Assessment of Ultrasonic Stress on Survival and β-Glucosidase Activity of Encapsulated Lactiplantibacillus plantarum BCRC 10357 in Fermentation of Black Soymilk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Lactiplantibacillus plantarum BCRC 10357 Culture
2.2. Encapsulation of L. plantarum BCRC 10357 by Extrusion
2.3. Characterization for the Microstructure of Encapsulated L. plantarum BCRC 10357
2.4. Ultrasound Treatment on the Encapsulated L. plantarum BCRC 10357
2.5. Determination of β-Glucosidase Activity
2.6. Determination of Isoflavones
2.7. Statistical Analysis
3. Results and Discussion
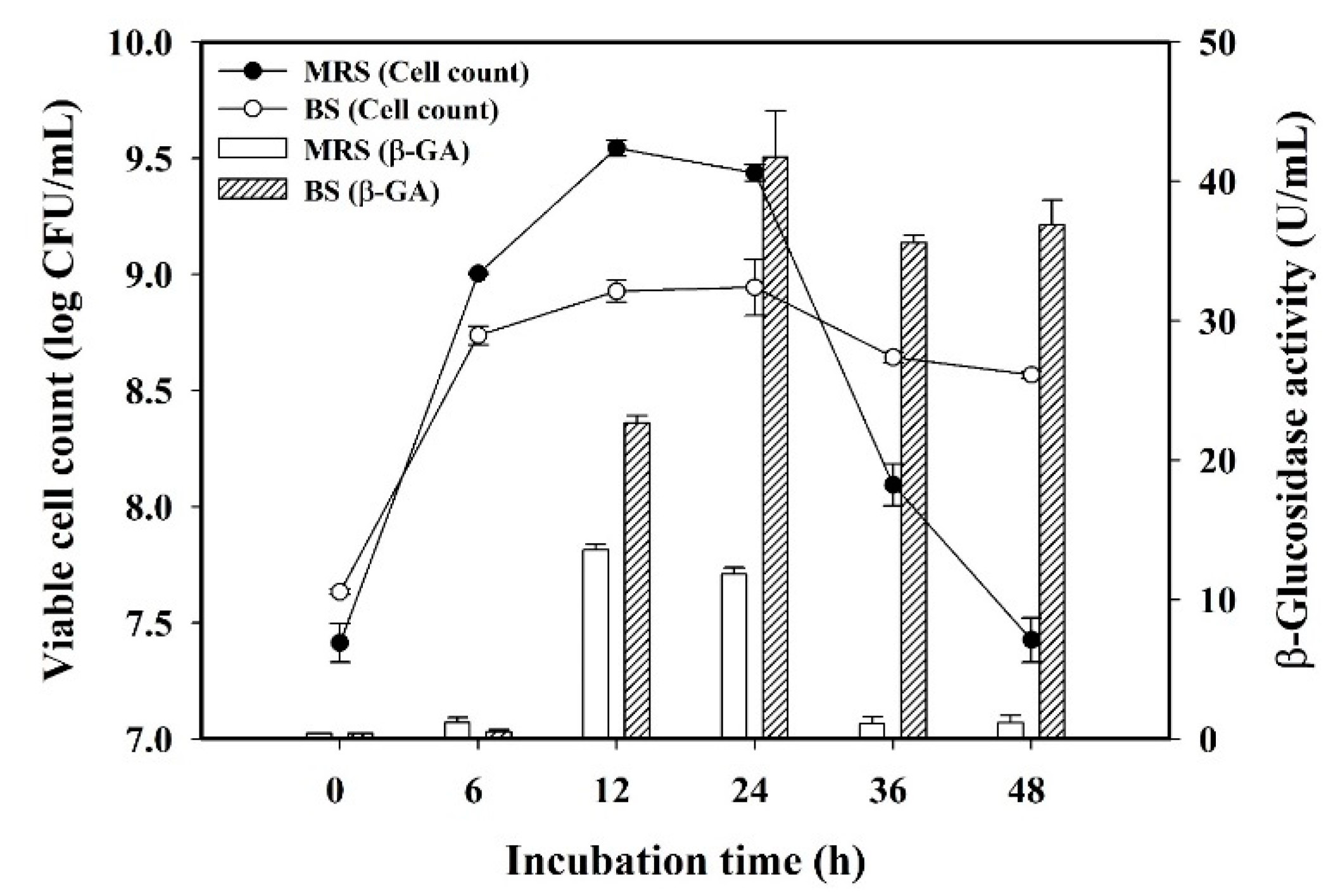
3.1. Effect of Culture Medium on L. plantarum BCRC 10357
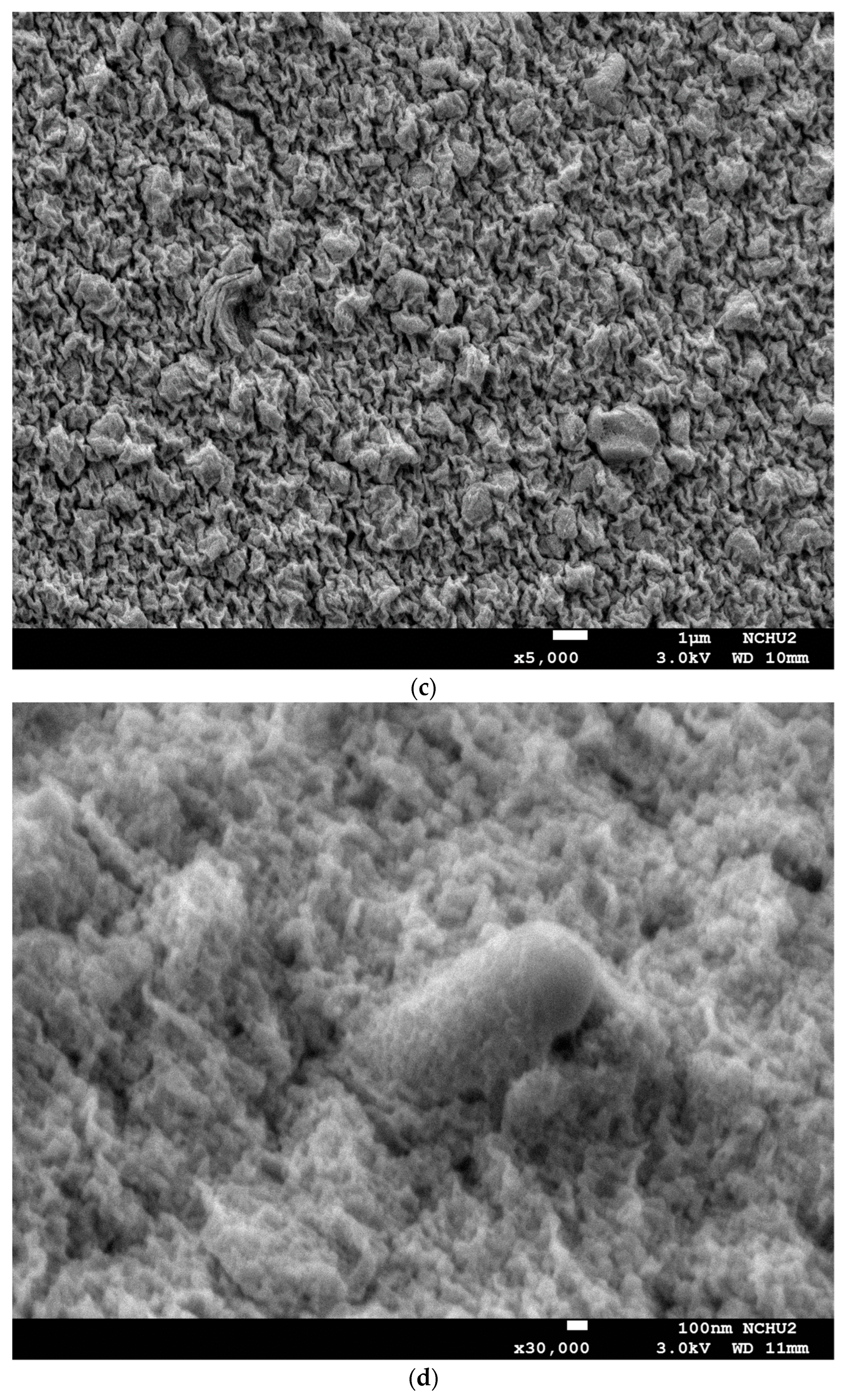
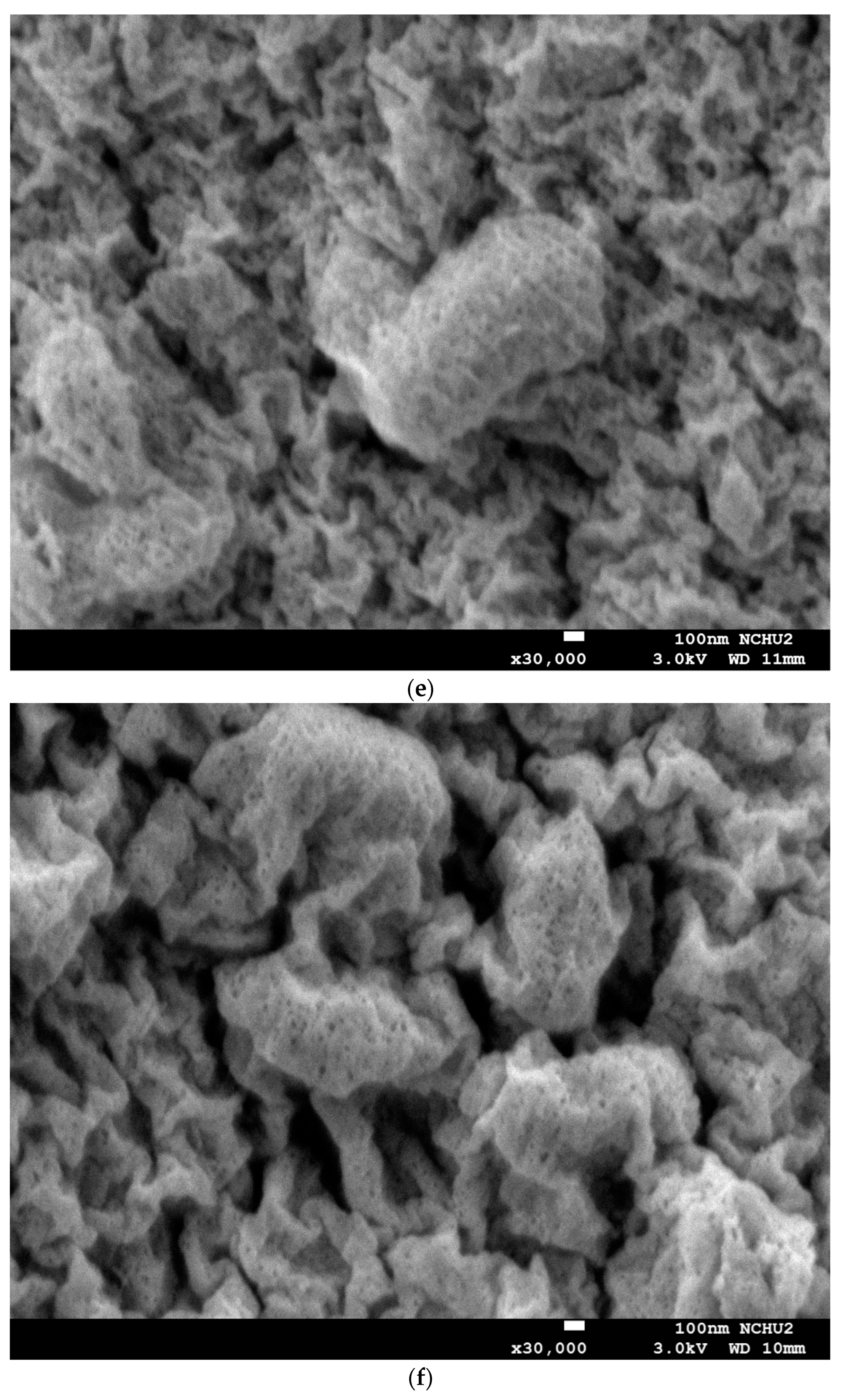
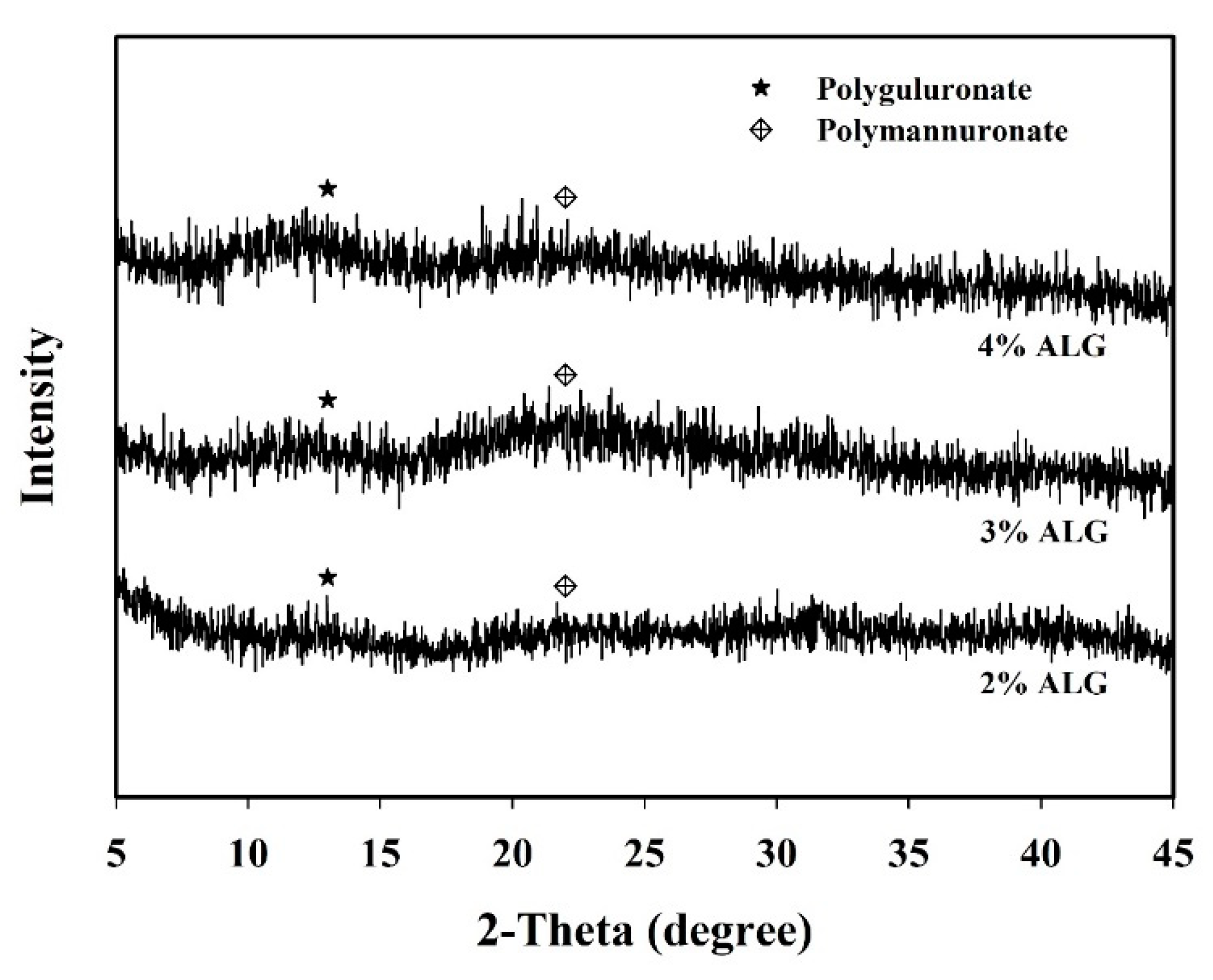
3.2. Morphological Structure and XRD Analysis of Capsules Loaded with L. plantarum BCRC 10357
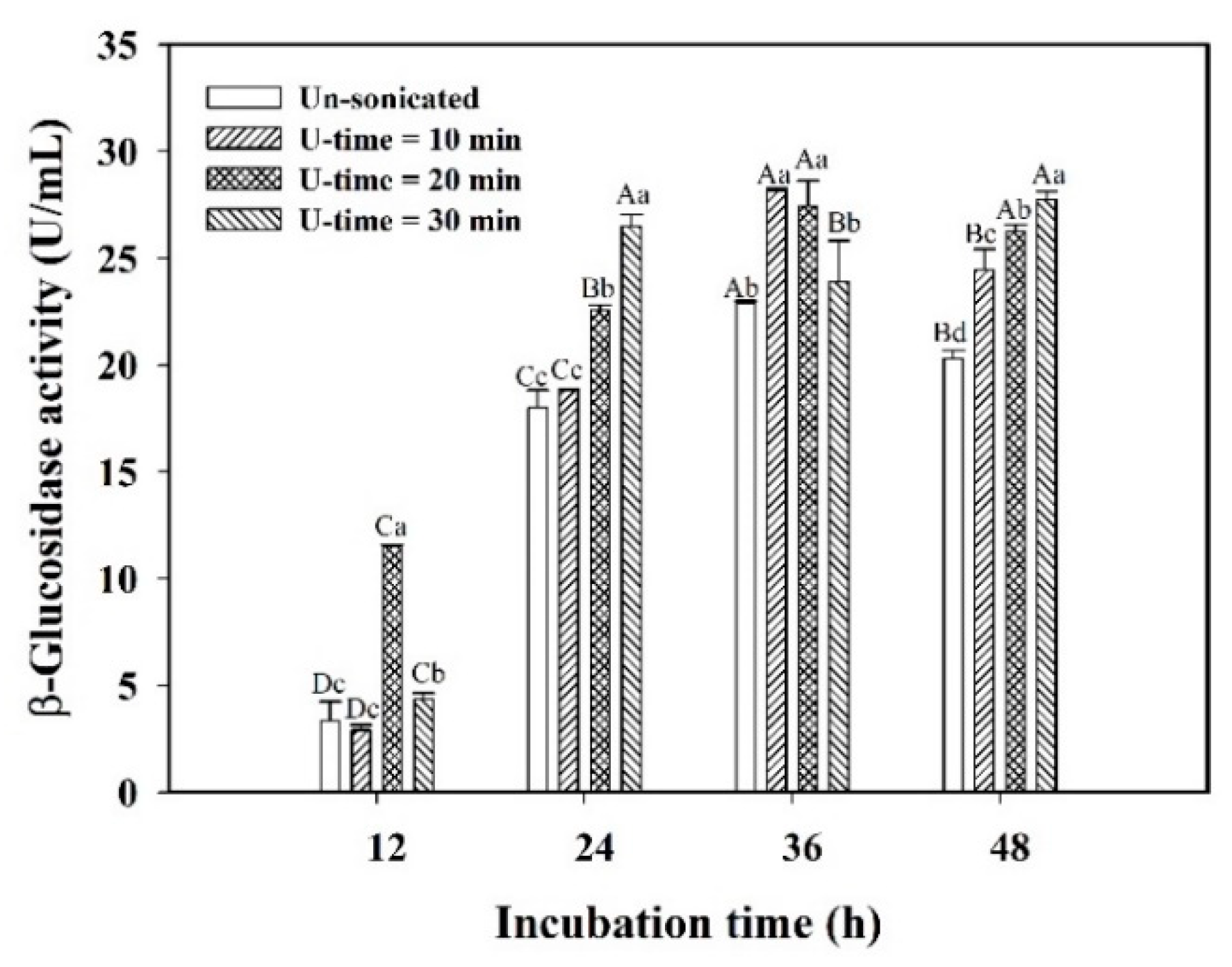
3.3. Stress Response of Encapsulated L. plantarum BCRC 10357 by Ultrasonic Treatment
3.4. Effect of Encapsulation Modes on L. plantarum BCRC 10357 in the Fermentation of Black Soymilk
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parvez, S.; Malik, K.A.; Ah Kang, S.; Kim, H.Y. Probiotics and their fermented food products are beneficial for health. J. Appl. Microbiol. 2006, 100, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Nuraida, L. A review: Health promoting lactic acid bacteria in traditional Indonesian fermented foods. Food Sci. Hum. Wellness 2015, 4, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Śliżewska, K.; Chlebicz-Wójcik, A. Growth kinetics of probiotic Lactobacillus strains in the alternative, cost-efficient semi-solid 547 fermentation medium. Biology 2020, 9, 423. [Google Scholar] [CrossRef]
- Tsui, C.Y.; Yang, C.Y. Evaluation of semi-solid-state fermentation of Elaeocarpus serratus L. leaves and black soymilk by Lactobacillus plantarum on bioactive compounds and antioxidant capacity. Foods. 2021, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Salas-Jara, M.J.; Ilabaca, A.; Vega, M.; García, A. Biofilm forming Lactobacillus: New challenges for the development of probiotics. Microorganisms 2016, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Petraitytė, S.; Šipailienė, A. Enhancing encapsulation efficiency of alginate capsules containing lactic acid bacteria by using different divalent cross-linkers sources. LWT—Food Sci. Technol. 2019, 110, 307–315. [Google Scholar] [CrossRef]
- Varela-Pérez, A.; Romero-Chapol, O.O.; Castillo-Olmos, A.G.; García, H.S.; Suárez-Quiroz, M.L.; Singh, J.; Figueroa-Hernández, C.Y.; Viveros-Contreras, R.; Cano-Sarmiento, C. Encapsulation of Lactobacillus gasseri: Characterization, probiotic survival, in vitro evaluation and viability in apple juice. Foods 2022, 11, 740. [Google Scholar] [CrossRef] [PubMed]
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of probiotic living cells: From laboratory scale to industrial applications. J. Food Eng. 2011, 104, 467–493. [Google Scholar] [CrossRef]
- Gortzi, O.; Rovoli, M.; Katsoulis, K.; Graikou, K.; Karagkini, D.A.; Stagos, D.; Kouretas, D.; Tsaknis, J.; Chinou, I. Study of stability, cytotoxic and antimicrobial activity of chios mastic gum fractions (neutral, acidic) after encapsulation in liposomes. Foods 2022, 11, 271. [Google Scholar] [CrossRef]
- Lavelli, V.; Sereikaitė, J. Kinetic study of encapsulated β-carotene degradation in dried systems: A review. Foods 2022, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Maryam, A. Encapsulation of lactic acid bacteria and Bifidobacteria using starch-sodium alginate nanofibers to enhance viability in food model. J. Food Process. Preserv. 2021, 45, e16048. [Google Scholar] [CrossRef]
- Luca, L.; Oroian, M. Influence of different prebiotics on viability of Lactobacillus casei, Lactobacillus plantarum and Lactobacillus rhamnosus encapsulated in alginate microcapsules. Foods 2021, 10, 710. [Google Scholar] [CrossRef]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. Evaluation of encapsulation techniques of probiotics for yogurt. Int. Dairy J. 2003, 13, 3–13. [Google Scholar] [CrossRef]
- Westman, J.O.; Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Effects of encapsulation of microorganisms on product formation during microbial fermentations. Appl. Microbiol. Biotechnol. 2012, 96, 1441–1454. [Google Scholar] [CrossRef]
- Liu, W.S.; Yang, C.Y.; Fang, T.J. Strategic ultrasound-induced stress response of lactic acid bacteria on enhancement of β-glucosidase activity for bioconversion of isoflavones in soymilk. J. Microbiol. Methods 2018, 148, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry-A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Peng, H.T.; Yang, C.Y.; Fang, T.J. Enhanced β-glucosidase activity of Lactobacillus plantarum by a strategic ultrasound treatment for biotransformation of isoflavones in okara. Food Sci. Technol. Res. 2018, 24, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Gaucher, F.; Bonnassie, S.; Rabah, H.; Marchand, P.; Blanc, P.; Jeantet, R.; Jan, G. Review: Adaptation of beneficial propionibacteria, lactobacilli, and bifidobacteria improves tolerance toward technological and digestive stresses. Front. Microbiol. 2019, 10, 841. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Boekhorst, J.; van Kranenburg, R.; Molenaar, D.; Kuipers, O.P.; Leer, R.; Tarchini, R.; Peters, S.A.; Sandbrink, H.M.; Fiers, M.W.E.J.; et al. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 2003, 100, 1990–1995. [Google Scholar] [CrossRef] [Green Version]
- Russo, P.; Arena, M.P.; Fiocco, D.; Capozzi, V.; Drider, D.; Spano, G. Lactobacillus plantarum with broad antifungal activity: A promising approach to increase safety and shelf-life of cereal-based products. Int. J. Food Microbiol. 2017, 247, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Abdelazez, A.; Abdelmotaal, H.; Zhu, Z.T.; Jia, F.F.; Sami, R.; Zhang, L.J.; Al-Tawaha, A.R.; Meng, X.C. Potential benefits of Lactobacillus plantarum as probiotic and its advantages in human health and industrial applications: A review. Adv. Environ. Biol. 2018, 12, 16–27. [Google Scholar]
- Kubota, H.; Senda, S.; Nomura, N.; Tokuda, H.; Uchiyama, H. Biofilm formation by lactic acid bacteria and resistance to environmental stress. J. Biosci. Bioeng. 2008, 106, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Antioxidant capacity of seed coat, dehulled bean, and whole black soybeans in relation to their distributions of total phenolics, phenolic acids, anthocyanins, and isoflavones. J. Agric. Food Chem. 2008, 56, 8365–8373. [Google Scholar] [CrossRef]
- Vasile, M.A.; Milea, Ș.A.; Enachi, E.; Barbu, V.; Cîrciumaru, A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Functional enhancement of bioactives from black beans and lactic acid bacteria into an innovative food ingredient by comicroencapsulation. Food Bioprocess Technol. 2020, 13, 978–987. [Google Scholar] [CrossRef]
- Andrade, J.E.; Twaddle, N.C.; Helferich, W.G.; Doerge, D.R. Absolute bioavailability of isoflavones from soy protein isolate containing food in female balb/c mice. J. Agric. Food Chem. 2010, 58, 4529–4536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mbye, M.; Baig, M.A.; AbuQamar, S.F.; El-Tarabily, K.A.; Obaid, R.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Turner, M.S.; Shah, N.P.; Ayyash, M.M. Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1110–1124. [Google Scholar] [CrossRef] [Green Version]
- Comaposada, J.; Gou, P.; Marcos, B.; Arnau, J. Physical properties of sodium alginate solutions and edible wet calcium alginate coatings. LWT—Food Sci. Technol. 2015, 64, 212–219. [Google Scholar] [CrossRef]
- Kavitake, D.; Sujatha Kandasamy, S.; Devi, P.B.; Shettya, P.H. Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods—A review. Food Biosci. 2018, 21, 34–44. [Google Scholar] [CrossRef]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; del Carmen Villarán, M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef]
- Chen, K.N.; Chen, M.J.; Liu, J.R.; Lin, C.W.; Chiu, H.Y. Optimization of incorporated prebiotics as coating materials for probiotic microencapsulation. J. Food Sci. 2005, 70, M260–M266. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundarrajan, P.; Eswaran, P.; Marimuthu, A.; Subhadra, L.B.; Kannaiyan, P. One pot synthesis and characterization of alginate stabilized semiconductor nanoparticles. Bull. Korean Chem. Soc. 2012, 33, 3218–3224. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.A.; Yang, C.Y. Effect of ultrasound on the extraction of bioactive aglycone isoflavones for the green valorization of black soybean residue (okara). J. Food Process. Preserv. 2019, 43, e13944. [Google Scholar] [CrossRef]
- Allan-Wojtas, P.; Truelstrup Hansen, L.; Paulson, A.T. Microstructural studies of probiotic bacteria-loaded alginate microcapsules using standard electron microscopy techniques and anhydrous fixation. LWT—Food Sci. Technol. 2008, 41, 101–108. [Google Scholar] [CrossRef]
- Thu, B.; Bruheim, P.; Espevik, T.; Smidsrød, O.; Soon-Shiong, P.; Skjåk-Bræk, G. Alginate polycation microcapsules. I. Interaction between alginate and polycation. Biomaterials 1996, 17, 1031–1040. [Google Scholar] [CrossRef]
- Fontes, G.C.; Calado, V.M.A.; Rossi, A.M.; da Rocha-Leão, M.H.M. Characterization of antibiotic-loaded alginate-osa starch microbeads produced by ionotropic pregelation. Biomed Res. Int. 2013, 2013, 472626. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, L.; Peng, T.; Zhong, W. Effects of Ca2+ bridge cross-linking on structure and pervaporation of cellulose/alginate blend membranes. J. Membr. Sci. 2000, 175, 53–60. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, X.; Du, Y.; Kennedy, J.F. Alginate/starch blend fibers and their properties for drug controlled release. Carbohydr. Polym. 2010, 82, 842–847. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Crecchio, C.; De Virgilio, C.; De Angelis, M.; Gobbetti, M. Transcriptional reprogramming and phenotypic switching associated with the adaptation of Lactobacillus plantarum C2 to plant niches. Sci. Rep. 2016, 6, 27392. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Xu, J.; Chen, Q.; Wang, H.; Kong, B. Physiological, morphological and antioxidant responses of Pediococcus pentosaceus R1 and Lactobacillus fermentum R6 isolated from harbin dry sausages to oxidative stress. Foods 2021, 10, 1203. [Google Scholar] [CrossRef]


| U-Time (Min) | Viable Cell Count (Log CFU/mL) at Incubation Time (h) * | |||
|---|---|---|---|---|
| 12 | 24 | 36 | 48 | |
| Un-sonicated | 8.45 ± 0.03 a | 8.70 ± 0.02 b | 8.90 ± 0.15 a | 8.73 ± 0.03 b |
| 10 | 8.28 ± 0.14 a | 8.80 ± 0.10 ab | 8.81 ± 0.27 a | 8.77 ± 0.08 ab |
| 20 | 8.47 ± 0.09 a | 8.99 ± 0.17 a | 8.81 ± 0.15 a | 8.78 ± 0.02 ab |
| 30 | 8.49 ± 0.18 a | 8.79 ± 0.05 b | 8.83 ± 0.14 a | 8.82 ± 0.01 a |
| Items | Fermentation Time (h) | Isoflavones (μg/g—Dried Black Soymilk) | AI/TI (%) | |||
|---|---|---|---|---|---|---|
| Daidzin | Genistin | Daidzein | Genistein | |||
| 2AN | 12 | 132.67 ± 20.24 | 166.11 ± 17.64 | 46.09 ± 9.15 | 22.22 ± 3.65 | 18.65 |
| 2AC | 12 | 83.89 ± 32.87 | 63.35 ± 24.70 | 143.25 ± 30.24 | 65.36 ± 15.73 | 59.56 |
| 4AN | 12 | 70.17 ± 15.37 | 11.43 ± 1.67 | 151.01 ± 34.50 | 64.07 ± 18.60 | 72.34 |
| 2AN | 48 | 2.36 ± 0.19 | 2.44 ± 0.07 | 195.40 ± 30.67 | 162.95 ± 28.16 | 98.65 |
| 2AC | 48 | 14.50 ± 7.29 | 28.69 ± 15.87 | 161.68 ± 68.47 | 101.48 ± 47.47 | 86.46 |
| 4AN | 48 | 18.33 ± 5.04 | 39.93 ± 12.14 | 193.14 ± 31.42 | 109.26 ± 17.70 | 84.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, H.-C.; Yang, C.-Y. Assessment of Ultrasonic Stress on Survival and β-Glucosidase Activity of Encapsulated Lactiplantibacillus plantarum BCRC 10357 in Fermentation of Black Soymilk. Foods 2022, 11, 1234. https://doi.org/10.3390/foods11091234
Tseng H-C, Yang C-Y. Assessment of Ultrasonic Stress on Survival and β-Glucosidase Activity of Encapsulated Lactiplantibacillus plantarum BCRC 10357 in Fermentation of Black Soymilk. Foods. 2022; 11(9):1234. https://doi.org/10.3390/foods11091234
Chicago/Turabian StyleTseng, Hung-Chih, and Chun-Yao Yang. 2022. "Assessment of Ultrasonic Stress on Survival and β-Glucosidase Activity of Encapsulated Lactiplantibacillus plantarum BCRC 10357 in Fermentation of Black Soymilk" Foods 11, no. 9: 1234. https://doi.org/10.3390/foods11091234