Optimization of Astaxanthin Recovery in the Downstream Process of Haematococcus pluvialis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Haematococcus Pluvialis Cultivation
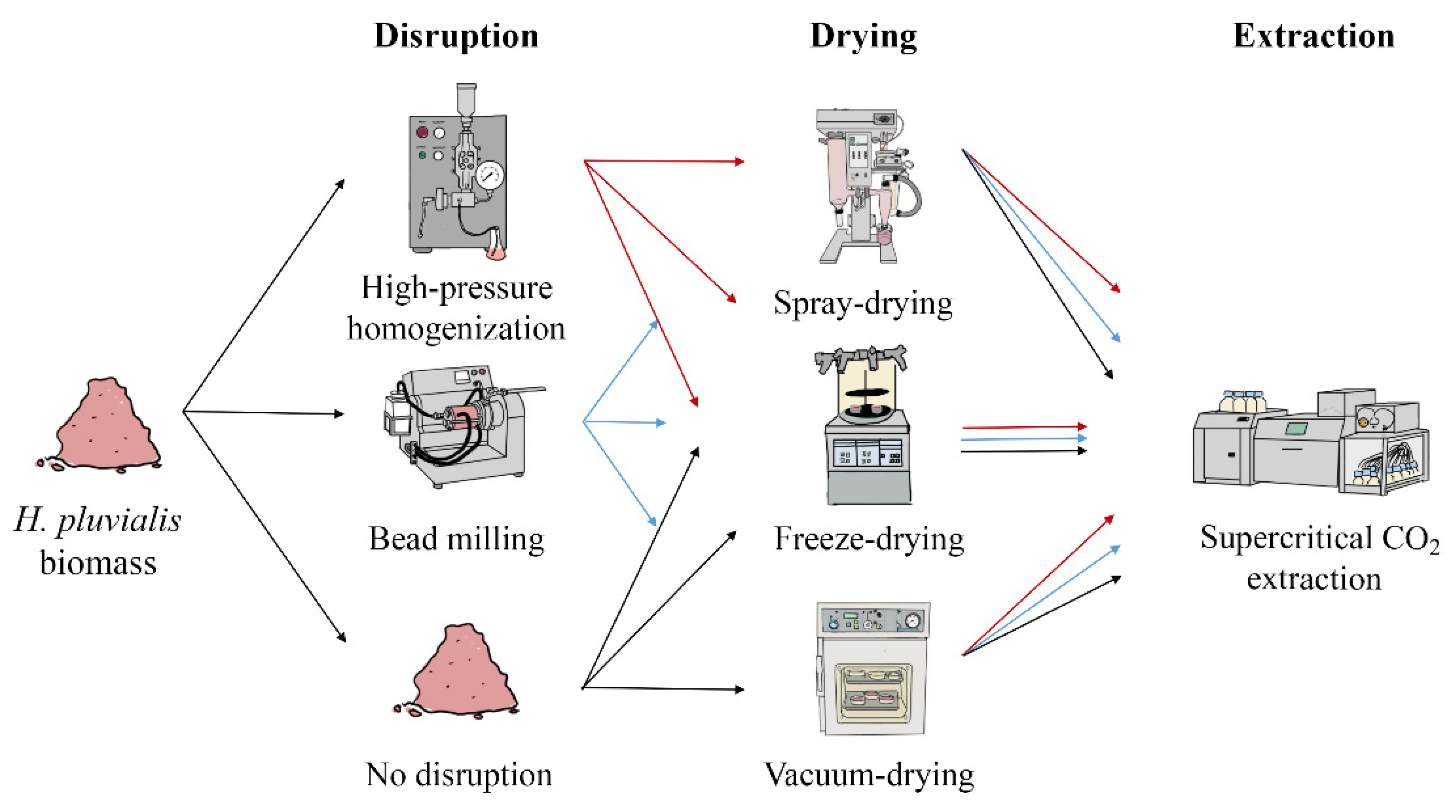
2.3. Downstream Processing—Laboratory Scale
2.3.1. Disruption of H. pluvialis Biomass
2.3.2. Drying of H. pluvialis Biomass
2.3.3. Supercritical CO2 Extraction of Astaxanthin
2.4. Downstream Processing—Pilot Scale
2.5. Disintegration Rate
2.6. Dry Weight
2.7. Astaxanthin Analysis
2.8. Evaluation of Significance
2.9. Effort Estimation
3. Results and Discussion
3.1. Disruption of H. pluvialis Biomass
3.1.1. High-Pressure Homogenization
3.1.2. Bead Milling
3.2. Drying of H. pluvialis Biomass
3.2.1. Freeze-Drying
3.2.2. Spray-Drying
3.2.3. Vacuum-Drying
3.3. Supercritical CO2 Extraction of Astaxanthin
3.4. Overall Astaxanthin Yield
3.5. Effort Estimation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Conn, P.F.; Schalch, W.; Truscott, T.G. The singlet oxygen and carotenoid interaction. J. Photochem. Photobiol. B Biol. 1991, 11, 41–47. [Google Scholar] [CrossRef]
- Miki, W. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991, 63, 141–146. [Google Scholar] [CrossRef]
- Shimidzu, N.; Goto, M.; Miki, W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish. Sci. 1996, 62, 134–137. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhang, X.; Sun, Y.; Lin, W. Antioxidative capacity and enzyme activity in Haematococcus pluvialis cells exposed to superoxide free radicals. Chin. J. Oceanol. Limnol. 2010, 28, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, E.; Mariutti, L.R.B.; Mercadante, A.Z. Scavenging capacity of marine carotenoids against reactive oxygen and nitrogen species in a membrane-mimicking system. Mar. Drugs 2012, 10, 1784–1798. [Google Scholar] [CrossRef]
- Liu, X.; Shibata, T.; Hisaka, S.; Osawa, T. Astaxanthin inhibits reactive oxygen species-mediated cellular toxicity in dopaminergic SH-SY5Y cells via mitochondria-targeted protective mechanism. Brain Res. 2009, 1254, 18–27. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Lee, P.-C.; Wu, Y.-L.; Liu, L.-Y. In vivo effects of free form astaxanthin powder on anti-oxidation and lipid metabolism with high-cholesterol diet. PLoS ONE 2015, 10, e0134733. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, L.; Shen, L.; Chen, Z.; Xu, L.; Zhang, J.; Yu, X. Trans.-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 2016, 1649, 30–37. [Google Scholar] [CrossRef]
- Xue, Y.; Qu, Z.; Fu, J.; Zhen, J.; Wang, W.; Cai, Y.; Wang, W. The protective effect of astaxanthin on learning and memory deficits and oxidative stress in a mouse model of repeated cerebral ischemia/reperfusion. Brain Res. Bull. 2017, 131, 221–228. [Google Scholar] [CrossRef]
- Farruggia, C.; Kim, M.-B.; Bae, M.; Lee, Y.; Pham, T.X.; Yang, Y.; Han, M.J.; Park, Y.-K.; Lee, J.-Y. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J. Nutr. Biochem. 2018, 62, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Sharma, D.; Sharma, M.; Sharma, N.; Bidve, P.; Prajapati, N.; Kalia, K.; Tiwari, V. Astaxanthin ameliorates behavioral and biochemical alterations in in-vitro and in-vivo model of neuropathic pain. Neurosci. Lett. 2018, 674, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Williamson, E.M. Astaxanthin: How much is too much? A safety review. Phytother. Res. 2019, 33, 3090–3111. [Google Scholar] [CrossRef] [PubMed]
- Capelli, B.; Bagchi, D.; Cysewski, G.R. Synthetic astaxanthin is significantly inferior to algal-based astaxanthin as an antioxidant and may not be suitable as a human nutraceutical supplement. Nutrafoods 2013, 12, 145–152. [Google Scholar] [CrossRef]
- Edwards, J.A.; Bellion, P.; Beilstein, P.; Rümbeli, R.; Schierle, J. Review of genotoxicity and rat carcinogenicity investigations with astaxanthin. Regul. Toxicol. Pharmacol. 2016, 75, 5–19. [Google Scholar] [CrossRef]
- Del Campo, J.A.; Rodríguez, H.; Moreno, J.; Vargas, M.Á.; Rivas, J.; Guerrero, M.G. Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta). Appl. Microbiol. Biotechnol. 2004, 64, 848–854. [Google Scholar] [CrossRef]
- Abe, K.; Hattori, H.; Hirano, M. Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem. 2007, 100, 656–661. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.H.; Lee, Y.K.; Ng, M.L.; Phang, S.M. Composition and accumulation of secondary carotenoids in Chlorococcum sp. J. Appl. Phycol. 1997, 9, 147–155. [Google Scholar] [CrossRef]
- Czeczuga, B. Carotenoids in Euglena rubida mainx. Comp. Biochem. Physiol. 1974, 48B, 349–354. [Google Scholar] [CrossRef]
- Czeczuga, B. Characteristic carotenoids in some phytobentos species in the coastal area of the Adriatic Sea. Acta Soc. Bot. Pol. 1986, 55, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Müller, T.; Bleiß, W.; Martin, C.D.; Rogaschewski, S.; Fuhr, G. Snow algae from northwest Svalbard: Their identification, distribution, pigment and nutrient content. Polar Biol. 1998, 20, 14–32. [Google Scholar] [CrossRef]
- Procházková, L.; Remias, D.; Holzinger, A.; Řezanka, T.; Nedbalová, L. Ecophysiological and ultrastructural characterisation of the circumpolar orange snow alga Sanguina aurantia compared to the cosmopolitan red snow alga Sanguina nivaloides (Chlorophyta). Polar Biol. 2021, 44, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.R.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-producing green microalga Haematococcus pluvialis: From single cell to high value commercial products. Front. Plant. Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, P.R.; Velani, S.; Kumari, S.; Lali, A.M.; Prakash, G. Isolation and optimization of a novel thraustochytrid strain for DHA rich and astaxanthin comprising biomass as aquafeed supplement. 3 Biotech. 2021, 11, 71. [Google Scholar] [CrossRef]
- Aki, T.; Hachida, K.; Yoshinaga, M.; Katai, Y.; Yamasaki, T.; Kawamoto, S.; Kakizono, T.; Maoka, T.; Shigeta, S.; Suzuki, O.; et al. Thraustochytrid as a potential source of carotenoids. J. Am. Oil Chem. Soc. 2003, 80, 789–794. [Google Scholar] [CrossRef]
- Park, H.; Kwak, M.; Seo, J.; Ju, J.; Heo, S.; Park, S.; Hong, W. Enhanced production of carotenoids using a thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess. Biosyst. Eng. 2018, 41, 1355–1370. [Google Scholar] [CrossRef]
- Yokoyama, A.; Izumida, H.; Miki, W. Production of astaxanthin and 4-ketozeaxanthin by the marine bacterium, Agrobacterium aurantiacum. Biosci. Biotechnol. Biochem. 1994, 58, 1842–1844. [Google Scholar] [CrossRef] [Green Version]
- Tsubokura, A.; Yoneda, H.; Mizuta, H. Paracoccus carotinifaciens sp. nov., a new aerobic gram-negative astaxanthin-producing bacterium. Int. J. Syst. Biol. 1999, 49 Pt 1, 277–282. [Google Scholar] [CrossRef]
- Yokoyama, A.; Miki, W.; Izumida, H.; Shizuri, Y. New trihydroxy-keto-carotenoids isolated from an astaxanthin-producing marine bacterium. Biosci. Biotechnol. Biochem. 1996, 60, 200–203. [Google Scholar] [CrossRef] [Green Version]
- Osanjo, G.O.; Muthike, E.W.; Tsuma, L.; Okoth, M.W.; Lünsdorf, H.; Abraham, W.-R.; Dion, M.; Timmis, K.N.; Golyshin, N.; Mulaa, F.J. A salt lake extremophile, Paracoccus bogoriensis sp. nov., efficiently produces xanthophyll carotenoids. Afr. J. Microbiol. Res. 2009, 3, 426–433. [Google Scholar]
- Iizuka, H.; Nishimura, Y. Microbiological studies on petroleum and natural Gas. X. Carotenoid pigments of hydrocarbon-utilizing bacteria. J. Gen. Appl. Microbiol. 1969, 15, 127–134. [Google Scholar] [CrossRef]
- Calo, P.; de Miguel, T.; Sieiro, C.; Velazquez, J.B.; Villa, T.G. Ketocarotenoids in halobacteria: 3-hydroxy-echinenone and trans-astaxanthin. J. Appl. Bacteriol. 1995, 79, 282–285. [Google Scholar] [CrossRef]
- Andrewes, A.G.; Phaff, H.J.; Starr, M.P. Carotenoids of Phaffia rhodozyma, a red-pigmented fermenting yeast. Phytochemistry 1976, 15, 1003–1007. [Google Scholar] [CrossRef]
- Tran, T.N.; Tran, Q.-V.; Huynh, H.T.; Hoang, N.-S.; Nguyen, H.C.; Ngo, D.-N. Astaxanthin production by newly isolated Rhodosporidium toruloides: Optimization of medium compositions by response surface Methodology. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 320–327. [Google Scholar] [CrossRef] [Green Version]
- Seybold, A.; Goodwin, T. Occurrence of astaxanthin in the flower petals in Adonis annua. Nature 1959, 184, 1714–1715. [Google Scholar] [CrossRef]
- Li, Y.; Gong, F.; Guo, S.; Yu, W.; Liu, J. Adonis amurensis is a promising alternative to Haematococcus as a resource for natural esterified (3S,3′S)-astaxanthin production. Plants 2021, 10, 1059. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, J.; Zhang, L. Cell cycles and proliferation patterns in Haematococcus pluvialis. Chin. J. Oceanol. Limnol. 2017, 35, 1205–1211. [Google Scholar] [CrossRef]
- Borowitzka, M.A.; Huisman, J.M.; Osborn, A. culture of the astaxanthin-producing green alga Haematococcus pluvialis. J. Appl. Phycol. 1991, 3, 295–304. [Google Scholar] [CrossRef]
- Kobayashi, M.; Kurimura, Y.; Kakizono, T.; Nishio, N.; Tsuji, Y. Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J. Ferment. Bioeng. 1997, 84, 94–97. [Google Scholar] [CrossRef]
- Boussiba, S.; Vonshak, A. Astaxanthin accumulation in the green alga Haematococcus pluvialis. Plant. Cell Physiol. 1991, 32, 1077–1082. [Google Scholar] [CrossRef] [Green Version]
- Boussiba, S. Carotenogenesis in the green alga Haematococcus pluvialis: Cellular physiology and stress response. Physiol. Plant. 2000, 108, 111–117. [Google Scholar] [CrossRef]
- Olaizola, M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000, 12, 499–506. [Google Scholar] [CrossRef]
- Torzillo, G.; Goksan, T.; Faraloni, C.; Kopecky, J.; Masojídek, J. Interplay between photochemical activities and pigment composition in an outdoor culture of Haematococcus pluvialis during the shift from the green to red stage. J. Appl. Phycol. 2003, 15, 127–136. [Google Scholar] [CrossRef]
- Aflalo, C.; Meshulam, Y.; Zarka, A.; Boussiba, S. On the relative efficiency of two- vs. one stage production of astaxanthin by the green alga Haematococcus pluvialis. Biotechnol. Bioeng. 2007, 98, 300–305. [Google Scholar] [CrossRef]
- Rao, R.; Sarada, A.R.; Baskaran, V.; Ravishankar, G.A. Identification of carotenoids from green alga Haematococcus pluvialis by HPLC and LC-MS (APCI) and their antioxidant properties. J. Microbiol. Biotechnol. 2009, 19, 1333–1341. [Google Scholar]
- Wang, J.; Han, D.; Sommerfeld, M.R.; Lu, C.; Hu, Q. Effect of initial biomass density on growth and astaxanthin production of Haematococcus pluvialis in an outdoor photobioreactor. J. Appl. Phycol. 2013, 25, 253–260. [Google Scholar] [CrossRef]
- Liyanaarachchi, V.C.; Nishshanka, G.K.S.H.; Premaratne, R.G.M.M.; Ariyadasa, T.U.; Nimarshana, P.H.V.; Malik, A. Astaxanthin accumulation in the green microalga Haematococcus pluvialis: Effect of initial phosphate concentration and stepwise/continuous light stress. Biotechnol. Rep. 2020, 28, e00538. [Google Scholar] [CrossRef]
- Rodríguez-Sifuentes, L.; Marszalek, J.E.; Hernández-Carbajal, G.; Chuck-Hernández, C. Importance of downstream processing of natural astaxanthin for pharmaceutical application. Front. Chem. Eng. 2021, 2, 29. [Google Scholar] [CrossRef]
- ’t Lam, G.P.; Vermuë, M.H.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Multi-product microalgae biorefineries: From concept towards reality. Trends Biotechnol. 2018, 36, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, D.; Niu, J.; Shen, S.; Wang, G. An economic assessment of astaxanthin production by large scale cultivation of Haematococcus pluvialis. Biotechnol. Adv. 2011, 29, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Nobre, B.; Marcelo, F.; Passos, R.; Beirão, L.; Palavra, A.; Gouveia, L.; Mendes, R. Supercritical carbon dioxide extraction of astaxanthin and other carotenoids from the microalga Haematococcus pluvialis. Eur. Food Res. Technol. 2006, 223, 787–790. [Google Scholar] [CrossRef]
- Valderrama, J.O.; Perrut, M.; Majewski, W. Extraction of astaxantine and phycocyanine from microalgae with supercritical carbon dioxide. J. Chem. Eng. Data 2003, 48, 827–830. [Google Scholar] [CrossRef]
- Damiani, M.C.; Leonardi, P.I.; Pieroni, O.I.; Cáceres, E.J. Ultrastructure of the cyst wall of Haematococcus pluvialis (Chlorophyceae): Wall development and behaviour during cyst germination. Phycologia 2006, 45, 616–623. [Google Scholar] [CrossRef]
- Hagen, C.; Siegmund, S.; Braune, W. Ultrastructural and chemical changes in the cell wall of Haematococcus pluvialis (Volvocales, Chlorophyta) during aplanospore formation. Eur. J. Phycol. 2002, 37, 217–226. [Google Scholar] [CrossRef]
- Sarada, R.; Vidhyavathi, R.; Usha, D.; Ravishankar, G.A. An efficient method for extraction of astaxanthin from green alga Haematococcus pluvialis. J. Agric. Food Chem. 2006, 54, 7585–7588. [Google Scholar] [CrossRef]
- Ruen-ngam, D.; Shotipruk, A.; Pavasant, P. Comparison of extraction methods for recovery of astaxanthin from Haematococcus pluvialis. Sep. Sci. Technol. 2010, 46, 64–70. [Google Scholar] [CrossRef]
- Dong, S.; Huang, Y.; Zhang, R.; Wang, S.; Liu, Y. Four different methods comparison for extraction of astaxanthin from green alga Haematococcus pluvialis. Sci. World J. 2014, 2014, 694305. [Google Scholar] [CrossRef] [Green Version]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT-Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Pinto, M.M.; Raposo, M.F.J.; Bowen, J.; Young, A.J.; Morais, R. Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: Effects on astaxanthin recovery and implications for bio-availability. J. Appl. Phycol. 2001, 13, 19–24. [Google Scholar] [CrossRef]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, A.; Minceva, M. Direct extraction of astaxanthin from the microalgae Haematococcus pluvialis using liquid–liquid chromatography. RSC Adv. 2019, 9, 22779–22789. [Google Scholar] [CrossRef] [Green Version]
- Praveenkumar, R.; Lee, K.; Lee, J.; Oh, Y.-K. Breaking dormancy: An energy-efficient means of recovering astaxanthin from microalgae. Green Chem. 2015, 17, 1226–1234. [Google Scholar] [CrossRef]
- Choi, S.-A.; Oh, Y.-K.; Lee, J.; Sim, S.J.; Hong, M.E.; Park, J.-Y.; Kim, M.-S.; Kim, S.W.; Lee, J.-S. High-efficiency cell disruption and astaxanthin recovery from Haematococcus pluvialis cyst cells using room-temperature imidazolium-based ionic liquid/water mixtures. Bioresour. Technol. 2019, 274, 120–126. [Google Scholar] [CrossRef]
- Praveenkumar, R.; Lee, J.; Vijayan, D.; Lee, S.Y.; Lee, K.; Sim, S.J.; Hong, M.E.; Kim, Y.-E.; Oh, Y.-K. Morphological change and cell disruption of Haematococcus pluvialis cyst during high-pressure homogenization for astaxanthin recovery. Appl. Sci. 2020, 10, 513. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, X.; Li, D.; Chen, W.; Zhang, K.; Chen, S. Preparation of stable microcapsules from disrupted cell of Haematococcus pluvialis by spray drying. Int. J. Food Sci. Technol. 2016, 51, 1834–1843. [Google Scholar] [CrossRef]
- Irshad, M.; Myint, A.A.; Hong, M.E.; Kim, J.; Sim, S.J. One-pot, simultaneous cell wall disruption and complete extraction of astaxanthin from Haematococcus pluvialis at room temperature. ACS Sustain. Chem. Eng. 2019, 7, 13898–13910. [Google Scholar] [CrossRef]
- Boonnoun, P.; Kurita, Y.; Kamo, Y.; Wahyudiono; Machmudah, S.; Okita, Y.; Ohashi, E.; Kanda, H.; Goto, M. Wet extraction of lipids and astaxanthin from Haematococcus pluvialis by liquefied dimethyl ether. J. Nutr. Food Sci. 2014, 4, 1–4. [Google Scholar] [CrossRef]
- Ahmed, F.; Li, Y.; Fanning, K.; Netzel, M.; Schenk, P.M. Effect of drying, storage temperature and air exposure on astaxanthin stability from Haematococcus pluvialis. Food Res. Int. 2015, 74, 231–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, M.F.J.; Morais, A.M.M.B.; Morais, R.M.S.C. Effects of spray-drying and storage on astaxanthin content of Haematococcus pluvialis biomass. World J. Microbiol. Biotechnol. 2012, 28, 1253–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wang, X.; Duan, C.; Yi, S.; Gao, Z.; Xiao, C.; Agathos, S.N.; Wang, G.; Li, J. Biotechnological production of astaxanthin from the microalga Haematococcus pluvialis. Biotechnol. Adv. 2020, 43, 107602. [Google Scholar] [CrossRef] [PubMed]
- Molina Grima, E.; Belarbi, E.-H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Etoh, H.; Suhara, M.; Tokuyama, S.; Kato, H.; Nakahigashi, R.; Maejima, Y.; Ishikura, M.; Terada, Y.; Maoka, T. Auto-oxidation products of astaxanthin. J. Oleo Sci. 2012, 61, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef]
- Perrut, M. Supercritical fluid applications: Industrial developments and economic issues. Ind. Eng. Chem. Res. 2000, 39, 4531–4535. [Google Scholar] [CrossRef]
- Álvarez, C.E.; Vardanega, R.; Salinas-Fuentes, F.; Ramírez, J.P.; Muñoz, W.B.; Jiménez-Rondón, D.; Meireles, M.A.A.; Mezquita, P.C.; Ruiz-Domínguez, M.C. Effect of CO2 flow rate on the extraction of astaxanthin and fatty acids from Haematococcus pluvialis using supercritical fluid technology. Molecules 2020, 25, 6044. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Shotipruk, A.; Goto, M.; Pavasant, P. Supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis with vegetable oils as co-solvent. Bioresour. Technol. 2008, 99, 5556–5560. [Google Scholar] [CrossRef]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of astaxanthin from Haematococcus pluvialis using supercritical CO2 and ethanol as entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Iovine, A.; Larocca, V.; Di Sanzo, G.; Martino, M.; Casella, P.; Chianese, S.; Musmarra, D. Extraction of astaxanthin and lutein from microalga Haematococcus pluvialis in the red phase using CO2 supercritical fluid extraction technology with ethanol as co-solvent. Mar. Drugs 2018, 16, 432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.-L.; Wang, H.-M.; Chen, C.-Y.; Chang, J.-S. Extraction of astaxanthin from Haematococcus pluvialis by supercritical carbon dioxide fluid with ethanol modifier: Supercritical CO2 fluid extraction of astaxanthin from microalgae. Eng. Life Sci. 2012, 12, 638–647. [Google Scholar] [CrossRef]
- Reyes, F.A.; Mendiola, J.A.; Ibañez, E.; del Valle, J.M. Astaxanthin extraction from Haematococcus pluvialis using CO2-expanded ethanol. J. Supercrit. Fluids 2014, 92, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Sanzo, G.; Mehariya, S.; Martino, M.; Larocca, V.; Casella, P.; Chianese, S.; Musmarra, D.; Balducchi, R.; Molino, A. Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from Haematococcus pluvialis microalgae. Mar. Drugs 2018, 16, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thana, P.; Machmudah, S.; Goto, M.; Sasaki, M.; Pavasant, P.; Shotipruk, A. Response surface methodology to supercritical carbon dioxide extraction of astaxanthin from Haematococcus pluvialis. Bioresour. Technol. 2008, 99, 3110–3115. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yang, B.; Yan, B.; Yao, X. Supercritical fluid extraction of astaxanthin from Haematococcus pluvialis and its antioxidant potential in sunflower oil. Innov. Food Sci. Emerg. Technol. 2012, 13, 120–127. [Google Scholar] [CrossRef]
- Pérez-López, P.; González-García, S.; Jeffryes, C.; Agathos, S.N.; McHugh, E.; Walsh, D.; Murray, P.; Moane, S.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of the red antioxidant carotenoid astaxanthin by microalgae: From lab to pilot scale. J. Clean. Prod. 2014, 64, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Koopmann, I.K.; Kramer, A.; Labes, A. Development and validation of reliable astaxanthin quantification from natural sources. PLoS ONE 2022, submitted.
- Euglert, G.; Vecchi, M. Trans./Cis isomerization of astaxanthin diacetate/isolation by HPLC. and identification by 1H-NMR. spectroscopy of three mono-cis- and six di-cis-isomers. Helv. Chim. Acta 1980, 63, 1711–1718. [Google Scholar] [CrossRef]
- Casella, P.; Iovine, A.; Mehariya, S.; Marino, T.; Musmarra, D.; Molino, A. Smart method for carotenoids characterization in Haematococcus pluvialis red phase and evaluation of astaxanthin thermal stability. Antioxidants 2020, 9, 422. [Google Scholar] [CrossRef]
- Subramanian, B.; Tchoukanova, N.; Djaoued, Y.; Pelletier, C.; Ferron, M.; Robichaud, J. Investigations on the geometrical isomers of astaxanthin: Raman spectroscopy of conjugated polyene chain with electronic and mechanical confinement: Investigations on the geometrical isomers of astaxanthin. J. Raman Spectrosc. 2014, 45, 299–304. [Google Scholar] [CrossRef]
- de Bruijn, W.J.C.; Weesepoel, Y.; Vincken, J.-P.; Gruppen, H. Fatty acids attached to all-trans-astaxanthin alter its cis–trans equilibrium, and consequently its stability, upon light-accelerated autoxidation. Food Chem. 2016, 194, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, E.A.; Kulikova, I.S.; Vasilov, R.G.; Selishcheva, A.A. The effect of the solvent nature and lighting on isomerization and oxidative degradation of astaxanthin. Biophysics 2020, 65, 433–442. [Google Scholar] [CrossRef]
- Bjerkeng, B.; Følling, M.; Lagocki, S.; Storebakken, T.; Olli, J.J.; Alsted, N. Bioavailability of all-E-astaxanthin and Z-isomers of astaxanthin in rainbow trout (Oncorhynchus mykiss). Aquaculture 1997, 157, 63–82. [Google Scholar] [CrossRef]
- Spiden, E.M.; Yap, B.H.J.; Hill, D.R.A.; Kentish, S.E.; Scales, P.J.; Martin, G.J.O. Quantitative evaluation of the ease of rupture of industrially promising microalgae by high pressure homogenization. Bioresour. Technol. 2013, 140, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Yap, B.H.J.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2015, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kleinig, A.R.; Mansell, C.J.; Nguyen, Q.D.; Badalyan, A.; Middelberg, A.P.J. Influence of broth dilution on the disruption of Escherichia coli. Biotechnol. Technol. 1995, 9, 759–762. [Google Scholar] [CrossRef]
- Halim, R.; Rupasinghe, T.W.T.; Tull, D.L.; Webley, P.A. Mechanical cell disruption for lipid extraction from microalgal biomass. Bioresour. Technol. 2013, 140, 53–63. [Google Scholar] [CrossRef]
- Middelberg, A.P.J. Microbial cell disruption by high-pressure homogenization. In Downstream Processing of Proteins; Desai, M.A., Ed.; Methods in Biotechnology; Humana Press: Totowa, NJ, USA, 2000; Volume 9, pp. 11–21. [Google Scholar]
- Brookman, J.S.G. Mechanism of cell disintegration in a high pressure homogenizer. Biotechnol. Bioeng. 1974, 16, 371–383. [Google Scholar] [CrossRef]
- Miao, F.; Geng, Y.; Lu, D.; Zuo, J.; Li, Y. Stability and changes in astaxanthin ester composition from Haematococcus pluvialis during storage. Chin. J. Oceanol. Limnol. 2013, 31, 1181–1189. [Google Scholar] [CrossRef] [Green Version]
- Niamnuy, C.; Devahastin, S.; Soponronnarit, S.; Vijaya Raghavan, G.S. Kinetics of astaxanthin degradation and color changes of dried shrimp during storage. J. Food Eng. 2008, 87, 591–600. [Google Scholar] [CrossRef]
- Yuan, J.-P.; Chen, F. Isomerization of trans-astaxanthin to cis-isomers in organic solvents. J. Agric. Food Chem. 1999, 47, 3656–3660. [Google Scholar] [CrossRef]
- Honda, M.; Takahashi, N.; Kuwa, T.; Takehara, M.; Inoue, Y.; Kumagai, T. Spectral characterisation of Z-isomers of lycopene formed during heat treatment and solvent effects on the E/Z isomerisation process. Food Chem. 2015, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Honda, M.; Takemura, R.; Fukaya, T.; Kubota, M.; Wahyudiono; Kanda, H.; Goto, M. The thermal Z-isomerization-induced change in solubility and physical properties of (all-E)-lycopene. Biochem. Biophys. Res. Commun. 2017, 491, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Honda, M.; Wahyudiono; Kanda, H.; Goto, M. Thermal isomerization of (all-E)-lycopene and separation of the Z-isomers by using a low boiling solvent: Dimethyl ether. Sep. Sci. Technol. 2017, 52, 2573–2582. [Google Scholar] [CrossRef]
- Honda, M.; Sowa, T.; Kawashima, Y. Thermal- and photo-induced isomerization of all-E- and Z-isomer-rich xanthophylls: Astaxanthin and its structurally--related xanthophylls, adonirubin, and adonixanthin. Eur. J. Lipid Sci. Technol. 2020, 122, 1900462. [Google Scholar] [CrossRef]
- Aman, R.; Schieber, A.; Carle, R. Effects of heating and illumination on trans-cis isomerization and degradation of β-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef]
- Floury, J.; Desrumaux, A.; Lardières, J. Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water wmulsions. Innov. Food Sci. Emerg. Technol. 2000, 1, 127–134. [Google Scholar] [CrossRef]
- Kwade, A. Determination of the most important grinding mechanism in stirred media mills by calculating stress intensity and stress number. Powder Technol. 1999, 105, 382–388. [Google Scholar] [CrossRef]
- Kwade, A.; Schwedes, J. Breaking Characteristics of different materials and their effect on stress intensity and stress number in stirred media mills. Powder Technol. 2002, 122, 109–121. [Google Scholar] [CrossRef]
- Suarez Garcia, E.; Lo, C.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Understanding mild cell disintegration of microalgae in bead mills for the release of biomolecules. Chem. Eng. Sci. 2019, 203, 380–390. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Mogren, H.; Lindblom, M.; Hedenskog, G. Mechanical disintegration of microorganisms in an industrial homogenizer. Biotechnol. Bioeng. 1974, 16, 261–274. [Google Scholar] [CrossRef]
- Postma, P.R.; Suarez-Garcia, E.; Safi, C.; Yonathan, K.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Energy efficient bead milling of microalgae: Effect of bead size on disintegration and release of proteins and carbohydrates. Bioresour. Technol. 2017, 224, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Montalescot, V.; Rinaldi, T.; Touchard, R.; Jubeau, S.; Frappart, M.; Jaouen, P.; Bourseau, P.; Marchal, L. Optimization of bead milling parameters for the cell disruption of microalgae: Process modeling and application to Porphyridium cruentum and Nannochloropsis oculata. Bioresour. Technol. 2015, 196, 339–346. [Google Scholar] [CrossRef]
- Suarez Garcia, E.; van Leeuwen, J.; Safi, C.; Sijtsma, L.; Eppink, M.H.M.; Wijffels, R.H.; van den Berg, C. Selective and energy efficient extraction of functional proteins from microalgae for food applications. Bioresour. Technol. 2018, 268, 197–203. [Google Scholar] [CrossRef]
- Irshad, M.; Hong, M.E.; Myint, A.A.; Kim, J.; Sim, S.J. Safe and complete extraction of astaxanthin from Haematococcus pluvialis by efficient mechanical disruption of cyst cell wall. Int. J. Food Eng. 2019, 15, 10. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Alhattab, M.; Kermanshahi-Pour, A.; Brooks, M.S.-L. Microalgae disruption techniques for product recovery: Influence of cell wall composition. J. Appl. Phycol. 2019, 31, 61–88. [Google Scholar] [CrossRef]
- Wileman, A.; Ozkan, A.; Berberoglu, H. Rheological properties of algae slurries for minimizing harvesting energy requirements in biofuel production. Bioresour. Technol. 2012, 104, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Pataro, G.; Capitoli, M.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M.; Olivieri, G.; Ferrari, G. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Kasper, J.C.; Winter, G.; Friess, W. Recent advances and further challenges in lyophilization. Eur. J. Pharm. Biopharm. 2013, 85, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Krokida, M. Effect of drying and extraction conditions on the recovery of bioactive compounds from Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 2947–2960. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Muylaert, K.; Eeckhout, M.; Ruyssen, T.; Foubert, I. Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J. Agric. Food Chem. 2011, 59, 11063–11069. [Google Scholar] [CrossRef]
- Chang, C.-H.; Lin, H.-Y.; Chang, C.-Y.; Liu, Y.-C. Comparisons on the antioxidant properties of fresh, freeze-dried and hot-air-dried tomatoes. J. Food Eng. 2006, 77, 478–485. [Google Scholar] [CrossRef]
- Vanamala, J.; Cobb, G.; Turner, N.D.; Lupton, J.R.; Yoo, K.S.; Pike, L.M.; Patil, B.S. Bioactive compounds of grapefruit (Citrus paradisi cv. rio red) respond differently to postharvest irradiation, storage, and freeze drying. J. Agric. Food Chem. 2005, 53, 3980–3985. [Google Scholar] [CrossRef]
- Simonne, A.H.; Smith, M.; Weaver, D.B.; Vail, T.; Barnes, S.; Wei, C.I. Retention and changes of soy isoflavones and carotenoids in immature soybean seeds (Edamame) during processing. J. Agric. Food Chem. 2000, 48, 6061–6069. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Fu, L.; Zhu, H.; Zhang, B. Effect of extraction and drying methods on antioxidant activity of astaxanthin from Haematococcus pluvialis. Food Bioprod. Process. 2016, 99, 197–203. [Google Scholar] [CrossRef]
- Cong, X.-Y.; Miao, J.-K.; Zhang, H.-Z.; Sun, W.-H.; Xing, L.-H.; Sun, L.-R.; Zu, L.; Gao, Y.; Leng, K.-L. Effects of drying methods on the content, structural isomers, and composition of astaxanthin in antarctic krill. ACS Omega 2019, 4, 17972–17980. [Google Scholar] [CrossRef] [Green Version]
- Focaroli, S.; Mah, P.T.; Hastedt, J.E.; Gitlin, I.; Oscarson, S.; Fahy, J.V.; Healy, A.M. A Design of experiment (DoE) approach to optimise spray drying process conditions for the production of trehalose/leucine formulations with application in pulmonary delivery. Int. J. Pharm. 2019, 562, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Ståhl, K.; Claesson, M.; Lilliehorn, P.; Lindén, H.; Bäckström, K. The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int. J. Pharm. 2002, 233, 227–237. [Google Scholar] [CrossRef]
- Broadhead, J.; Rouan, S.K.E.; Hau, I.; Rhodes, C.T. The Effect of process and formulation variables on the properties of spray-dried β-Galactosidase. J. Pharm. Pharmacol. 1994, 46, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Linehan, B.; Tseng, Y.-C. Optimization of the Büchi B-90 spray drying process using central composite design for preparation of solid dispersions. Int. J. Pharm. 2015, 491, 208–217. [Google Scholar] [CrossRef]
- Büchi Application Note No. 245/2016, Spray Drying of Microalgae. 2016. Available online: https://www.buchi.com/en/knowledge/applications/spray-drying-microalgae (accessed on 11 April 2022).
- Leach, G.; Oliveira, G.; Morais, R. Spray-drying of Dunaliella salina to produce β-carotene rich powder. J. Ind. Microbiol. Biotechnol. 1998, 20, 82–85. [Google Scholar] [CrossRef]
- Bhosale, P.; Jogdand, V.V.; Gadre, R.V. Stability of β-Carotene in spray dried preparation of Rhodotorula glutinis mutant 32. J. Appl. Microbiol. 2003, 95, 584–590. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β -carotene by spray drying: Effect of wall material concentration and drying inlet temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef] [Green Version]
- Bustamante, A.; Masson, L.; Velasco, J.; del Valle, J.M.; Robert, P. Microencapsulation of H. pluvialis oleoresins with different fatty acid composition: Kinetic stability of astaxanthin and alpha-tocopherol. Food Chem. 2016, 190, 1013–1021. [Google Scholar] [CrossRef]
- Larrosa, A.P.Q.; Comitre, A.A.; Vaz, L.B.; Pinto, L.A.A. Influence of air temperature on physical characteristics and bioactive compounds in vacuum drying of Arthrospira spirulina: Spirulina dried in vacuum dryer. J. Food Process. Eng. 2017, 40, e12359. [Google Scholar] [CrossRef]
- Karasu, S.; Kilicli, M.; Baslar, M.; Arici, M.; Sagdic, O.; Karaagacli, M. Dehydration kinetics and changes of bioactive compounds of tulip and poppy petals as a natural colorant under vacuum and oven conditions: Best drying condition of tulip and poppy petals. J. Food Process. Preserv. 2015, 39, 2096–2106. [Google Scholar] [CrossRef]
- Brunner, G. Gas Extraction; Topics in Physical Chemistry; Springer: Berlin/Heidelberg, Germany, 1994; Volume 4, ISBN 978-3-662-07382-7. [Google Scholar]
- Huang, Z.; Shi, X.; Jiang, W. Theoretical models for supercritical fluid extraction. J. Chromatogr. A 2012, 1250, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Gamlieli-Bonshtein, I.; Korin, E.; Cohen, S. Selective separation of cis-trans geometrical isomers of β-carotene via CO2 supercritical fluid extraction. Biotechnol. Bioeng. 2002, 80, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Honda, M.; Higashiura, T.; Fukaya, T.; Machmudah, S.; Wahyudiono; Kanda, H.; Goto, M. Rapid and selective concentration of lycopene Z-isomers from tomato pulp by supercritical CO2 with co-solvents. Solvent Extr. Res. Dev. Jpn. 2018, 25, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorganica Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Gomez, P.I.; Inostroza, I.; Pizarro, M.; Perez, J. From genetic improvement to commercial-scale mass culture of a Chilean strain of the green microalga Haematococcus pluvialis with enhanced productivity of the red ketocarotenoid astaxanthin. AoB Plants 2013, 5, plt026. [Google Scholar] [CrossRef] [Green Version]
- Nobre, B.P.; Palavra, A.F.; Pessoa, F.L.P.; Mendes, R.L. Supercritical CO2 extraction of trans-lycopene from Portuguese tomato industrial waste. Food Chem. 2009, 116, 680–685. [Google Scholar] [CrossRef]
- Yi, C.; Shi, J.; Xue, S.J.; Jiang, Y.; Li, D. Effects of supercritical fluid extraction parameters on lycopene yield and antioxidant activity. Food Chem. 2009, 113, 1088–1094. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-drying of plant-based foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.; Jakubczyk, E. The freeze-drying of foods—The characteristic of the process course and the effect of its parameters on the physical properties of food materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Filková, I.; Huang, L.X.; Mujumdar, A.S. Industrial spray drying systems. In Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA, 2006; pp. 215–256. [Google Scholar]
- Ratti, C. Hot air and freeze-frying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Blázquez, E.; Rodríguez, C.; Ródenas, J.; Saborido, N.; Solà-Ginés, M.; Pérez de Rozas, A.; Campbell, J.M.; Segalés, J.; Pujols, J.; Polo, J. Combined effects of spray-drying conditions and postdrying storage time and temperature on Salmonella choleraesuis and Salmonella typhimurium survival when inoculated in liquid porcine plasma. Lett. Appl. Microbiol. 2018, 67, 205–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LiCari, J.J.; Potter, N.N. Salmonella survival during spray drying and subsequent handling of skimmilk powder. II. Effects of drying conditions. J. Dairy Sci. 1970, 53, 871–876. [Google Scholar] [CrossRef]
- Zgheib, N.; Saade, R.; Khallouf, R.; Takache, H. Extraction of astaxanthin from microalgae: Process design and economic feasibility study. IOP Conf. Ser. Mater. Sci. Eng. 2018, 323, 012011. [Google Scholar] [CrossRef]


| Linear Model | Quadratic Model | Quadratic Model + Interactions | ||||
|---|---|---|---|---|---|---|
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| A (Intercept) | 7.175 | 27.5392 | −23.6551 | |||
| b1 (S a) | −0.0178 | 0.0006 | −0.0848 | 0.212 | −0.0349 | 0.48853 |
| b2 (F b) | 0.0294 | 0.2422 | −0.0326 | 0.9482 | −6.4457 | 0.01817 |
| b3 (T c) | 0.0196 | 0.7315 | −0.0295 | 0.9082 | 0.7736 | 0.07361 |
| c1 (S2) | 0.0001 | 0.3117 | 0.0001 | 0.06927 | ||
| c2 (F2) | 0.0031 | 0.8963 | −0.0661 | 0.04148 | ||
| c3 (T2) | 0.0001 | 0.859 | −0.002 | 0.08029 | ||
| d12 (S*F) | 0.0044 | 0.02337 | ||||
| d13 (S*T) | −0.0008 | 0.04314 | ||||
| d23 (F*T) | 0.0337 | 0.02003 | ||||
| R2 | 0.7739 | 0.8085 | 0.9612 | |||
| R2 adjusted | 0.7061 | 0.6444 | 0.8739 | |||
| Optimized parameters | ||||||
| F | 15 | 15 | 10.4 | |||
| S | 400 | 400 | 400 | |||
| T | 180 | 180 | 180 | |||
| Disruption | Drying | Recovery (%) |
|---|---|---|
| No disruption | Freeze-drying | 13.7 ± 0.35 |
| No disruption | Spray-drying | 9.4 ± 0.21 |
| No disruption | Vacuum-drying | 14.0 ± 0.27 |
| Milling | Freeze-drying | 78.1 ± 0.95 |
| Milling | Spray-drying | 79.0 ± 1.88 |
| Milling | Vacuum-drying | 30.9 ± 0.71 |
| High-pressure homogenization | Freeze-drying | 85.4 ± 1.36 |
| High-pressure homogenization | Spray-drying | 81.6 ± 1.56 |
| High-pressure homogenization | Vacuum-drying | 60.5 ± 0.69 |
| Cell Disruption | Biomass Drying | |||
|---|---|---|---|---|
| Bead Mill | High-Pressure Homogenizer | Spray-Dryer | Freeze-Dryer | |
| Throughput | 10 L/h | 3 L/h | 5 L/h | 0.4 L/h |
| Power consumption | 4 kW | 4 kW | 5 kW | 4.5 kW |
| Disruption efficiency | high | high | n.a. | n.a. |
| Residual moisture | n.a. | n.a. | <9% | <9% |
| Time for setup | <0.5 h | <0.5 h | 1 h | <0.5 h |
| Time for processing | 15 h | 50 h | 10 h a/30 h b | 120 h a/360 h b |
| Cleaning time | 1 h | <1 h | 1–2 h | <0.5 h |
| Cleaning procedure | simple | very simple | difficult | very simple |
| Overall workload | 32 h | 61 h | 12 h | 2 h |
| Product sanitization | n.a. | n.a. | very high | high |
| Usability | simple | simple | moderate | simple |
| Scalability | yes | yes | yes | yes |
| Acquisition costs | EUR 70,000 | EUR 40,000 | EUR 250,000 | EUR 27,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koopmann, I.K.; Möller, S.; Elle, C.; Hindersin, S.; Kramer, A.; Labes, A. Optimization of Astaxanthin Recovery in the Downstream Process of Haematococcus pluvialis. Foods 2022, 11, 1352. https://doi.org/10.3390/foods11091352
Koopmann IK, Möller S, Elle C, Hindersin S, Kramer A, Labes A. Optimization of Astaxanthin Recovery in the Downstream Process of Haematococcus pluvialis. Foods. 2022; 11(9):1352. https://doi.org/10.3390/foods11091352
Chicago/Turabian StyleKoopmann, Inga K., Simone Möller, Clemens Elle, Stefan Hindersin, Annemarie Kramer, and Antje Labes. 2022. "Optimization of Astaxanthin Recovery in the Downstream Process of Haematococcus pluvialis" Foods 11, no. 9: 1352. https://doi.org/10.3390/foods11091352
APA StyleKoopmann, I. K., Möller, S., Elle, C., Hindersin, S., Kramer, A., & Labes, A. (2022). Optimization of Astaxanthin Recovery in the Downstream Process of Haematococcus pluvialis. Foods, 11(9), 1352. https://doi.org/10.3390/foods11091352






