [CrossRef]
- Arterburn, L.M.; Oken, H.A.; Hoffman, J.P.; Bailey-Hall, E.; Chung, G.; Rom, D.; Hamersley, J.; Mccarthy, D. Bioequivalence of Docosahexaenoic Acid from Different Algal Oils in Capsules and in a DHA-Fortified Food. Lipids 2007, 42, 1011. [Google Scholar] [CrossRef]
- Lien, E.L.; Richard, C.; Hoffman, D.R. DHA and ARA addition to infant formula: Current status and future research directions. Prostaglandins Leukot. Essent. Fat. Acids 2018, 128, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Desai, S.S.; Mane, V.K.; Enman, J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Futuristic food fortification with a balanced ratio of dietary ω-3/ω-6 omega fatty acids for the prevention of lifestyle diseases. Trends Food Sci. Technol. 2022, 120, 140–153. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Xu, J.; Wang, Y.-M.; Xue, C.-H. Health benefits of dietary marine DHA/EPA-enriched glycerophospholipids. Prog. Lipid Res. 2019, 75, 100997. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Keum, Y.-S. Omega-3 and omega-6 polyunsaturated fatty acids: Dietary sources, metabolism, and significance—A review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, C.A.; Senhorinho, G.N.A.; Laamanen, C.A.; Scott, J.A. Microalgae as an alternative to oil crops for edible oils and animal feed. Algal Res. 2022, 64, 102663. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar] [CrossRef]
- Diao, J.; Song, X.; Guo, T.; Wang, F.; Chen, L.; Zhang, W. Cellular engineering strategies toward sustainable omega-3 long chain polyunsaturated fatty acids production: State of the art and perspectives. Biotechnol. Adv. 2020, 40, 107497. [Google Scholar] [CrossRef]
- Draaisma, R.B.; Wijffels, R.H.; Slegers, P.; Brentner, L.B.; Roy, A.; Barbosa, M.J. Food commodities from microalgae. Curr. Opin. Biotechnol. 2013, 24, 169–177. [Google Scholar] [CrossRef]
- Cohen, Z.; Ratledge, C. Single Cell Oils: Microbial and Algal Oils, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Araujo, G.S.; Matos, L.J.B.L.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Bioprospecting for oil producing microalgal strains: Evaluation of oil and biomass production for ten microalgal strains. Bioresour. Technol. 2011, 102, 5248–5250. [Google Scholar] [CrossRef]
- San Pedro, A.; González-López, C.V.; Acién, F.G.; Molina-Grima, E. Outdoor pilot-scale production of Nannochloropsis gaditana: Influence of culture parameters and lipid production rates in tubular photobioreactors. Bioresour. Technol. 2014, 169, 667–676. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Beardall, J.; Raven, J.A. Lipid Metabolism in Microalgae. In The Physiology of Microalgae; Springer: Cham, Switzerland, 2016; Chapter 18; pp. 413–484. [Google Scholar] [CrossRef]
- Cohen, Z. Monodus subterraneus. In Chemicals from Microalgae; Cohen, Z., Ed.; CRC Press: Boca Raton, FL, USA, 1999; pp. 25–44. [Google Scholar]
- Singh, I.P.; Sidana, J. Functional Ingredients from Algae for Foods and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Lang, I.; Hodac, L.; Friedl, T.; Feussner, I. Fatty acid profiles and their distribution patterns in microalgae: A comprehensive analysis of more than 2000 strains from the SAG culture collection. BMC Plant Biol. 2011, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Chapman, P. The biochemistry of dinoflagellate lipids, with particular reference to the fatty acid and sterol composition of a Karenia brevis bloom. Phycologia 2003, 42, 324. [Google Scholar]
- Mooney, B.D.; Nichols, P.D.; Salas, M.; Hallegraeff, G.M. Lipid, fatty acid, and sterol composition of eight species of Kareniaceae (Dinophyta): Chemotaxonomy and putative lipid phycotoxins. J. Phycol. 2007, 43, 101–111. [Google Scholar] [CrossRef]
- Abida, H.; Dolch, L.-J.; Meï, C.; Villanova, V.; Conte, M.; Block, M.A.; Finazzi, G.; Bastien, O.; Tirichine, L.; Bowler, C.; et al. Membrane Glycerolipid Remodeling Triggered by Nitrogen and Phosphorus Starvation in Phaeodactylum tricornutum. Plant Physiol. 2015, 167, 118–136. [Google Scholar] [CrossRef]
- Liang, Y.; Maeda, Y.; Yoshino, T.; Matsumoto, M.; Tanaka, T. Profiling of Polar Lipids in Marine Oleaginous Diatom Fistulifera solaris JPCC DA0580: Prediction of the Potential Mechanism for Eicosapentaenoic Acid-Incorporation into Triacylglycerol. Mar. Drugs 2014, 12, 3218–3230. [Google Scholar] [CrossRef]
- Arao, T.; Kawaguchi, A.; Yamada, M. Positional distribution of fatty acids in lipids of the marine diatom Phaeodactylum tricornutum. Phytochemistry 1987, 26, 2573–2576. [Google Scholar] [CrossRef]
- Miller, M.R.; Quek, S.Y.; Staehler, K.; Nalder, T.; Packer, M.A. Changes in oil content, lipid class and fatty acid composition of the microalga Chaetoceros calcitrans over different phases of batch culture. Aquac. Res. 2015, 45, 1634–1647. [Google Scholar] [CrossRef]
- Callejón, M.; Medina, A.R.; Moreno, P.; Cerdán, L.; Grima, E.M. Simultaneous extraction and fractionation of lipids from the microalga Nannochloropsis sp. for the production of EPA-rich polar lipid concentrates. J. Appl. Phycol. 2020, 32, 1117–1128. [Google Scholar] [CrossRef]
- Costa, E.D.; Amaro, H.M.; Melo, T.; Guedes, A.C.; Domingues, M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of Gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. J. Appl. Phycol. 2020, 32, 3015–3030. [Google Scholar] [CrossRef]
- Klyachko-Gurvich, G.L.; Tsoglin, L.N.; Doucha, J.; Kopetskii, J.; Ryabykh, I.B.S.; Semenenko, V.E. Desaturation of fatty acids as an adaptive response to shifts in light intensity 1. Physiol. Plant. 1999, 107, 240–249. [Google Scholar] [CrossRef]
- Adarme-Vega, T.; Lim, D.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Lee Chang, K.J.; Nichols, C.M.; Blackburn, S.I.; Dunstan, G.A.; Koutoulis, A.; Nichols, P.D. Comparison of Thraustochytrids Aurantiochytrium sp. Schizochytrium sp. Thraustochytrium sp. and Ulkenia sp. for Production of Biodiesel, Long-Chain Omega-3 Oils, and Exopolysaccharide. Mar. Biotechnol. 2014, 16, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.; Dietrich, D.; Becker, J.; Kohlstedt, M.; Wittmann, C. Microbial production of polyunsaturated fatty acids—High-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr. Opin. Biotechnol. 2021, 69, 199–211. [Google Scholar] [CrossRef] [PubMed]
- James, G.O.; Hocart, C.H.; Hillier, W.; Chen, H.; Kordbacheh, F.; Price, G.D.; Djordjevic, M.A. Fatty acid profiling of Chlamydomonas reinhardtii under nitrogen deprivation. Bioresour. Technol. 2011, 102, 3343–3351. [Google Scholar] [CrossRef]
- De Swaaf, M.E.; Sijtsma, L.; Pronk, J.T. High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol. Bioeng. 2003, 81, 666–672. [Google Scholar] [CrossRef]
- Wynn, J.; Behrens, P.; Sundararajan, A.; Hansen, J.; Apt, K. 6-Production of Single Cell Oils by Dinoflagellates. In Single Cell Oils, 2nd ed.; Cohen, Z., Ratledge, C., Eds.; AOCS Press: Champaign, IL, USA, 2010; pp. 115–129. [Google Scholar]
- Rodolfi, L.; Chini Zittelli, G.; Bassi, N.; Padovani, G.; Biondi, N.; Bonini, G.; Tredici, M.R. Microalgae for oil: Strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol. Bioeng. 2009, 102, 100–112. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; Hwang, J.-H.; Cho, Y.; Min, B.; Jeon, B.-H. Characterization of microalgal species isolated from fresh water bodies as a potential source for biodiesel production. Appl. Energy 2011, 88, 3300–3306. [Google Scholar] [CrossRef]
- Manikan, V.; Nazir, M.Y.M.; Kalil, M.S.; Isa, M.H.M.; Kader, A.J.A.; Yusoff, W.M.W.; Hamid, A.A. A new strain of docosahexaenoic acid producing microalga from Malaysian coastal waters. Algal Res. 2015, 9, 40–47. [Google Scholar] [CrossRef]
- Chang, G.; Luo, Z.; Gu, S.; Wu, Q.; Chang, M.; Wang, X. Fatty acid shifts and metabolic activity changes of Schizochytrium sp. S31 cultured on glycerol. Bioresour. Technol. 2013, 142, 255–260. [Google Scholar] [CrossRef]
- Bonnefond, H.; Moelants, N.; Talec, A.; Mayzaud, P.; Bernard, O.; Sciandra, A. Coupling and uncoupling of triglyceride and beta-carotene production by Dunaliella salina under nitrogen limitation and starvation. Biotechnol. Biofuels 2017, 10, 25. [Google Scholar] [CrossRef]
- Sprague, M.; Betancor, M.B.; Tocher, D.R. Microbial and genetically engineered oils as replacements for fish oil in aquaculture feeds. Biotechnol. Lett. 2017, 39, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, K.S.; Relph, L.E.; Armstrong, M.C.; Rahman, P.K. Biofuel production: Tapping into microalgae despite challenges. Biofuels 2017, 8, 261–271. [Google Scholar] [CrossRef][Green Version]
- Remmers, I.M.; Martens, D.E.; Wijffels, R.H.; Lamers, P.P. Dynamics of triacylglycerol and EPA production in Phaeodactylum tricornutum under nitrogen starvation at different light intensities. PLoS ONE 2017, 12, e0175630. [Google Scholar] [CrossRef]
- Trentacoste, E.M.; Shrestha, R.P.; Smith, S.R.; Glé, C.; Gerwick, W.H. Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19748–19753. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Mary, H.; Stephen, P.; Johnathan, N.; Olga, S. Heterotrophic Production of Omega-3 Long-Chain Polyunsaturated Fatty Acids by Trophically Converted Marine Diatom Phaeodactylum tricornutum. Mar. Drugs 2016, 14, 53. [Google Scholar]
- Geng, L.; Chen, S.; Sun, X.; Hu, X.; Ji, X.; Huang, H.; Ren, L. Fermentation performance and metabolomic analysis of an engineered high-yield PUFA-producing strain of Schizochytrium sp. Bioprocess Biosyst. Eng. 2019, 42, 71–81. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, S.; Feng, Y.; Wan, W.; Zhang, H.; Bai, X.; Cui, Q.; Song, X. Obtaining High-Purity Docosahexaenoic Acid Oil in Thraustochytrid Aurantiochytrium through a Combined Metabolic Engineering Strategy. J. Agric. Food Chem. 2021, 69, 10215–10222. [Google Scholar] [CrossRef]
- Anderson, M.S.; Muff, T.J.; Georgianna, D.R.; Mayfield, S.P. Towards a syntheti c nuclear transcription system in green algae: Characterization of Chlamydomonas reinhardtii nuclear transcription factors and identification of targeted promoters. Algal Res. 2017, 22, 47–55. [Google Scholar] [CrossRef]
- Fields, F.J.; Ostrand, J.T.; Mayfield, S.P. Fed-batch mixotrophic cultivation of Chlamydomonas reinhardtii for high-density cultures. Algal Res. 2018, 33, 109–117. [Google Scholar] [CrossRef]
- Rasala, B.A.; Chao, S.S.; Matthew, P.; Barrera, D.J.; Mayfield, S.P.; Ning, C.W. Enhanced Genetic Tools for Engineering Multigene Traits into Green Algae. PLoS ONE 2014, 9, e94028. [Google Scholar] [CrossRef] [PubMed]
- Molino, J.; Carvalho, J.; Mayfield, S.P. Comparison of secretory signal peptides for heterologous protein expression in microalgae: Expanding the secretion portfolio for Chlamydomonas reinhardtii. PLoS ONE 2018, 13, e0192433. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.G.; Jose, A.; Lee, S.-L.; Wang, T.; Harrata, K.A. Microalgae Lipid Characterization. J. Agric. Food Chem. 2015, 63, 1773. [Google Scholar] [CrossRef]
- Wu, S.; Huang, W.G.A.; Li, Y.; Kumar, M.; Lim, P.E.; Huan, L.; Gao, S.; Wang, G. Elevated CO2 improves both lipid accumulation and growth rate in the glucose-6-phosphate dehydrogenase engineered Phaeodactylum tricornutum. Microb. Cell Factories 2019, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Chen, P.; Min, A.; Zhang, R.; Ruan, R. Carbon-dependent alleviation of ammonia toxicity for algae cultivation and associated mechanisms exploration. Bioresour. Technol. 2017, 249, 99. [Google Scholar]
- Brown, A.P.; Slabas, A.R.; Rafferty, J.B. Fatty Acid Biosynthesis in Plants—Metabolic Pathways, Structure and Organization. In Lipids in Photosynthesis; Springer: Dordrecht, The Netherlands, 2009; pp. 11–34. [Google Scholar]
- Monroig, Ó.; Tocher, D.; Navarro, J. Biosynthesis of Polyunsaturated Fatty Acids in Marine Invertebrates: Recent Advances in Molecular Mechanisms. Mar. Drugs 2013, 11, 3998–4018. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Sommerfeld, M.; Jarvis, E.; Ghirardi, M.; Posewitz, M.; Seibert, M.; Darzins, A. Microalgal triacylglycerols as feedstocks for biofuel production: Perspectives and advances. Plant J. 2010, 54, 621–639. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef]
- Chang, H.X.; Huang, Y.; Fu, Q.; Liao, Q.; Zhu, X. Kinetic characteristics and modeling of microalgae Chlorella vulgaris growth and CO2 biofixation considering the coupled effects of light intensity and dissolved inorganic carbon. Bioresour. Technol. 2016, 206, 231–238. [Google Scholar] [CrossRef]
- Bohutskyi, P.; Kligerman, D.C.; Byers, N.; Nasr, L.K.; Cua, C.; Chow, S.; Su, C.; Tang, Y.; Betenbaugh, M.J.; Bouwer, E.J. Effects of inoculum size, light intensity, and dose of anaerobic digestion centrate on growth and productivity of Chlorella and Scenedesmus microalgae and their poly-culture in primary and secondary wastewater. Algal Res. 2016, 19, 278–290. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Mimouni, V.; Tremblin, G.; Ulmann, L. Light Intensity Regulates LC-PUFA Incorporation into Lipids of Pavlova lutheri and the Final Desaturase and Elongase Activities Involved in Their Biosynthesis. J. Agric. Food Chem. 2015, 63, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Krzeminska, I.; Pawlik-Skowronska, B.; Trzcinska, M.; Tys, J. Influence of photoperiods on the growth rate and biomass productivity of green microalgae. Bioprocess Biosyst. Eng. 2014, 37, 735–741. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Delles, R.; Fusconi, G. Effects of a DHA-rich unextracted microalgae as a dietary supplement on performance, carcass traits and meat fatty acid profile in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Khoeyi, Z.A.; Seyfabadi, J.; Ramezanpour, Z. Effect of light intensity and photoperiod on biomass and fatty acid composition of the microalgae, Chlorella vulgaris. Aquac. Int. 2012, 20, 41–49. [Google Scholar] [CrossRef]
- Floreto, E.; Teshima, S. The Fatty Acid Composition of Seaweeds Exposed to Different Levels of Light Intensity and Salinity. Bot. Mar. 1998, 41, 467–482. [Google Scholar] [CrossRef]
- Manoharan, K.; Lee, T.K.; Cha, J.M.; Kim, J.H.; Jin, H.C. Acclimation of Prorocentrum minimum (Dinophyceae) to prolonged darkness by use of an alternative carbon source from triacylglycerides and galactolipids. J. Phycol. 2010, 35, 287–292. [Google Scholar] [CrossRef]
- Mclarnon-Riches, C.J.; Rolph, C.E.; Greenway, D.; Robinson, P.K. Effects of environmental factors and metals on selenastrum capricornutum lipids. Phytochemistry 1998, 49, 1241–1247. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Thelen, J.J.; Fedosejevs, E.; Harwood, J.L. The lipid biochemistry of eukaryotic algae. Prog. Lipid Res. 2019, 74, 31–68. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, P.; Cheng, J.J. A comprehensive comparable study of the physiological properties of four microalgal species under different light wavelength conditions. Planta 2018, 248, 489–498. [Google Scholar] [CrossRef]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; Borghi, M.D. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]
- Aussant, J.; Guihéneuf, F.; Stengel, D.B. Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, E.; Ruiz-Dominguez, M.C.; Cuaresma, M.; Vaquero, I.; Ramos-Merchante, A.; Vega, J.M.; Vílchez, C.; Garbayo, I. Production of lutein, and polyunsaturated fatty acids by the acidophilic eukaryotic microalga Coccomyxa onubensis under abiotic stress by salt or ultraviolet light. J. Biosci. Bioeng. 2018, 125, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.S.; Chai, T.J. Reduction in omega-3 fatty acids by UV-B irradiation in microalgae. J. Appl. Phycol. 1994, 6, 415–422. [Google Scholar] [CrossRef]
- Pohl, P.; Zurheide, F. Fatty acids and lipids of marine algae and the control of their biosynthesis by environmental factors. In Marine Algae in Pharmaceutical Science; Hoppe, H.A., Levring, T., Tanaka, Y., Eds.; 1979; Available online: https://www.degruyter.com/document/doi/10.1515/9783110882049/pdf#page=487 (accessed on 9 March 2022).
- Lu, Q.; Li, J.; Wang, J.; Li, K.; Li, J.; Han, P.; Chen, P.; Zhou, W. Exploration of a mechanism for the production of highly unsaturated fatty acids in Scenedesmus sp. at low temperature grown on oil crop residue based medium. Bioresour. Technol. 2017, 244, 542–551. [Google Scholar] [CrossRef]
- Moller, A. Carotenoid-dependent signals: Indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 2000, 11, 137–159. [Google Scholar]
- Safdar, W.; Zan, X.; Song, Y. Synergistic Effects of pH, Temperature and Agitation on Growth Kinetics and Docosahexaenoic Acid Production of C. cohnii Cultured on Different Carbon Sources. Int. J. Res. Agric. Sci. 2017, 4, 2348–3997. [Google Scholar]
- Winwood, R.J. Recent developments in the commercial production of DHA and EPA rich oils from micro-algae. OCL 2013, 20, D604. [Google Scholar] [CrossRef]
- Bajhaiya, A.K.; Mandotra, S.K.; Suseela, M.R.; Toppo, K.; Ranade, S. Algal Biodiesel: The next generation biofuel for India. Asian J. Exp. Biol. Sci. 2010, 1, 728–739. [Google Scholar]
- Willette, S.; Gill, S.S.; Dungan, B.; Schaub, T.M.; Jarvis, J.M.; Hilaire, R.S.; Holguin, F.O. Alterations in lipidome and metabolome profiles of Nannochloropsis salina in response to reduced culture temperature during sinusoidal temperature and light. Algal Res. 2018, 32, 79–92. [Google Scholar] [CrossRef]
- Hegde, M.V.; Zanwar, A.A.; Adekar, S.P. Importance of Polyunsaturated Fatty Acids from Marine Algae. In Omega-3 Fatty Acids; Springer: Cham, Switzerland, 2016; Chapter 9; pp. 101–126. [Google Scholar] [CrossRef]
- Zhu, C.J.; Lee, Y.K.; Chao, T.M. Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. J. Appl. Phycol. 1997, 9, 451–457. [Google Scholar] [CrossRef]
- Kavitha, M.; Kathiresan, S.; Bhattacharya, S.; Sarada, R. Culture media optimization of Porphyridium purpureum: Production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. J. Food Sci. Technol. 2016, 53, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Emily, H.; Rahul, K.; Seetharaman, V.; Gilmour, D.; Phillip, W. The Search for a Lipid Trigger: The Effect of Salt Stress on the Lipid Profile of the Model Microalgal Species Chlamydomonas reinhardtii for Biofuels Production. Curr. Biotechnol. 2016, 5, 305–313. [Google Scholar]
- Xia, L.; Rong, J.; Yang, H.; He, Q.; Zhang, D.; Hu, C. NaCl as an effective inducer for lipid accumulation in freshwater microalgae Desmodesmus abundans. Bioresour. Technol. 2014, 161, 402–409. [Google Scholar] [CrossRef]
- Suzuki, H.; Hulatt, C.J.; Wijffels, R.H.; Kiron, V. Growth and LC-PUFA production of the cold-adapted microalga Koliella antarctica in photobioreactors. J. Appl. Phycol. 2019, 31, 981–997. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.K.; Chandra, P.; Singh, D.P. Sodium chloride incites Reactive Oxygen Species in green algae Chlorococcum humicola and Chlorella vulgaris: Implication on lipid synthesis, mineral nutrients and antioxidant system. Bioresour. Technol. 2018, 270, 489–497. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Xi, B. Effect of NaCl salinity on the growth, metabolites, and antioxidant system of Microcystis aeruginosa. J. Freshw. Ecol. 2013, 28, 477–487. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum tricornutum microalgae as a rich source of omega-3 oil: Progress in lipid induction techniques towards industry adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef]
- Azachi, M.; Sadka, A.; Fisher, M.; Goldshlag, P.; Gokhman, I.; Zamir, A. Salt induction of fatty acid elongase and membrane lipid modifications in the extreme halotolerant alga Dunaliella salina. Plant Physiol. 2002, 129, 1320–1329. [Google Scholar] [CrossRef]
- Nitsos, C.; Filali, R.; Taidi, B.; Lemaire, J. Current and novel approaches to downstream processing of microalgae: A review. Biotechnol. Adv. 2020, 45, 107650. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.; Mahlia, T.M.I. Experimental investigation of energy properties for Stigonematales sp. microalgae as potential biofuel feedstock. Int. J. Sustain. Eng. 2019, 12, 123–130. [Google Scholar] [CrossRef]
- Morais, K.; Conceio, D.; Vargas, J.; Mitchell, D.A.; Mariano, A.B.; Ordonez, J.C.; Galli-Terasawa, L.V.; Kava, V.M. Enhanced microalgae biomass and lipid output for increased biodiesel productivity. Renew. Energy 2021, 163, 138–145. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.-L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization—A review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Lohman, E.J.; Gardner, R.D.; Halverson, L.D.; Peyton, B.M.; Gerlach, R. Carbon partitioning in lipids synthesized by Chlamydomonas reinhardtii when cultured under three unique inorganic carbon regimes. Algal Res. 2014, 5, 171–180. [Google Scholar] [CrossRef]
- Freddy, G.; Dagmar, B.S. LC-PUFA-Enriched Oil Production by Microalgae: Accumulation of Lipid and Triacylglycerols Containing n-3 LC-PUFA Is Triggered by Nitrogen Limitation and Inorganic Carbon Availability in the Marine Haptophyte Pavlova lutheri. Mar. Drugs 2013, 11, 4246–4266. [Google Scholar]
- Sato, N.; Tsuzuki, M.; Kawaguchi, A. Glycerolipid synthesis in Chlorella kessleri 11h. I. Existence of a eukaryotic pathway. Biochim. Biophys. Acta 2003, 1633, 27–34. [Google Scholar] [CrossRef]
- Muradyan, E.A.; Klyachko-Gurvich, G.L.; Tsoglin, L.N.; Sergeyenko, T.V.; Pronina, N.A. Changes in Lipid Metabolism during Adaptation of the Dunaliella salina Photosynthetic Apparatus to High CO2 Concentration. Russ. J. Plant Physiol. 2004, 51, 53–62. [Google Scholar] [CrossRef]
- Mercer, P.; Armenta, R.E. Developments in oil extraction from microalgae. Eur. J. Lipid Sci. Technol. 2011, 113, 539–547. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Ismail, N.; Hossain, N.; Silitonga, A.S.; Shamsuddin, A.H. Palm oil and its wastes as bioenergy sources: A comprehensive review. Environ. Sci. Pollut. Res. Int. 2019, 26, 14849–14866. [Google Scholar] [CrossRef]
- López, G.; Yate, C.; Ramos, F.A.; Cala, M.P.; Restrepo, S.; Baena, S. Production of Polyunsaturated Fatty Acids and Lipids from Autotrophic, Mixotrophic and Heterotrophic cultivation of Galdieria sp. strain USBA-GBX-832. Sci. Rep. 2019, 9, 10791. [Google Scholar] [CrossRef]
- Moraes, L.; Rosa, G.; Cardias, B.B.; Santos, L.; Costa, J. Microalgal biotechnology for greenhouse gas control: Carbon dioxide fixation by Spirulina sp. at different diffusers. Ecol. Eng. 2016, 91, 426–431. [Google Scholar] [CrossRef]
- Yang, L.; Li, H.; Liu, T.; Zhong, Y.; Ji, C.; Lu, Q.; Fan, L.; Li, J.; Leng, L.; Li, K.; et al. Microalgae biotechnology as an attempt for bioregenerative life support systems: Problems and prospects. J. Chem. Technol. Biotechnol. 2019, 94, 3039–3048. [Google Scholar] [CrossRef]
- Hellebust, J.A.; Ahmad, I. Regulation of Nitrogen Assimilation in Green Microalgae. Biol. Oceanogr. 1989, 6, 241–255. [Google Scholar]
- Costa, F.D.; Grand, F.L.; Quéré, C.; Bougaran, G.; Soudant, P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res. 2017, 27, 177–189. [Google Scholar] [CrossRef]
- Chen, L.H.; Xing, R.L.; Jiang, A.L.; Yao, Y.N.; Zhou, G.F. Effects of nitrogen source and N/P on growth and photosynthesis in the invasive marine macroalga Chaetomorpha valida. Environ. Sci. Pollut. Res. 2020, 27, 24272–24283. [Google Scholar]
- Breuer, G.; Lamers, P.P.; Martens, D.E.; Draaisma, R.B.; Wijffels, R.H. The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour. Technol. 2012, 124, 217–226. [Google Scholar] [CrossRef]
- Gris, B.; Morosinotto, T.; Giacometti, G.M.; Bertucco, A.; Sforza, E. Cultivation of Scenedesmus obliquus in Photobioreactors: Effects of Light Intensities and Light–Dark Cycles on Growth, Productivity, and Biochemical Composition. Appl. Biochem. Biotechnol. 2014, 172, 2377–2389. [Google Scholar] [CrossRef]
- Han, D.; Jia, J.; Li, J.; Sommerfeld, M.; Xu, J.; Hu, Q.; Han, D. Metabolic Remodeling of Membrane Glycerolipids in the Microalga Nannochloropsis oceanica under Nitrogen Deprivation. Front. Mar. Sci. 2017, 7, 7026. [Google Scholar] [CrossRef]
- Simionato, D.; Block, M.A.; La Rocca, N.; Jouhet, J.; Marechal, E.; Finazzi, G.; Morosinotto, T. The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot. Cell 2013, 12, 665–676. [Google Scholar] [CrossRef]
- Fan, J.; Cui, Y.; Wan, M.; Wang, W.; Li, Y. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosaunder three nutrition stressors. Biotechnol. Biofuels 2014, 7, 17. [Google Scholar] [CrossRef]
- Alonso, D.L.; Belarbi, E.H.; Fernández-Sevilla, J.; Rodríguez-Ruiz, J.; Grima, E.M. Acyl lipid composition variation related to culture age and nitrogen concentration in continuous culture of the microalga Phaeodactylum tricornutum. Phytochemistry 2000, 54, 461–471. [Google Scholar] [CrossRef]
- Huang, X.; Huang, Z.; Wen, W.; Yan, J. Effects of nitrogen supplementation of the culture medium on the growth, total lipid content and fatty acid profiles of three microalgae (Tetraselmis subcordiformis, Nannochloropsis oculata and Pavlova viridis). J. Appl. Phycol. 2013, 25, 129–137. [Google Scholar] [CrossRef]
- Boyle, N.R.; Page, M.D.; Liu, B.; Blaby, I.K.; Casero, D.; Kropat, J.; Cokus, S.J.; Hong-Hermesdorf, A.; Shaw, J.; Karpowicz, S.J.; et al. Three acyltransferases and nitrogen-responsive regulator are implicated in nitrogen starvation-induced triacylglycerol accumulation in Chlamydomonas. J. Biol. Chem. 2012, 287, 15811–15825. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Nedbal, L.; Ebenhöh, O. Modelling phosphorus uptake in microalgae. Biochem. Soc. Trans. 2018, 46, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Riekhof, W.R. The Sulfolipids 2′-O-Acyl-Sulfoquinovosyldiacylglycerol and Sulfoquinovosyldiacylglycerol Are Absent from a Chlamydomonas reinhardtii Mutant Deleted in SQD1. Plant Physiol. 2003, 133, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Khozin-Goldberg, I.; Cohen, Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 2006, 67, 696–701. [Google Scholar] [CrossRef]
- Siron, R.; Giusti, G.; Berland, B. Changes in the fatty acid composition of Phaeodactylum tricornutum and Dunaliella tertiolecta during growth and under phosphorus deficiency. Mar. Ecol. Prog. Ser. 1989, 55, 95–100. [Google Scholar] [CrossRef]
- Gopal, S.G.; Chandra, G.P.; Ruma, P. Efficacy of EDTA and Phosphorous on Biomass Yield and Total Lipid Accumulation in Two Green Microalgae with Special Emphasis on Neutral Lipid Detection by Flow Cytometry. Adv. Biol. 2016, 2016, 8712470. [Google Scholar]
- Baek, J.; Choi, J.I. Effect of Nutrient Limitation on Lipid Content and Fatty Acid Composition of Mutant Chlamydomonas reinhardtii. Korean Soc. Biotechnol. Bioeng. J. 2015, 30, 91–95. [Google Scholar] [CrossRef][Green Version]
- Reitan, K.I.; Rainuzzo, J.R.; Olsen, Y. Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J. Phycol. 1994, 30, 972–979. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Rady, A. Effect of phosphorus starvation on growth, photosynthesis and some metabolic processes in the unicellular green alga Chlorella kessleri. Phyton 1995, 35, 139–151. [Google Scholar]
- Guschina, I.A.; Harwood, J.L. Algal Lipids and Effect of the Environment on Their Biochemistry; Lipids in Aquatic Ecosystems; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sato, N.; Hagio, M.; Wada, H.; Tsuzuki, M. Environmental effects on acidic lipids of thylakoid membranes. Biochem. Soc. Trans. 2000, 28, 912. [Google Scholar] [CrossRef] [PubMed]
- Solovchenko, A.E.; Ismagulova, T.T.; Lukyanov, A.A.; Vasilieva, S.G.; Gorelova, O.A. Luxury phosphorus uptake in microalgae. J. Appl. Phycol. 2019, 31, 2755–2770. [Google Scholar] [CrossRef]
- Hanikenne, M.; Merchant, S.S.; Hamel, P. Chapter 10—Transition Metal Nutrition: A Balance Between Deficiency and Toxicity. In The Chlamydomonas Sourcebook, 2nd ed.; Harris, E.H., Stern, D.B., Witman, G.B., Eds.; Academic Press: London, UK, 2009; pp. 333–399. [Google Scholar]
- Shamshad, I.; Khan, S.; Waqas, M.; Ahmad, N.; Ur-Rehman, K.; Khan, K. Removal and bioaccumulation of heavy metals from aqueous solutions using freshwater algae. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2015, 71, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Urzica, E.I.; Vieler, A.; Hong-Hermesdorf, A.; Page, M.D.; Casero, D.; Gallaher, S.D.; Kropat, J.; Pellegrini, M.; Benning, C.; Merchant, S.S. Remodeling of Membrane Lipids in Iron-starved Chlamydomonas. J. Biol. Chem. 2013, 288, 30246–30258. [Google Scholar] [CrossRef]
- Page, M.; Allen, M.; Kropat, J.; Urzica, E.I.; Karpowicz, S.J.; Hsieh, S.I.; Merchant, L. Fe Sparing and Fe Recycling Contribute to Increased Superoxide Dismutase Capacity in Iron-Starved Chlamydomonas reinhardtii. Plant Cell 2012, 24, 2649–2665. [Google Scholar] [CrossRef][Green Version]
- Mercedes, R.; González-Rodríguez, A.; Belén, N.; Pilar, B.B.; Lindahl, A.M.; Manuel, H.; Navarro, J.A.; Ortega, J.M. Iron Deficiency Induces a Partial Inhibition of the Photosynthetic Electron Transport and a High Sensitivity to Light in the Diatom Phaeodactylum tricornutum. Front. Plant Sci. 2016, 7, 1050. [Google Scholar]
- Hemschemeier, A.; Casero, D.; Liu, B.; Benning, D.C.; Pellegrini, D.M. COPPER RESPONSE REGULATOR1–Dependent and–Independent Responses of the Chlamydomonas reinhardtii Transcriptome to Dark AnoxiaW. Plant Cell 2013, 25, 3186–3211. [Google Scholar] [CrossRef]
- Ben Amor-Ben Ayed, H.; Taidi, B.; Ayadi, H.; Pareau, D.; Stambouli, M. Effect of magnesium ion concentration in autotrophic cultures of Chlorella vulgaris. Algal Res. 2015, 9, 291–296. [Google Scholar] [CrossRef]
- Ayed, H.; Taidi, B.; Ayadi, H.; Pareau, D.; Stambouli, M. Magnesium Uptake by the Green Microalga Chlorella vulgaris in Batch Cultures. J. Microbiol. Biotechnol. 2016, 26, 503–510. [Google Scholar] [CrossRef]
- Vandamme, D.; Foubert, I.; Muylaert, K. Flocculation as a low-cost method for harvesting microalgae for bulk biomass production. Trends Biotechnol. 2013, 31, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ende, S.S.W.; Noke, A. Heterotrophic microalgae production on food waste and by-products. J. Appl. Phycol. 2019, 31, 1565–1571. [Google Scholar] [CrossRef]
- Mathimani, T.; Uma, L.; Prabaharan, D. Optimization of direct solvent lipid extraction kinetics on marine trebouxiophycean alga by central composite design—Bioenergy perspective. Energy Convers. Manag. 2017, 142, 334–346. [Google Scholar] [CrossRef]
- Oliveira, C.; Rabello, D.; Daniel, N.M. Biomass production and harvesting of Desmodesmus subspicatus cultivated in flat plate photobioreactor using chitosan as flocculant agent. J. Appl. Phycol. 2019, 31, 857–866. [Google Scholar]
- Cui, H.; Meng, F.; Li, F.; Wang, Y.; Duan, W.; Lin, Y. Two-stage mixotrophic cultivation for enhancing the biomass and lipid productivity of Chlorella vulgaris. AMB Express 2017, 7, 187. [Google Scholar] [CrossRef]
- Demirbas, A.; Demirbas, M.F. Algae Energy: Algae as a New Source of Biodiesel; Green Energy and Technology, 2010; Volume 36, Available online: https://books.google.com.hk/books?hl=zh-CN&lr=&id=Cv_3jJ5hp0AC&oi=fnd&pg=PA1&dq=Algae+Energy:+Algae+as+a+New+Source+of+Biodiesel&ots=od98QGOWML&sig=9-41ZaDgeUreGJn9YxdMbhFrsMQ&redir_esc=y#v=onepage&q=Algae%20Energy%3A%20Algae%20as%20a%20New%20Source%20of%20Biodiesel&f=false (accessed on 9 March 2022).
- Mr, A.; Nrma, B.; Dp, C. Luminescent solar concentrator panels for increasing the efficiency of mass microalgal production. Renew. Sustain. Energy Rev. 2019, 101, 47–59. [Google Scholar]
- Rayen, F.; Behnam, T.; Dominique, P. Optimization of a raceway pond system for wastewater treatment: A review. Crit. Rev. Biotechnol. 2019, 39, 422–435. [Google Scholar] [CrossRef]
- Lee, Y.K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Eing, C.; Goettel, M.; Straessner, R.; Gusbeth, C. Pulsed Electric Field Treatment of Microalgae—Benefits for Microalgae Biomass Processing. IEEE Trans. Plasma Sci. 2013, 41 Pt 1, 2901–2907. [Google Scholar] [CrossRef]
- Norsker, N.H.; Barbosa, M.J.; Vermu, M.H.; Wijffels, R.H. Microalgal production—A close look at the economics. Biotechnol. Adv. 2011, 29, 24–27. [Google Scholar] [CrossRef]
- Ugwu, C.U.; Aoyagi, H. Microalgal Culture Systems: An Insight into their Designs, Operation and Applications. Biotechnology 2012, 11, 127–132. [Google Scholar] [CrossRef]
- Acién, F.; Molina, E.; Reis, A.; Torzillo, G.; Masojídek, J. Photobioreactors for the Production of Microalgae. In Microalgae-Based Biofuels and Bioproducts. Woodhead Publishing, 2017. Available online: https://www.sciencedirect.com/science/article/pii/B9780081010235000017 (accessed on 9 March 2022).
- Segečová, A.; Červený, J.; Roitsch, T. Cultivation of photoautotrophic plant suspension cultures in photobioreactors. Nature 2015, 262, 47–48. [Google Scholar]
- Taya, M. Effective Cultures of Photoautotrophic Cells in Photobioreactors; The Society for Biotechnology: Osaka, Japan, 1997. [Google Scholar]
- Richmond, A.; Qiang, H. Principles for efficient utilization of light for mass production of photoautotrophic microorganisms. Appl. Biochem. Biotechnol. 1997, 63–65, 649. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A.; Demirbas, M.F. Importance of algae oil as a source of biodiesel. Energy Convers. Manag. 2011, 52, 163–170. [Google Scholar] [CrossRef]
- Jorquera, O.; Kiperstok, A.; Sales, E.A.; Embiruçu, M.; Ghirardi, M.L. Comparative energy life-cycle analyses of microalgal biomass production in open ponds and photobioreactors. Bioresour. Technol. 2010, 101, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Schade, S.; Meier, T. Distinct microalgae species for food—Part 1: A methodological (top-down) approach for the life cycle assessment of microalgae cultivation in tubular photobioreactors. J. Appl. Phycol. 2020, 32, 2977–2995. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Fernández Sevilla, J.M.; Molina Grima, E. Photobioreactors for the production of microalgae. Rev. Environ. Sci. Bio/Technol. 2013, 12, 131–151. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Silva, S.O.; Baptista, J.M.; Malcata, F.X. Light requirements in microalgal photobioreactors: An overview of biophotonic aspects. Appl. Microbiol. Biotechnol. 2011, 89, 1275–1288. [Google Scholar] [CrossRef]
- Mayers, J.J.; Ekman Nilsson, A.; Svensson, E.; Albers, E. Integrating Microalgal Production with Industrial Outputs-Reducing Process Inputs and Quantifying the Benefits. Ind. Biotechnol. 2016, 12, 219–234. [Google Scholar] [CrossRef]
- Chang, H.; Fu, Q.; Zhong, N.; Yang, X.; Quan, X.; Li, S.; Fu, J.; Xiao, C. Microalgal lipids production and nutrients recovery from landfill leachate using membrane photobioreactor. Bioresour. Technol. 2019, 277, 18–26. [Google Scholar] [CrossRef]
- Gupta, S.; Pawar, S.B.; Pandey, R.A.; Kanade, G.S.; Lokhande, S.K. Outdoor microalgae cultivation in airlift photobioreactor at high irradiance and temperature conditions: Effect of batch and fed-batch strategies, photoinhibition, and temperature stress. Bioprocess Biosyst. Eng. 2019, 42, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Khoobkar, Z.; Shariati, F.P.; Safekordi, A.A.; Amrei, H.D. Performance Assessment of a Novel Pyramid Photo-Bioreactor for Cultivation of Microalgae using External and Internal Light Sources. Food Technol. Biotechnol. 2019, 57, 68. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-M.; Ren, L.-J.; Zhao, Q.-Y.; Ji, X.-J.; Huang, H. Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnol. Biofuels 2018, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Paul, H.; Nichols, P.D.; Koutoulis, A.; Blackburn, S.I. Australian thraustochytrids: Potential production of dietary long-chain omega-3 oils using crude glycerol. J. Funct. Foods 2015, 6, 810–820. [Google Scholar] [CrossRef]
- Li, J.; Liu, R.; Chang, G.; Li, X.; Chang, M.; Liu, Y.; Jin, Q.; Wang, X. A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Bioresour. Technol. 2015, 177, 51–57. [Google Scholar] [CrossRef]
- Marchan, L.F.; Chang, K.L.; Nichols, P.D.; Polglase, J.L.; Mitchell, W.J.; Gutierrez, T. Screening of new British thraustochytrids isolates for docosahexaenoic acid (DHA) production. J. Appl. Phycol. 2017, 29, 2831–2843. [Google Scholar] [CrossRef]
- Najafabadi, H.A.; Malekzadeh, M.; Jalilian, F.; Vossoughi, M.; Pazuki, G. Effect of various carbon sources on biomass and lipid production of Chlorella vulgaris during nutrient sufficient and nitrogen starvation conditions. Bioresour. Technol. 2015, 180, 311–317. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Labavitch, J.; Vandergheynst, J.S. Organic and Inorganic Nitrogen Impact Chlorella variabilis Productivity and Host Quality for Viral Production and Cell Lysis. Appl. Biochem. Biotechnol. 2015, 176, 326–331. [Google Scholar] [CrossRef]
- Chen, Y.H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef]
- Serio, M.; Tesser, R.; Santacesaria, E. A kinetic and mass transfer model to simulate the growth of baker’s yeast in industrial bioreactors. Chem. Eng. J. 2001, 82, 347–354. [Google Scholar] [CrossRef]
- Gayen, K.; Bhowmik, T.K.; Maity, S.K. Sustainable Downstream Processing of Microalgae for Industrial Application; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Katiyar, R.; Bharti, R.K.; Gurjar, B.R.; Kumar, A.; Biswas, S.; Pruthi, V. Utilization of de-oiled algal biomass for enhancing vehicular quality biodiesel production from Chlorella sp. in mixotrophic cultivation systems. Renew. Energy 2018, 122, 80–88. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Bhatnagar, M.; Chinnasamy, S.; Das, K.C. Chlorella minutissima—A promising fuel alga for cultivation in municipal wastewaters. Appl. Biochem. Biotechnol. 2010, 161, 523–536. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Roostaei, J.; Zhang, Y.; Gopalakrishnan, K.; Ochocki, A.J. Mixotrophic Microalgae Biofilm: A Novel Algae Cultivation Strategy for Improved Productivity and Cost-efficiency of Biofuel Feedstock Production. Sci. Rep. 2018, 8, 12528. [Google Scholar] [CrossRef]
- Berner, F.; Heimann, K.; Sheehan, M. Microalgal biofilms for biomass production. J. Appl. Phycol. 2015, 27, 1793–1804. [Google Scholar] [CrossRef]
- Gross, M.; Mascarenhas, V.; Wen, Z. Evaluating algal growth performance and water use efficiency of pilot-scale revolving algal biofilm (RAB) culture systems. Biotechnol. Bioeng. 2015, 112, 2040–2050. [Google Scholar] [CrossRef]
- Ozkan, A.; Kinney, K.; Katz, L.; Berberoglu, H. Reduction of water and energy requirement of algae cultivation using an algae biofilm photobioreactor. Bioresour. Technol. 2012, 114, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Muylaert, K.; Bastiaens, L.; Vandamme, D.; Gouveia, L. 5-Harvesting of microalgae: Overview of process options and their strengths and drawbacks. In Microalgae-Based Biofuels and Bioproducts; Gonzalez-Fernandez, C., Muñoz, R., Eds.; Woodhead Publishing, 2017; pp. 113–132. Available online: https://www.sciencedirect.com/science/article/pii/B9780081010235000054 (accessed on 9 March 2022).
- Guedes, A.C.; Amaro, H.M.; Barbosa, C.R.; Pereira, R.D.; Malcata, F.X. Fatty acid composition of several wild microalgae and cyanobacteria, with a focus on eicosapentaenoic, docosahexaenoic and α-linolenic acids for eventual dietary uses. Food Res. Int. 2011, 44, 2721–2729. [Google Scholar] [CrossRef]
- Alexandra, K.; Kristína, G. Microalgae Harvesting: A Review. Res. Pap. Fac. Mater. Sci. Technol. Slovak Univ. Technol. 2019, 27, 129–143. [Google Scholar]
- Depraetere, O.; Pierre, G.; Deschoenmaeker, F.; Badri, H.; Foubert, I.; Leys, N.; Markou, G.; Wattiez, R.; Michaud, P.; Muylaert, K. Harvesting carbohydrate-rich Arthrospira platensis by spontaneous settling. Bioresour. Technol. 2015, 180, 16–21. [Google Scholar] [CrossRef]
- Chtourou, H.; Dahmen, I.; Jebali, A.; Karray, F.; Hassairi, I.; Abdelkafi, S.; Ayadi, H.; Sayadi, S.; Dhouib, A. Characterization of Amphora sp. a newly isolated diatom wild strain, potentially usable for biodiesel production. Bioprocess Biosyst. Eng. 2015, 38, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhao, P.; He, C.; Li, J.; Tang, X.; Zhou, J.; Huang, Z. Isolation of a novel strain of Monoraphidium sp. and characterization of its potential application as biodiesel feedstock. Bioresour. Technol. 2012, 121, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Zheng, L.; Bai, Y.; Saroussi, S.; Grossman, A.R. Flocculation of Chlamydomonas reinhardtii with Different Phenotypic Traits by Metal Cations and High pH. Front. Plant Sci. 2017, 8, 1997. [Google Scholar] [CrossRef] [PubMed]
- Şirin, S.; Clavero, E.; Salvadó, J. Efficient harvesting of Chaetoceros calcitrans for biodiesel production. Environ. Technol. 2015, 36, 1902–1912. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Kilimtzidi, E.; Devaere, J.; Goiris, K.; Gonzalez-Fernandez, C.; Wattiez, R.; Muylaert, K. Harvesting of Arthrospira platensis with helicoidal and straight trichomes using filtration and centrifugation. Sep. Sci. Technol. 2020, 55, 2381–2390. [Google Scholar] [CrossRef]
- Wang, S.; Yerkebulan, M.; Abomohra, A.E.-F.; El-Khodary, S.; Wang, Q. Microalgae harvest influences the energy recovery: A case study on chemical flocculation of Scenedesmus obliquus for biodiesel and crude bio-oil production. Bioresour. Technol. 2019, 286, 121371. [Google Scholar] [CrossRef]
- Niaghi, M.; Mahdavi, M.A.; Gheshlaghi, R. Optimization of dissolved air flotation technique in harvesting microalgae from treated wastewater without flocculants addition. J. Renew. Sustain. Energy 2015, 7, 013130. [Google Scholar] [CrossRef]
- Laamanen, C.A.; Scott, J.A. Development of heat-aided flocculation for flotation harvesting of microalgae. Biomass Bioenergy 2017, 107, 150–154. [Google Scholar] [CrossRef]
- Jana, A.; Ghosh, S.; Majumdar, S. Energy efficient harvesting of Arthrospira sp. using ceramic membranes: Analyzing the effect of membrane pore size and incorporation of flocculant as fouling control strategy. J. Chem. Technol. Biotechnol. 2018, 93, 1085–1096. [Google Scholar] [CrossRef]
- Ansari, F.A.; Gupta, S.K.; Nasr, M.; Rawat, I.; Bux, F. Evaluation of various cell drying and disruption techniques for sustainable metabolite extractions from microalgae grown in wastewater: A multivariate approach. J. Clean. Prod. 2018, 182, 634–643. [Google Scholar] [CrossRef]
- Raja, R.; Coelho, A.; Hemaiswarya, S.; Kumar, P.; Carvalho, I.S.; Alagarsamy, A. Applications of microalgal paste and powder as food and feed: An update using text mining tool. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 740–747. [Google Scholar] [CrossRef]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef]
- Al Hattab, M.; Ghaly, A.; Hammouda, A. Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis. J. Fundam. Renew. Energy Appl. 2015, 5, 1000154. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sustain. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Le, T.V.A.; Show, P.L.; Nguyen, T.T.; Tran, M.H.; Tran, T.N.T.; Lee, S.Y. Bioflocculation formation of microalgae-bacteria in enhancing microalgae harvesting and nutrient removal from wastewater effluent. Bioresour. Technol. 2019, 272, 34–39. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X. Microalgal harvesting using foam flotation: A critical review. Biomass Bioenergy 2019, 120, 176–188. [Google Scholar] [CrossRef]
- Baudelet, P.-H.; Ricochon, G.; Linder, M.; Muniglia, L. A new insight into cell walls of Chlorophyta. Algal Res. 2017, 25, 333–371. [Google Scholar] [CrossRef]
- Montsant, A.; Zarka, A.; Boussiba, S. Presence of a Nonhydrolyzable Biopolymer in the Cell Wall of Vegetative Cells and Astaxanthin-Rich Cysts of Haematococcus pluvialis (Chlorophyceae). Mar. Biotechnol. 2001, 3, 515–521. [Google Scholar] [CrossRef]
- Duan, Z.; Tan, X.; Guo, J.; Kahehu, C.W.; Yang, H.; Zheng, X.; Zhu, F. Effects of biological and physical properties of microalgae on disruption induced by a low-frequency ultrasound. J. Appl. Phycol. 2017, 29, 2937–2946. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, J.M.; Chang, Y.K.; Oh, Y.-K. Cell disruption and lipid extraction for microalgal biorefineries: A review. Bioresour. Technol. 2017, 244, 1317–1328. [Google Scholar] [CrossRef]
- Gong, M.; Hu, T.; Yedahalli, S.; Bassi, A. Oil Extraction Processes in Microalgae. In Recent Advances in Renewable Energy; Pires, M., Ed.; Bentham Science Publisher: Sharjah, United Arab Emirate, 2017; Volume 1, pp. 377–411. [Google Scholar]
- Posada, J.A.; Brentner, L.B.; Ramirez, A.; Patel, M.K. Conceptual design of sustainable integrated microalgae biorefineries: Parametric analysis of energy use, greenhouse gas emissions and techno-economics. Algal Res. 2016, 17, 113–131. [Google Scholar] [CrossRef]
- Masoumi, S.; Boahene, P.E.; Dalai, A.K. Biocrude oil and hydrochar production and characterization obtained from hydrothermal liquefaction of microalgae in methanol-water system. Energy 2021, 217, 119344. [Google Scholar] [CrossRef]
- Fu, Q.; Xiao, C.; Liao, Q.; Huang, Y.; Xia, A.; Zhu, X. Kinetics of hydrolysis of microalgae biomass during hydrothermal pretreatment. Biomass Bioenergy 2021, 149, 106074. [Google Scholar] [CrossRef]
- Qiu, Y.; Frear, C.; Chen, S.; Ndegwa, P.; Harrison, J.; Yao, Y.; Ma, J. Accumulation of long-chain fatty acids from Nannochloropsis salina enhanced by breaking microalgae cell wall under alkaline digestion. Renew. Energy 2020, 149, 691–700. [Google Scholar] [CrossRef]
- Callejo-López, J.A.; Ramírez, M.; Cantero, D.; Bolívar, J. Versatile method to obtain protein- and/or amino acid-enriched extracts from fresh biomass of recalcitrant microalgae without mechanical pretreatment. Algal Res. 2020, 50, 102010. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Awad, F.N.; Qi, X.; Sahu, J.N. Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renew. Sustain. Energy Rev. 2019, 105, 105–128. [Google Scholar] [CrossRef]
- García-Cubero, M.T.; González-Benito, G.; Indacoechea, I.; Coca, M.; Bolado, S. Effect of ozonolysis pretreatment on enzymatic digestibility of wheat and rye straw. Bioresour. Technol. 2009, 100, 1608–1613. [Google Scholar] [CrossRef]
- Keris-Sen, U.D.; Gurol, M.D. Using ozone for microalgal cell disruption to improve enzymatic saccharification of cellular carbohydrates. Biomass Bioenergy 2017, 105, 59–65. [Google Scholar] [CrossRef]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined bead milling and enzymatic hydrolysis for efficient fractionation of lipids, proteins, and carbohydrates of Chlorella vulgaris microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Casazza, A.A.; Donsì, F.; Perego, P.; Ferrari, G.; Pataro, G. Effect of pulsed electric fields and high pressure homogenization on the aqueous extraction of intracellular compounds from the microalgae Chlorella vulgaris. Algal Res. 2018, 31, 60–69. [Google Scholar] [CrossRef]
- Zhang, R.; Grimi, N.; Marchal, L.; Lebovka, N.; Vorobiev, E. Effect of ultrasonication, high pressure homogenization and their combination on efficiency of extraction of bio-molecules from microalgae Parachlorella kessleri. Algal Res. 2019, 40, 101524. [Google Scholar] [CrossRef]
- Papachristou, I.; Silve, A.; Jianu, A.; Wüstner, R.; Nazarova, N.; Müller, G.; Frey, W. Evaluation of pulsed electric fields effect on the microalgae cell mechanical stability through high pressure homogenization. Algal Res. 2020, 47, 101847. [Google Scholar] [CrossRef]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Suo, H.; Peng, H.; Xu, P.; Gao, X.; Du, S. Simulation and exploration of cavitation process during microalgae oil extracting with ultrasonic-assisted for hydrogen production. Int. J. Hydrogen Energy 2021, 46, 2890–2898. [Google Scholar] [CrossRef]
- ’tLam, G.P.; Postma, P.R.; Fernandes, D.A.; Timmermans, R.A.H.; Vermuë, M.H.; Barbosa, M.J.; Eppink, M.H.M.; Wijffels, R.H.; Olivieri, G. Pulsed Electric Field for protein release of the microalgae Chlorella vulgaris and Neochloris oleoabundans. Algal Res. 2017, 24, 181–187. [Google Scholar]
- Guo, B.; Yang, B.; Silve, A.; Akaberi, S.; Scherer, D.; Papachristou, I.; Frey, W.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of residual microalgae biomass after pulsed electric field-assisted valuables extraction. Algal Res. 2019, 43, 101650. [Google Scholar] [CrossRef]
- Buchmann, L.; Brändle, I.; Haberkorn, I.; Hiestand, M.; Mathys, A. Pulsed electric field based cyclic protein extraction of microalgae towards closed-loop biorefinery concepts. Bioresour. Technol. 2019, 291, 121870. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, X.; Zhen, F.; Wang, Z.; Kong, X.; Sun, Y. Assessment of enzyme addition strategies on the enhancement of lipid yield from microalgae. Biochem. Eng. J. 2021, 177, 108198. [Google Scholar] [CrossRef]
- Constantino, A.; Rodrigues, B.; Leon, R.; Barros, R.; Raposo, S. Alternative chemo-enzymatic hydrolysis strategy applied to different microalgae species for bioethanol production. Algal Res. 2021, 56, 102329. [Google Scholar] [CrossRef]
- Howlader, M.S.; French, W.T.; Shields-Menard, S.A.; Amirsadeghi, M.; Green, M.; Rai, N. Microbial cell disruption for improving lipid recovery using pressurized CO2: Role of CO2 solubility in cell suspension, sugar broth, and spent media. Biotechnol. Prog. 2017, 33, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Günerken, E.; D’Hondt, E.; Eppink, M.H.M.; Wijffels, R.H.; Elst, K. Disruption of microalgae with a novel continuous explosive decompression device. Algal Res. 2019, 39, 101376. [Google Scholar] [CrossRef]
- Zhang, R.; Marchal, L.; Lebovka, N.; Vorobiev, E.; Grimi, N. Two-step procedure for selective recovery of bio-molecules from microalga Nannochloropsis oculata assisted by high voltage electrical discharges. Bioresour. Technol. 2020, 302, 122893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Parniakov, O.; Grimi, N.; Lebovka, N.; Marchal, L.; Vorobiev, E. Emerging techniques for cell disruption and extraction of valuable bio-molecules of microalgae Nannochloropsis sp. Bioprocess Biosyst. Eng. 2019, 42, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Heo, Y.M.; Lee, H.; Lee, C.; Kang, J.; Ahn, J.-W.; Lee, Y.M.; Kang, K.-Y.; Choi, Y.-E.; Kim, J.-J. An integrative process for obtaining lipids and glucose from Chlorella vulgaris biomass with a single treatment of cell disruption. Algal Res. 2017, 27, 286–294. [Google Scholar] [CrossRef]
- Yoo, G.; Park, W.-K.; Kim, C.W.; Choi, Y.-E.; Yang, J.-W. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour. Technol. 2012, 123, 717–722. [Google Scholar] [CrossRef]
- Orr, V.C.A.; Plechkova, N.V.; Seddon, K.R.; Rehmann, L. Disruption and Wet Extraction of the Microalgae Chlorella vulgaris Using Room-Temperature Ionic Liquids. ACS Sustain. Chem. Eng. 2016, 4, 591–600. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of Hydrothermal Pretreatment of Lignocellulosic Biomass in the Bioethanol Production Process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Kim, S.; Kim, Y.-S. Chlorella virus-mediated disruption of microalgal cell wall for biodiesel production. Korean J. Microbiol. 2018, 54, 140–145. [Google Scholar]
- Cheng, Y.-S.; Zheng, Y.; Labavitch, J.M.; VanderGheynst, J.S. Virus infection of Chlorella variabilis and enzymatic saccharification of algal biomass for bioethanol production. Bioresour. Technol. 2013, 137, 326–331. [Google Scholar] [CrossRef]
- Onumaegbu, C.; Mooney, J.; Alaswad, A.; Olabi, A.G. Pre-treatment methods for production of biofuel from microalgae biomass. Renew. Sustain. Energy Rev. 2018, 93, 16–26. [Google Scholar] [CrossRef]
- Clavijo Rivera, E.; Montalescot, V.; Viau, M.; Drouin, D.; Bourseau, P.; Frappart, M.; Monteux, C.; Couallier, E. Mechanical cell disruption of Parachlorella kessleri microalgae: Impact on lipid fraction composition. Bioresour. Technol. 2018, 256, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Skorupskaite, V.; Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Microalgae Chlorella sp. cell disruption efficiency utilising ultrasonication and ultrahomogenisation methods. J. Appl. Phycol. 2019, 31, 2349–2354. [Google Scholar] [CrossRef]
- Kapoore, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Ranjith Kumar, R.; Hanumantha Rao, P.; Arumugam, M. Lipid extraction methods from microalgae: A comprehensive review. Front. Energy Res. 2015, 2, 61. [Google Scholar] [CrossRef]
- Prabakaran, P.; Ravindran, A.D. A comparative study on effective cell disruption methods for lipid extraction from microalgae. Lett. Appl. Microbiol. 2011, 53, 150–154. [Google Scholar] [CrossRef]
- Guo, H.; Chen, H.; Fan, L.; Linklater, A.; Zheng, B.; Jiang, D.; Qin, W. Enzymes produced by biomass-degrading bacteria can efficiently hydrolyze algal cell walls and facilitate lipid extraction. Renew. Energy 2017, 109, 195–201. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef]
- Chauton, M.S.; Reitan, K.I.; Norsker, N.H.; Tveterås, R.; Kleivdal, H.T. A techno-economic analysis of industrial production of marine microalgae as a source of EPA and DHA-rich raw material for aquafeed: Research challenges and possibilities. Aquaculture 2015, 436, 95–103. [Google Scholar] [CrossRef]
- Adam, F.; Abert-Vian, M.; Peltier, G.; Chemat, F. “Solvent-free” ultrasound-assisted extraction of lipids from fresh microalgae cells: A green, clean and scalable process. Bioresour. Technol. 2012, 114, 457–465. [Google Scholar] [CrossRef]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, M.; Gentili, F. A single-step method for rapid extraction of total lipids from green microalgae. PLoS ONE 2014, 9, e89643. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Friedman, C.; Ross, A.B. Hydrothermal microwave processing of microalgae as a pre-treatment and extraction technique for bio-fuels and bio-products. Bioresour. Technol. 2013, 136, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wiyarno, B.; Yunus, R.; Mel, M. Extraction of Algae Oil from Nannocloropsis sp.: A Study of Soxhlet and Ultrasonic-Assisted Extractions. J. Appl. Sci. 2011, 11, 3607–3612. [Google Scholar] [CrossRef]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved extraction of vegetable oils under high-intensity ultrasound and/or microwaves. Ultrason. Sonochem. 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Krienitz, L.; Wirth, M. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 2006, 36, 204–210. [Google Scholar] [CrossRef]
- Halim, R.; Danquah, M.K.; Webley, P.A. Extraction of oil from microalgae for biodiesel production: A review. Biotechnol. Adv. 2012, 30, 709–732. [Google Scholar] [CrossRef]
- Ghasemi Naghdi, F.; González González, L.M.; Chan, W.; Schenk, P.M. Progress on lipid extraction from wet algal biomass for biodiesel production. Microb. Biotechnol. 2016, 9, 718–726. [Google Scholar] [CrossRef]
- Paiva Pinheiro Pires, A.; Arauzo, J.; Fonts, I.; Dómine, M.; Arroyo, A.; García-Pérez, M.; Montoya, J.; Chejne Janna, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Saleh, J.; Dubé, M.A.; Tremblay, A.Y. Effect of Soap, Methanol, and Water on Glycerol Particle Size in Biodiesel Purification. Energy Fuels 2010, 24, 6179–6186. [Google Scholar] [CrossRef]
- Fábryová, T.; Cheel, J.; Kubáč, D.; Hrouzek, P.; Vu, D.L.; Tůmová, L.; Kopecký, J. Purification of lutein from the green microalgae Chlorella vulgaris by integrated use of a new extraction protocol and a multi-injection high performance counter-current chromatography (HPCCC). Algal Res. 2019, 41, 101574. [Google Scholar] [CrossRef]
- Abu-Nasr, A.M.; Potts, W.M.; Holman, R.T. Highly unsaturated fatty acids. II. Fractionation by urea inclusion compounds. J. Am. Oil Chem. Soc. 1954, 31, 16–20. [Google Scholar] [CrossRef]
- Mendes, A.; Lopes da Silva, T.; Reis, A. DHA Concentration and Purification from the Marine Heterotrophic Microalga Crypthecodinium cohnii CCMP 316 by Winterization and Urea Complexation. Food Technol. Biotechnol. 2006, 45, 38–44. [Google Scholar]
- Cao, X.J.; Hur, B.K. Separation of EPA and DHA from fish oil using modified zeolite 13X and supercritical CO2. J. Ind. Eng. Chem. 2005, 11, 762–768. [Google Scholar]
- Li, M.; Pham, P.J.; Pittman, C.U.; Li, T. SBA-15-supported ionic liquid compounds containing silver salts: Novel mesoporous π-complexing sorbents for separating polyunsaturated fatty acid methyl esters. Microporous Mesoporous Mater. 2009, 117, 436–443. [Google Scholar] [CrossRef]
- Létisse, M.; Rozières, M.; Hiol, A.; Sergent, M.; Comeau, L. Enrichment of EPA and DHA from sardine by supercritical fluid extraction without organic modifier: I. Optimization of extraction conditions. J. Supercrit. Fluids 2006, 38, 27–36. [Google Scholar] [CrossRef]
- Alkio, M.; Gonzalez, C.; Jäntti, M.; Aaltonen, O. Purification of polyunsaturated fatty acid esters from tuna oil with supercritical fluid chromatography. J. Am. Oil Chem. Soc. 2000, 77, 315–321. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, T.; Chowdhury, H.; Ahmed, A.; Nizamuddin, S.; Griffin, G.; Mahlia, T.M.; Park, Y.K. Edible bio-oil production from microalgae and application of nano-technology. Microalgae 2021, 91–116. [Google Scholar] [CrossRef]
- Goh, B.H.H.; Ong, H.C.; Cheah, M.Y.; Chen, W.-H.; Yu, K.L.; Mahlia, T.M.I. Sustainability of direct biodiesel synthesis from microalgae biomass: A critical review. Renew. Sustain. Energy Rev. 2019, 107, 59–74. [Google Scholar] [CrossRef]
- Bwapwa, J.K.; Anandraj, A.; Trois, C. Microalgae processing for jet fuel production. Biofuels Bioprod. Biorefining 2018, 12, 2018. [Google Scholar] [CrossRef]
- Hossain, N.; Bhuiyan, M.A.; Pramanik, B.K.; Nizamuddin, S.; Griffin, G. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review. J. Clean. Prod. 2020, 255, 120261. [Google Scholar] [CrossRef]
- Wang, Y.; Nie, J.; Zhao, M.; Ma, S.; Kuang, L.; Han, X.; Tang, S. Production of Biodiesel from Waste Cooking Oil via a Two-Step Catalyzed Process and Molecular Distillation. Energy Fuels 2010, 24, 2104–2108. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation using biopolymers as an alternative to produce food enhanced with phytosterols and omega-3 fatty acids: A review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
- Niu, B.; Shao, P.; Luo, Y.; Sun, P. Recent advances of electrosprayed particles as encapsulation systems of bioactives for food application. Food Hydrocoll. 2020, 99, 105376. [Google Scholar] [CrossRef]
- Jafari, S.M. Nanotechnology Approaches for Increasing Nutrient Bioavailability. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2017; Volume 81, pp. 1–30. [Google Scholar]
- Giroldi, M.; Grambusch, I.; Lehn, D.; Souza, C. Encapsulation of dairy protein hydrolysates: Recent trends and future prospects. Dry. Technol. 2021, 39, 1–16. [Google Scholar] [CrossRef]
- Bakry, A.M.; Abbas, S.; Ali, B.; Majeed, H.; Abouelwafa, M.Y.; Mousa, A.; Liang, L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Ence Food Saf. 2016, 15, 143–182. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Giménez, B.; Oyarzún-Ampuero, F.; Robert, P. Conventional spray-drying and future trends for the microencapsulation of fish oil. Trends Food Sci. Technol. 2016, 56, 46–60. [Google Scholar] [CrossRef]
- Łozińska, N.; Gowacz-Róyńska, A.; Artichowicz, W.; Lu, Y.; Jungnickel, C. Microencapsulation of fish oil–determination of optimal wall material and encapsulation methodology. J. Food Eng. 2019, 268, 109730. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of active ingredients in polysaccharide–protein complex coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex coacervation: Principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.G.; Lee, M.Y.; Cha, J.M.; Lee, J.K.; Lee, S.C.; Kim, J.; Hwang, Y.S.; Bae, H. Nanogels Derived from Fish Gelatin: Application to Drug Delivery System. Mar. Drugs 2019, 17, 246. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Zhao, Y.; Ding, J.; Lin, S. Investigation on complex coacervation between fish skin gelatin from cold-water fish and gum arabic: Phase behavior, thermodynamic, and structural properties. Food Res. Int. 2018, 107, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Nickerson, M.T. Encapsulation of omega 3-6-9 fatty acids-rich oils using protein-based emulsions with spray drying. J. Food Sci. Technol. 2018, 55, 2850–2861. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; Barrow, C.J.; Adhikari, B. Microencapsulation of omega-3 fatty acids: A review of microencapsulation and characterization methods. J. Funct. Foods 2015, 19, 868–881. [Google Scholar] [CrossRef]
- Esparza, Y.; Ngo, T.D.; Boluk, Y. Preparation of powdered oil particles by spray drying of cellulose nanocrystals stabilized Pickering hempseed oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124823. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.L.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Jafari, S.M.; Assadpoor, E.; He, Y.; Bhandari, B. Encapsulation Efficiency of Food Flavours and Oils during Spray Drying. Dry. Technol. 2008, 26, 816–835. [Google Scholar] [CrossRef]
- Fuchs, M.; Turchiuli, C.; Bohin, M.; Cuvelier, M.E.; Ordonnaud, C.; Peyrat-Maillard, M.N.; Dumoulin, E. Encapsulation of oil in powder using spray drying and fluidised bed agglomeration. J. Food Eng. 2017, 75, 27–35. [Google Scholar] [CrossRef]
- Icyer, N.C.; Toker, O.S.; Karasu, S.; Tornuk, F.; Arici, M. Microencapsulation of fig seed oil rich in polyunsaturated fatty acids by spray drying. J. Food Meas. Charact. 2017, 11, 50–57. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. Advances in Spray-Drying Encapsulation of Food Bioactive Ingredients: From Microcapsules to Nanocapsules. Annu. Rev. Food Ence Technol. 2019, 10, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pang, X.; Zhang, S.; Liu, L.; Lyu, J. Buttermilk as a wall material for microencapsulation of omega-3 oils by spray drying. LWT 2020, 127, 109320. [Google Scholar] [CrossRef]
- Drusch, S.; Mannino, S. Patent-based review on industrial approaches for the microencapsulation of oils rich in polyunsaturated fatty acids. Trends Food Sci. Technol. 2009, 20, 237–244. [Google Scholar] [CrossRef]
- Ubbink, J.; Krüger, J. Physical approaches for the delivery of active ingredients in foods. Trends Food Sci. Technol. 2006, 17, 244–254. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Yonekura, L.; Parmenter, C.; Fisk, I. Impact of Milk Protein Type on the Viability and Storage Stability of Microencapsulated Lactobacillus acidophilus NCIMB 701748 Using Spray Drying. Food Bioprocess Technol. 2014, 7, 1255–1268. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Rocha, F. Chapter 7: Application of Biopolymers in Microencapsulation Processes. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: New York, NY, USA, 2018; pp. 191–222. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Nickerson, M.; Yan, C.; Cloutier, S.; Zhang, W. Protection and Masking of Omega-3 and -6 Oils via Microencapsulation; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Pimentel-Moral, S.; Verardo, V.; Robert, P.; Segura-Carretero, A.; Martínez-Férez, A. Nanoencapsulation strategies applied to maximize target delivery of intact polyphenols. Encapsulations 2016, 559–595. [Google Scholar] [CrossRef]
- Subramanian, S.; Connolly, B.J.; Hendrickson, W.A. Encapsulated Labile Compound Compositions and Methods of Making the Same. EP: 2012. Available online: https://patents.google.com/patent/US8221809B2/en (accessed on 9 March 2022).
- Emami, F.; Vatanara, A.; Park, E.J.; Na, D.H. Drying Technologies for the Stability and Bioavailability of Biopharmaceuticals. Pharmaceutics 2018, 10, 131. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, X.; Wu, X.; Xu, Q.; Tian, W.; Li, Z. Inert particles as process aid in spray-freeze drying. Dry. Technol. 2019, 38, 71–79. [Google Scholar] [CrossRef]
- Adali, M.; Barresi, A.; Boccardo, G.; Pisano, R. Spray Freeze-Drying as a Solution to Continuous Manufacturing of Pharmaceutical Products in Bulk. Processes 2020, 8, 709. [Google Scholar] [CrossRef]
- Ray, S.; Raychaudhuri, U.; Chakraborty, R. An overview of encapsulation of active compounds used in food products by drying technology. Food Biosci. 2016, 13, 76–83. [Google Scholar] [CrossRef]
- Delshadi, R.; Bahrami, A.; Tafti, A.G.; Barba, F.J.; Williams, L.L. Micro and nano-encapsulation of vegetable and essential oils to develop functional food products with improved nutritional profiles. Trends Food Sci. Technol. 2020, 104, 72–83. [Google Scholar] [CrossRef]
- Eghbal, N.; Choudhary, R. Complex coacervation: Encapsulation and controlled release of active agents in food systems. LWT 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Voorn, M.J. Complex coacervation. I. General theoretical considerations. Recl. Trav. Chim. Pays-Bas 2015, 75, 317–330. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Wang, B.; Adhikari, R.; Adhikari, B. Advances in microencapsulation of polyunsaturated fatty acids (PUFAs)-rich plant oils using complex coacervation: A review. Food Hydrocoll. 2017, 69, 369–381. [Google Scholar] [CrossRef]
- Dubin, P.; Stewart, R.J. Complex coacervation. Soft Matter 2018, 14, 329. [Google Scholar] [CrossRef]
- Marfil, P.H.M.; Paulo, B.B.; Alvim, I.D.; Nicoletti, V.R. Production and characterization of palm oil microcapsules obtained by complex coacervation in gelatin/gum Arabic. J. Food Process Eng. 2018, 41, e12673. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Huang, X.; Gaenzle, M.; Wu, Z.; Nishinari, K.; Yang, N.; Fang, Y. Ambient storage of microencapsulated Lactobacillus plantarum ST-III by complex coacervation of type-A gelatin and gum arabic. Food Funct. 2018, 9, 1000–1008. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, H.; Gao, J.; Chen, L.; Vasanthan, T. Field pea protein isolate/chitosan complex coacervates: Formation and characterization. Carbohydr. Polym. 2020, 250, 116925. [Google Scholar] [CrossRef]
- Elmer, C.; Karaca, A.C.; Low, N.H.; Nickerson, M.T. Complex coacervation in pea protein isolateechitosan mixtures. Food Res. Int. 2011, 44, 1441–1446. [Google Scholar] [CrossRef]
- Klemmer, K.J.; Waldner, L.; Stone, A.; Low, N.H.; Nickerson, M.T. Complex coacervation of pea protein isolate and alginate polysaccharides. Food Chem. 2012, 130, 710–715. [Google Scholar] [CrossRef]
- Conto, L.C.D.; Grosso, C.R.F.; Gonalves, L.A.G. Chemometry as applied to the production of omega-3 microcapsules by complex coacervation with soy protein isolate and gum Arabic. LWT-Food Sci. Technol. 2013, 53, 218–224. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Colin, J.B.; Adhikari, B. Microencapsulation of chia seed oil using chia seed protein isolate-chia seed gum complex coacervates. Int. J. Biol. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Vongsvivut, J.; Tobin, M.J.; Adhikari, R.; Barrow, C.; Adhikari, B. Investigation of oil distribution in spray-dried chia seed oil microcapsules using synchrotron-FTIR microspectroscopy. Food Chem. 2019, 275, 457–466. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Gandhi, M. Nanoemulsions in food: Market demand. Environ. Chem. Lett. 2019, 17, 1003–1009. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.D.J.; Campos-Montiel, R.G.; Jiménez-Alvarado, R.; Almaraz-Buendía, I.; Fernández-Luqueo, F. Development and incorporation of nanoemulsions in food. Int. J. Food Stud. 2019, 8, 105–124. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S. Food Nanoemulsions: Stability, Benefits and Applications. 2018. Available online: https://link.springer.com/chapter/10.1007/978-981-10-6986-4_2 (accessed on 9 March 2022).
- Yalçınöz, Ş.; Erçelebi, E. Potential applications of nano-emulsions in the food systems: An update. Mater. Res. Express 2018, 5, 62001. [Google Scholar] [CrossRef]
- Abbasi, F.; Samadi, F.; Jafari, S.M.; Ramezanpour, S.; Shams Shargh, M. Ultrasound-assisted preparation of flaxseed oil nanoemulsions coated with alginate-whey protein for targeted delivery of omega-3 fatty acids into the lower sections of gastrointestinal tract to enrich broiler meat. Ultrason. Sonochem. 2019, 50, 208–217. [Google Scholar] [CrossRef]
- Lane, K.E.; Zhou, Q.; Robinson, S.; Li, W. The composition and oxidative stability of vegetarian omega-3 algal oil nanoemulsions suitable for functional food enrichment. J. Sci. Food Agric. 2020, 100, 695–704. [Google Scholar] [CrossRef]
- Hashim, A.F.; Hamed, S.F.; Abdel Hamid, H.A.; Abd-Elsalam, K.A.; Golonka, I.; Musiał, W.; El-Sherbiny, I.M. Antioxidant and antibacterial activities of omega-3 rich oils/curcumin nanoemulsions loaded in chitosan and alginate-based microbeads. Int. J. Biol. Macromol. 2019, 140, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Dhritlahre, R.K.; Ruchika; Padwad, Y.; Saneja, A. Self-emulsifying formulations to augment therapeutic efficacy of nutraceuticals: From concepts to clinic. Trends Food Sci. Technol. 2021, 115, 347–365. [Google Scholar] [CrossRef]
- Alsulays, B.B.; Park, J.B.; Alshehri, S.M.; Morott, J.T.; Kolter, K. Journal of Drug Delivery Science and Technology. J. Drug Deliv. Sci. Technol. 2015, 29, 189–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ameeduzzafar; El-Bagory, I.; Alruwaili, N.K.; Elkomy, M.H.; Alam, S. Development of novel dapagliflozin loaded solid self-nanoemulsifying oral delivery system: Physiochemical characterization and in vivo antidiabetic activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101279. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, X.; Ma, X.; Wang, Z. Preparation, characterization, and evaluation of antioxidant activity and bioavailability of a self-nanoemulsifying drug delivery system (SNEDDS) for buckwheat flavonoids. Acta Biochim. Biophys. Sin. 2020, 52, 1265–1274. [Google Scholar] [CrossRef]
- Singh, H.; Nathani, S.; Singh, N.; Roy, P.; Paul, S.; Sohal, H.S.; Jain, S.K. Development and characterization of Solid-SNEDDS formulation of DHA using hydrophilic carrier with improved shelf life, oxidative stability and therapeutic activity. J. Drug Deliv. Sci. Technol. 2019, 54, 101326. [Google Scholar] [CrossRef]
- Nunes, R.; Pereira, B.D.A.; Cerqueira, M.A.; Silva, P.; Pastrana, L.M.; Vicente, A.A.; Martins, J.T.; Bourbon, A.I. Lactoferrin-based nanoemulsions to improve the physical and chemical stability of omega-3 fatty acids. Food Funct. 2020, 11, 1966–1981. [Google Scholar] [CrossRef]
- Walker, R.; Decker, E.A.; Mcclements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2014, 6, 41–54. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Hasani, S. Characteristics and oxidative stability of fish oil nano-liposomes and its application in functional bread. J. Food Meas. Charact. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Wang, Q.; Lv, S.; Lu, J.; Jiang, S.; Lin, L. Characterization, Stability, andIn VitroRelease Evaluation of Carboxymethyl Chitosan Coated Liposomes Containing Fish Oil. J. Food Sci. 2015, 80, C1460–C1467. [Google Scholar] [CrossRef]
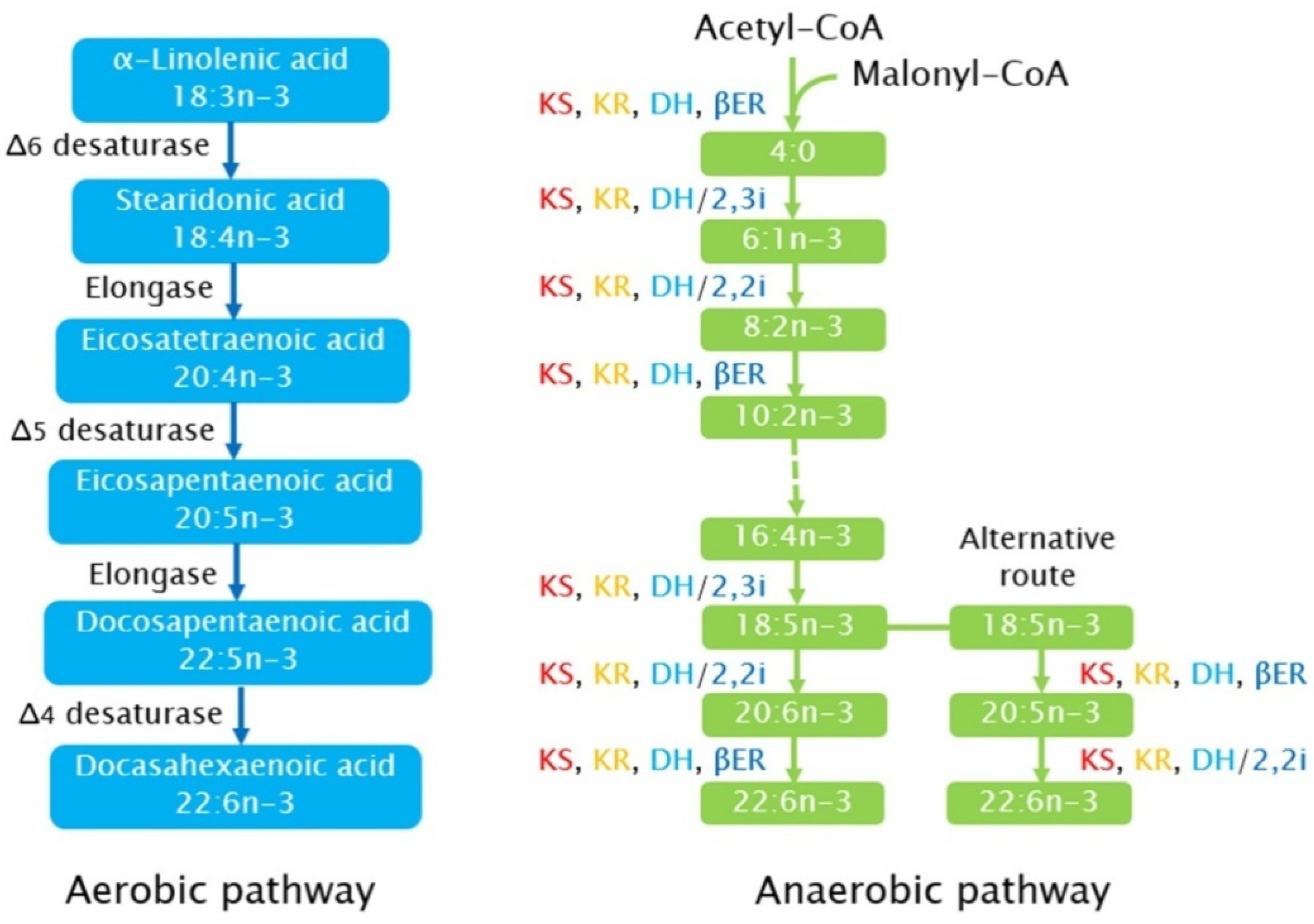

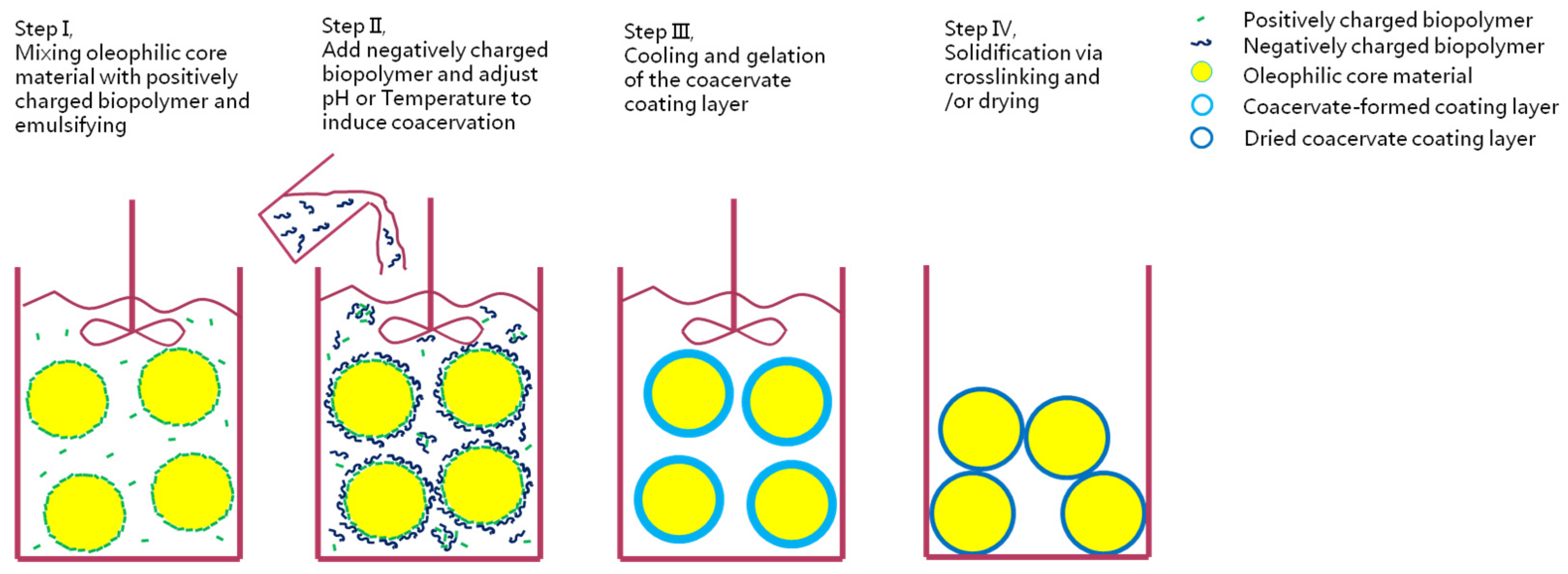
| Species | 14:0 | 16:0 | 16:1 | 18:0 | 18:1 | 18:2 | 18:3 | 20:5 EPA | 22:6 DHA | Total (%DM) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| % of Total Fatty Acids | |||||||||||
| Aurantiochytrium | 2.9 | 39.8 | 0.5 | 0.5 | 0.1 | 0.4 | 0.5 | 46.7 | 40–55 | [52,53] | |
| Chlamydomonas reinhardtii | 4–20 | 3.8 | 1–16 | 1–10 | 2–22 | 0–5.4 | 12–64 | [54] | |||
| Crypthecodiniumcohnii | 18 | 12–45 | 3 | 8 | 13–55 | 25–63 | [53,55,56] | ||||
| Dunaliella sp. | 10–28 | 12–16 | 8–11 | 5.9 | 12–36 | 14–21 | 12–46 | [36,53] | |||
| Emiliana huxleyi | 18.9 | 10.3 | 10.8 | 19.8 | 9.2 | [41] | |||||
| Euglena gracilis | 0.9 | 11.3 | 1.3 | 3.1 | 3.5 | 19.3 | 9.0 | [41] | |||
| Heterococcus chodati | 10.0 | 30.6 | 8.1 | 32.6 | [41] | ||||||
| Nannochloropsis oculata | 4.2 | 14–24 | 24–30 | 3–5 | 2.9 | 0–9 | 27–49 | 22–37 | [36,37] | ||
| Pavlova lutheri | 9.7 | 20.1 | 26.3 | 1.7 | 0.5 | 0.4 | 18.2 | 9.8 | 35 | [41] | |
| Phaeodactylum tricornutum | 4.4 | 14–16 | 40–60 | 8.1 | 1.0 | 20–30 | 18.4 | 1.4 | 32 | [57] | |
| Scenedesmus obliquus | 30.7 | 23.3 | 6–25 | 8–18 | 10–33 | 21–58 | [58] | ||||
| Schizochytrium | 2–8 | 20–45 | 4.8 | 38.4 | 7.9 | 1.2 | 5–12 | 5–50 | 51–71 | [53,59,60] | |
| Thraustochytrium sp. | 1.6 | 16.8 | 0.2 | 0.2 | 0.2 | 7.5 | 69 | 13 | [52] | ||
| Tribonema vulgare | 4.1 | 13.3 | 34.4 | 10.5 | 17.4 | [41] | |||||
| Ulkenia sp. | 25–30 | 10–12 | 5–15 | 15–30 | 20–52 | [52] | |||||
| Method | Description | Advantage | Disadvantage | Example | Ref. |
|---|---|---|---|---|---|
| Sedimentation | Natural gravity sedimentation relies on the particle size of microalgae cells and the density difference of culture environment to harvest; suitable for large biomass and fast sedimentation rate. | Simple; Inexpensive | Affected by cell morphology, not applicable to small-diameter and low-density algae | The filamentous Spirulina platensis having a sedimentation velocity of 0.64 m/h. | [201] |
| The diatom Amphora having a velocity of 2.91 m/h. | [202] | ||||
| Monoraphidium sp. can be harvested after 24 h with a yield of 98%. | [203] | ||||
| Coagulation- Flocculation | Coagulation and flocculation employ chemical (coagulant, zeta potential and pH) or physicochemical (e.g., hydrodynamics) principles to promote cell aggregation and form large particles for separation purposes | Efficient; Inexpensive | Possible coagulant contamination | At high pH, Fe3+, Ca2+ and Mg2+ induced coagulation of C. reinhardtii at <5 mM with >90% biomass harvesting efficiency. | [204] |
| Adjusted pH to 9.5 induced coagulation of Chaetoceros calcitrans with 89% of cells were harvested. | [205] | ||||
| Centrifugation | Centrifugal method uses acceleration to harvest cells. Various types of centrifugal equipment can be used to harvest microalgae, such as spiral plate centrifuge, decanter centrifuge, disk stack centrifuge, and hydrocyclone. | Efficient; No chemical pollution | High energy consumption; Expensive; Affected by algae morphology | A low biomass harvest efficiency of approx. 50% at 9000× g for Helical A. platensis filaments. | [206] |
| A harvest efficiency of 99.3% achieved at 3000× g for 10 min for S. obliquus cells. | [207] | ||||
| Flotation | Flotation is a method to transfer microalgae to the surface of culture medium by introducing bubbles (air or ozone), and then collect microalgae by skimming. | Efficient | High energy consumption | Using 3.8 L flotation cell and dissolved air flotation, the harvest efficiency reaches 91%. | [208] |
| The heat-induced flotation of Scenedesmus dimorphus at 85 °C, with a harvest efficiency around 80%. | [209] | ||||
| Membrane filtration | Membrane filtration can be employed as dead-end or tangential flow filtration mode with membrane pore size varied from 0.1 μm to 10 μm for microfiltration and a few nanometers to 0.1 μm for ultrafiltration membrane respectively. | Pollution-free | Easy to be corroded by medium; Blockages need to be cleaned | Driven by gravity, A. platensis cultures collected using 5 μm nylon membrane with over 90% harvest rate. | [206] |
| In the harvesting of Arthrospira sp. with ceramic microfiltration and ultrafiltration membranes, fluxes of 230 L m−2 h−1 and 93 L m−2 h−1 reported, respectively. | [210] | ||||
| Drying | The water content of microalgae can be reduced to 10%. There are many drying methods, such as sun-drying, freeze-drying, oven-drying, spray drying, and drum drying. | Lower moisture content; Efficient | Long time; High energy consumption; Uneconomical (except sun drying) | Sun drying is done under sunlight, usually at 18–27 °C; the efficiency is 400–1200 mmol m−2 s−1; takes 2–3 days. | [211] |
| Oven drying is done using hot air, usually at 60 °C, takes 12 h. | [211] |
| Method | Description | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| Chemical Method | ||||
| Hydrothermal | Hydrothermal pretreatment is based on cell wall rupture due to internal pressure build-up from the heating, and hydrolysis of cell wall components by steam explosion, autoclave and water bath treatment. | Unrestricted moisture content; Suitable for low value targets; No chemical reagent; Simple operation | High temperature may oxidize and degrade lipids and other bioactives; High energy consumption | [224,225] |
| Acid/Alkaline treatment | Inorganic acid or alkaline solution is used to catalyze and promote hydrolysis processes as an improved version of hydrothermal pretreatment | Efficient; Simple operation | Enhance the soluble chemical oxygen demand; Degradation of sensitive compounds | [226,227] |
| Oxidative pretreatment | Strong oxidant (such as ozone or hydrogen peroxide)is used togenerate hydroxyl radicals (OH-) that attack and disrupt the cell walls of microalgae. | Efficient; Suitable for the preparation of biofuels | Destroy highly oxidizable compounds | [228,229,230] |
| Physical method | ||||
| Pressing | A mechanical force is used to demolish the thick membrane of microalgae and release the oil content. Screw press, extruder, and biomass spraying are the main means of the mechanical pressing. | High purity of the target products; No chemical pollution | Require highdryness of the biomass | [231] |
| Bead beating | The membrane of microalgae is disrupted by the action of fast-moving spinning beads. | Simple equipment; Efficient; Wide application range | Need cooling equipment; high temperature destabilize target compounds; Emulsification of products | [232] |
| High-pressure homogenization (HPH) | HPH is typically used for emulsification but is also suitable for a large-scale disruption of microalgae cells. | Efficient; High biomass concentration; Reduction of viscosity | Product emulsification affects subsequent extraction | [233,234,235] |
| Ultrasonication | Highpressure bubbles and their cavitation generate shock waves, producing high shear forces. | Simple; Suitable for combination with other methods | Oxidation target product; Affect fatty acid chain length; Low efficiency | [236,237] |
| Pulsed Electric Field | An intense electric field for very short durations (pulses)applied to microalgae cells to induces reversible or irreversible pores creation (electroporation) on the cell membranes to aid their disruption. | Suitable for freshwater microalgae; Gentle | Low efficiency; Additional steps to remove salt (cost up); Not applicable to marine microalgae | [238,239,240] |
| Other novel pretreatment methods | ||||
| Enzymatic methods | It is a specific pretreatment method, and requires high selectivity of suitable enzymeson the cell wall structure and composition of a special typeof algae. | High specificity; Mild reaction conditions; Low energy consumption | High enzyme cost; Short process time | [232,241,242] |
| CO2 explosion | It pressurizes CO2 inside the cell and increases intracellular gas concentration, leading to excessive expansion and cell rupture. Other non-reactive gasses such as N2 are also used. | Prevent degradation of target products; Efficient | High-cost | [243,244] |
| Electricity-based methods | High voltage electric discharges (HVED) utilizes electrodes of needle-plate geometry to deliver high voltage pulses to microalgae suspensions. HVED additionally induces thermal and mechanical effects to the cells due to cavitation and shockwave formation. Non-thermal plasma is another electricity-based method where a needle-to-plate electrode geometry is placed in an argon filled reactor. | No chemical pollution; High extraction rate | Not suitable for extraction of unsaturated fatty acids | [233,245,246] |
| Osmotic shock | A week-long pretreatment in which microalgae cells are broken up due to the density difference between cytoplasm and high salt solution. | Simple; High extraction rate | Time consuming | [247,248] |
| Ionic liquids | Ionic liquids form a large number of hydrogen bonds that interact with polymers such as cellulose, and destroy the original hydrogen bonds in cellulose and break the cell wall. | High extraction rate; Room temperature | Loss of ions over time; Potential ions pollution | [249,250] |
| Viral cell lysis | Virus-assisted cell disruption is a novel method that appeals for low energy consumption. | No chemical pollution | Unknown control factors | [251,252] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Liu, Y.; Fan, C.; Hong, H.; Wu, W.; Zhang, W.; Wang, Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods 2022, 11, 1215. https://doi.org/10.3390/foods11091215
Ren X, Liu Y, Fan C, Hong H, Wu W, Zhang W, Wang Y. Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods. 2022; 11(9):1215. https://doi.org/10.3390/foods11091215
Chicago/Turabian StyleRen, Xiang, Yanjun Liu, Chao Fan, Hao Hong, Wenzhong Wu, Wei Zhang, and Yanwen Wang. 2022. "Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil" Foods 11, no. 9: 1215. https://doi.org/10.3390/foods11091215
APA StyleRen, X., Liu, Y., Fan, C., Hong, H., Wu, W., Zhang, W., & Wang, Y. (2022). Production, Processing, and Protection of Microalgal n-3 PUFA-Rich Oil. Foods, 11(9), 1215. https://doi.org/10.3390/foods11091215






