Myostatin Alteration in Pigs Enhances the Deposition of Long-Chain Unsaturated Fatty Acids in Subcutaneous Fat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sampling
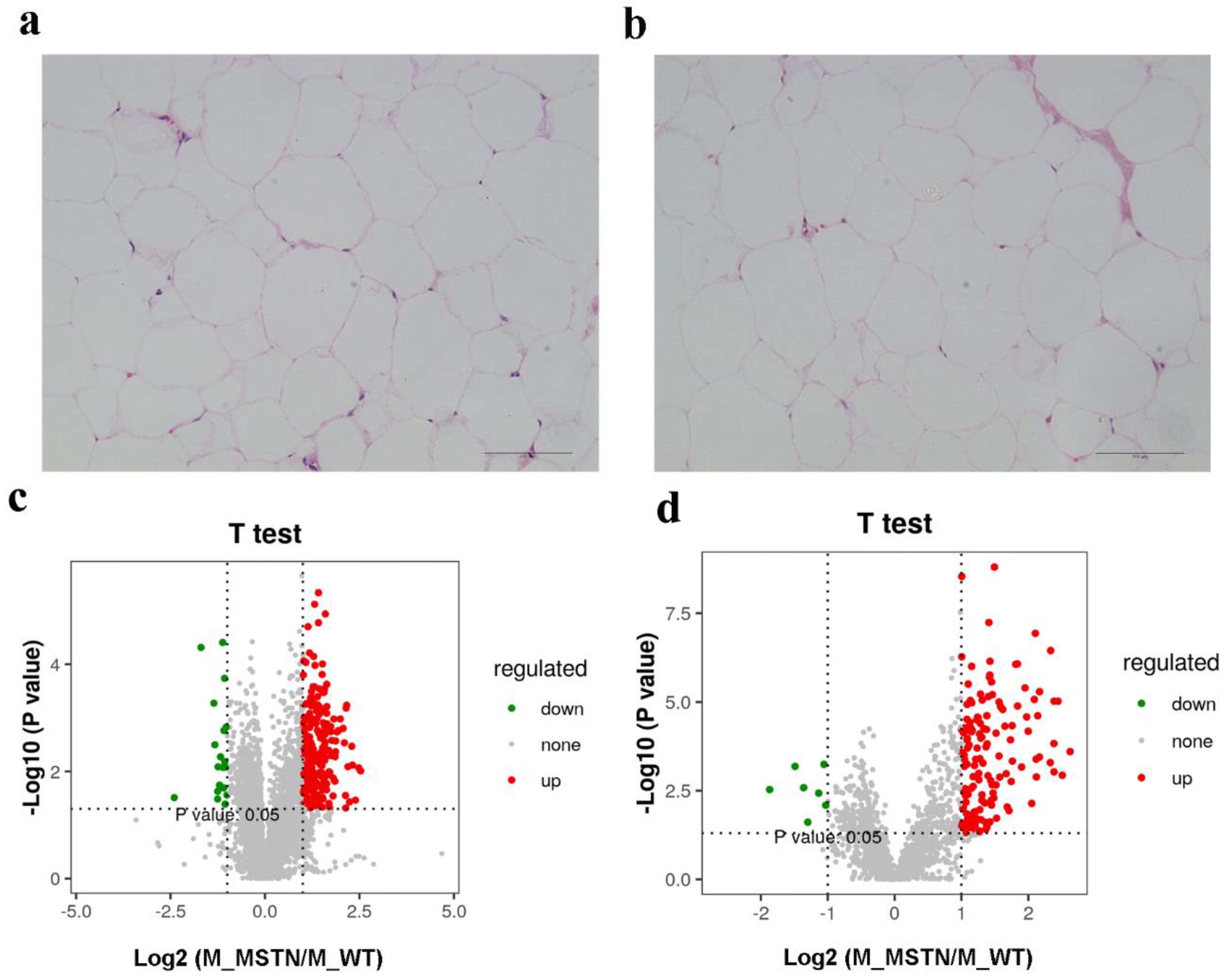
2.3. Histologic Analysis
2.4. Lipid Extraction
2.5. Lipidomics Study
2.6. Data Analysis
2.7. Pathway Analysis
3. Results
3.1. Basic Information on Experimental Pigs and LC-MS-Based Untargeted Lipidomics Results
3.2. PCA and PLS-DA Analysis of Lipid Metabolism Profiles
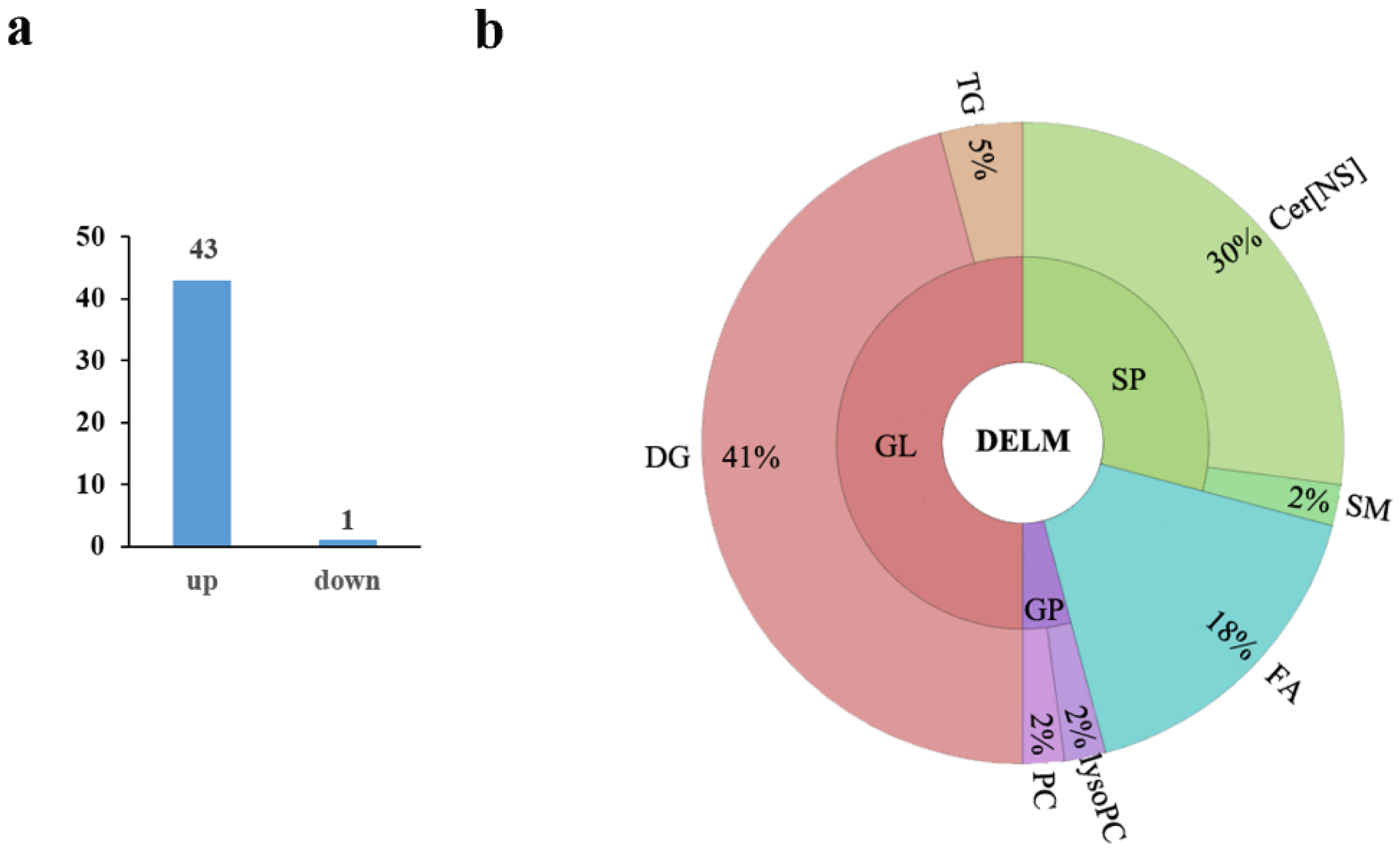
3.3. Screening the Differential Lipid Metabolites
3.4. Pathway Analysis of the Differentially Expressed Lipids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McPherron, A.C.; Lawler, A.M.; Lee, S.J. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997, 387, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wall, R.J.; Yang, J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem. Biophys. Res. Commun. 2005, 337, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ouyang, H.; Xie, Z.; Yao, C.; Guo, N.; Li, M.; Jiao, H.; Pang, D. Efficient Generation of Myostatin Mutations in Pigs Using the CRISPR/Cas9 System. Sci. Rep. 2015, 5, 16623. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Tang, M.; Yang, J.; Wang, Q.; Cai, C.; Jiang, S.; Li, H.; Jiang, K.; Gao, P.; Ma, D.; et al. Targeted mutations in myostatin by zinc-finger nucleases result in double-muscled phenotype in Meishan pigs. Sci. Rep. 2015, 5, 14435. [Google Scholar] [CrossRef]
- Rao, S.; Fujimura, T.; Matsunari, H.; Sakuma, T.; Nakano, K.; Watanabe, M.; Asano, Y.; Kitagawa, E.; Yamamoto, T.; Nagashima, H. Efficient modification of the myostatin gene in porcine somatic cells and generation of knockout piglets. Mol. Reprod. Dev. 2016, 83, 61–70. [Google Scholar] [CrossRef]
- Wang, K.; Tang, X.; Xie, Z.; Zou, X.; Li, M.; Yuan, H.; Guo, N.; Ouyang, H.; Jiao, H.; Pang, D. CRISPR/Cas9-mediated knockout of myostatin in Chinese indigenous Erhualian pigs. Transgenic Res. 2017, 26, 799–805. [Google Scholar] [CrossRef]
- Ren, H.; Xiao, W.; Qin, X.; Cai, G.; Chen, H.; Hua, Z.; Cheng, C.; Li, X.; Hua, W.; Xiao, H.; et al. Myostatin regulates fatty acid desaturation and fat deposition through MEF2C/miR222/SCD5 cascade in pigs. Commun. Biol. 2020, 3, 612. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Z.; Xu, K.; Wu, T.; Ruan, J.; Zheng, X.; Bao, S.; Mu, Y.; Sonstegard, T.; Li, K. Long-term, multidomain analyses to identify the breed and allelic effects in MSTN-edited pigs to overcome lameness and sustainably improve nutritional meat production. Sci. China Life Sci. 2022, 65, 362–375. [Google Scholar] [CrossRef]
- Mosler, S.; Relizani, K.; Mouisel, E.; Amthor, H.; Diel, P. Combinatory effects of siRNA-induced myostatin inhibition and exercise on skeletal muscle homeostasis and body composition. Physiol. Rep. 2014, 2, e00262. [Google Scholar] [CrossRef]
- Mcpherron, A.C.; Guo, T.; Wang, Q.; Portas, J. Soluble activin receptor type IIB treatment does not cause fat loss in mice with diet-induced obesity. Diabetes Obes. Metab. 2012, 14, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Liang, L.; Dean, R.G.; Hausman, D.B.; Hartzell, D.L.; Baile, C.A. Inhibition of preadipocyte differentiation by myostatin treatment in 3T3-L1 cultures. Biochem. Biophys. Res. Commun. 2001, 281, 902–906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.J.; Pan, H.; Zhang, X.Z.; Li, N.S.; Wang, L.J.; Yang, H.B.; Gong, F.Y. The effect of myostatin on proliferation and lipid accumulation in 3T3-L1 preadipocytes. J. Mol. Endocrinol. 2015, 54, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Hirai, S.; Matsumoto, H.; Hino, N.; Kawachi, H.; Matsui, T.; Yano, H. Myostatin inhibits differentiation of bovine preadipocyte. Domest. Anim. Endocrinol. 2007, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.X.; Dodson, M.V.; Jiang, Z.H.; Yu, S.G.; Chu, W.W.; Chen, J. Myostatin inhibits porcine intramuscular preadipocyte differentiation in vitro. Domest. Anim. Endocrinol. 2016, 55, 25–31. [Google Scholar] [CrossRef]
- Feldman, B.J.; Streeper, R.S.; Farese, R.V., Jr.; Yamamoto, K.R. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc. Natl. Acad. Sci. USA 2006, 103, 15675–15680. [Google Scholar] [CrossRef]
- Matsuo, N. Nutritional characteristics and health benefits of diacylglycerol in foods. Food Sci. Technol. Res. 2004, 10, 103–110. [Google Scholar] [CrossRef]
- Wang, B.W.; Zhang, M.G.; Ge, W.H.; He, K.L.; Cheng, F.S. Microencapsulated duck oil diacylglycerol: Preparation and application as anti-obesity agent. LWT-Food Sci. Technol. 2019, 101, 646–652. [Google Scholar] [CrossRef]
- Fischer, A.H.; Jacobson, K.A.; Rose, J.; Zeller, R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008, 2008, pdb-prot4986. [Google Scholar] [CrossRef]
- Blank, M.M.; Wentzensen, N.; Murphy, M.A.; Hollenbeck, A.; Park, Y. Dietary fat intake and risk of ovarian cancer in the NIH-AARP Diet and Health Study. Br. J. Cancer 2012, 106, 596–602. [Google Scholar] [CrossRef]
- Macke, R.A.; Nason, K.S.; Mukaisho, K.; Hattori, T.; Fujimura, T.; Sasaki, S.; Oyama, K.; Miyashita, T.; Ohta, T.; Miwa, K.; et al. Barrett’s esophagus and animal models. Ann. N. Y. Acad. Sci. 2011, 1232, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Bowers, K.; Tobias, D.K.; Yeung, E.; Hu, F.B.; Zhang, C. A prospective study of prepregnancy dietary fat intake and risk of gestational diabetes. Am. J. Clin. Nutr. 2012, 95, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Fan, Z.; Song, Y.; Chen, C.; Mu, Y.; Li, B.; Feng, Z.; Li, H.; Li, K. Viscera Characteristics of MSTN-Edited Heterozygous Pigs. Front. Genet. 2022, 13, 764965. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Zhang, F.; Wen, J.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Jiang, S. The function of myostatin in the regulation of fat mass in mammals. Nutr. Metab. 2017, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.F.; Li, N.; Rodgers, B.D. Myostatin regulates tissue potency and cardiac calcium-handling proteins. Endocrinology 2014, 155, 1771–1785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jackson, M.F.; Luong, D.; Vang, D.D.; Garikipati, D.K.; Stanton, J.B.; Nelson, O.L.; Rodgers, B.D. The aging myostatin null phenotype: Reduced adiposity, cardiac hypertrophy, enhanced cardiac stress response, and sexual dimorphism. J. Endocrinol. 2012, 213, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, R.; Cavanaugh, K.L.; Blot, W.J.; Ikizler, T.A.; Lipworth, L.; Kabagambe, E.K. Dietary polyunsaturated fatty acids and incidence of end-stage renal disease in the Southern Community Cohort Study. BMC Nephrol. 2016, 17, 152. [Google Scholar] [CrossRef]
- Sokoła-Wysoczańska, E.; Wysoczański, T.; Wagner, J.; Czyż, K.; Bodkowski, R.; Lochyński, S.; Patkowska-Sokoła, B. Polyunsaturated Fatty Acids and Their Potential Therapeutic Role in Cardiovascular System Disorders—A Review. Nutrients 2018, 10, 1561. [Google Scholar] [CrossRef]
- Kelly, O.J.; Gilman, J.C.; Kim, Y.; Ilich, J.Z. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr. Res. 2013, 33, 521–533. [Google Scholar] [CrossRef]
- Escrich, E.; Moral, R.; Grau, L.; Costa, I.; Solanas, M. Molecular mechanisms of the effects of olive oil and other dietary lipids on cancer. Mol. Nutr. Food Res. 2007, 51, 1279–1292. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vazquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Kumar, S.A.; Iyer, A.; Waanders, J.; Ward, L.C.; Brown, L. Responses to oleic, linoleic and alpha-linolenic acids in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Yang, X.; Koltes, J.E.; Park, C.A.; Chen, D.; Reecy, J.M. Gene co-expression network analysis provides novel insights into myostatin regulation at three different mouse developmental timepoints. PLoS ONE 2015, 10, e0117607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xin, X.B.; Yang, S.P.; Li, X.; Liu, X.F.; Zhang, L.L.; Ding, X.B.; Zhang, S.; Li, G.P.; Guo, H. Proteomics insights into the effects of MSTN on muscle glucose and lipid metabolism in genetically edited cattle. Gen. Comp. Endocrinol. 2020, 291, 113237. [Google Scholar] [CrossRef] [PubMed]
- Nagao, T.; Watanabe, H.; Goto, N.; Onizawa, K.; Taguchi, H.; Matsuo, N.; Yasukawa, T.; Tsushima, R.; Shimasaki, H.; Itakura, H. Dietary diacylglycerol suppresses accumulation of body fat compared to triacylglycerol in men in a double-blind controlled trial. J. Nutr. 2000, 130, 792–797. [Google Scholar] [CrossRef]
- Taguchi, H.; Watanabe, H.; Onizawa, K.; Nagao, T.; Gotoh, N.; Yasukawa, T.; Tsushima, R.; Shimasaki, H.; Itakura, H. Double-blind controlled study on the effects of dietary diacylglycerol on postprandial serum and chylomicron triacylglycerol responses in healthy humans. J. Am. Coll. Nutr. 2000, 19, 789–796. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, J.W.; Park, J.H.; Kim, I.H. Efficacy of 1,3-diacylglycerol as a fat emulsifier in low-density diet for broilers. Poult. Sci. 2017, 96, 1672–1678. [Google Scholar] [CrossRef]
- Karasawa, K.; Tanigawa, K.; Harada, A.; Yamashita, A. Transcriptional Regulation of Acyl-CoA:Glycerol-sn-3-Phosphate Acyltransferases. Int. J. Mol. Sci. 2019, 20, 964. [Google Scholar] [CrossRef]
- Chu, W.; Wei, W.; Han, H.; Gao, Y.; Liu, K.; Tian, Y.; Jiang, Z.; Zhang, L.; Chen, J. Muscle-specific downregulation of GR levels inhibits adipogenesis in porcine intramuscular adipocyte tissue. Sci. Rep. 2017, 7, 510. [Google Scholar] [CrossRef]
- Xuan, M.F.; Luo, Z.B.; Han, S.Z.; Li, Z.Y.; Gao, K.; Liu, X.Y.; Chang, S.Y.; Jin, Z.Y.; Choe, H.M.; Paek, H.J.; et al. Skeletal muscle-secreted myokine interleukin-6 induces white adipose tissue conversion into beige adipose tissue in myostatin gene knockout pigs. Domest. Anim. Endocrinol. 2022, 78, 106679. [Google Scholar] [CrossRef] [PubMed]
- Catalán, V.; Frühbeck, G.; Gómez-Ambrosi, J. Inflammatory and Oxidative Stress Markers in Skeletal Muscle of Obese Subjects. In Obesity; del Moral, A.M., Aguilera García, C.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 163–189. [Google Scholar]
- Kim, D.S.; Kim, S.Y.; Chung, J.H.; Kim, K.H.; Eun, H.C.; Park, K.C. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell. Signal. 2002, 14, 779–785. [Google Scholar] [CrossRef]
- Meckfessel, M.H.; Brandt, S. The structure, function, and importance of ceramides in skin and their use as therapeutic agents in skin-care products. J. Am. Acad. Dermatol. 2014, 71, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Shimamoto, T.; Nagano, H.; Tsuruno, M.; Okuhara, H.; Hatanaka, H.; Tojo, H.; Kodama, Y.; Funato, K. Producing human ceramide-NS by metabolic engineering using yeast Saccharomyces cerevisiae. Sci. Rep. 2015, 5, 16319. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.R.; Wertz, P.W. Structures of the ceramides from porcine palatal stratum corneum. Lipids 2009, 44, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Lee, B.M. Safety and risk assessment of ceramide 3 in cosmetic products. Food Chem. Toxicol. 2015, 84, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, B.; Zhou, S.; Zhu, H.; Qu, L.; Wang, X.; Chen, Y. RNA-seq reveals transcriptome changes in goats following myostatin gene knockout. PLoS ONE 2017, 12, e0187966. [Google Scholar] [CrossRef]
- Pei, Y.; Chen, C.; Mu, Y.; Yang, Y.; Feng, Z.; Li, B.; Li, H.; Li, K. Integrated Microbiome and Metabolome Analysis Reveals a Positive Change in the Intestinal Environment of Myostatin Edited Large White Pigs. Front. Microbiol. 2021, 12, 628685. [Google Scholar] [CrossRef]




| Mode | All Feature | All Annotated | MS2 | HMDB | KEGG |
|---|---|---|---|---|---|
| pos | 5259 | 2959 | 410 | 2551 | 1081 |
| neg | 1756 | 844 | 44 | 679 | 375 |
| MS2 Metabolites | WT | MSTN | FC (MSTN/WT) | t.Test_p.Value | VIP | Regulated | MS2class |
|---|---|---|---|---|---|---|---|
| FA 18:1 | 3546.793 | 9773.901481 | 2.7557 | <0.0001 | 2.0193 | up | FA |
| FA 18:2 | 143,397.6 | 391,532.6511 | 2.7304 | <0.0001 | 2.0036 | up | FA |
| FA 18:3 | 6932.366 | 17,210.03558 | 2.4826 | <0.0001 | 1.9423 | up | FA |
| FA 20:2 | 8642.403 | 22,567.74914 | 2.6113 | 0.0002 | 1.8699 | up | FA |
| FA 20:3 | 6461.461 | 19,078.51236 | 2.9527 | 0.0003 | 1.9203 | up | FA |
| FA 20:4 | 19,995.97 | 86,817.52091 | 4.3418 | 0.0004 | 2.1756 | up | FA |
| FA 22:4 | 5978.082 | 34,021.06063 | 5.6910 | 0.0012 | 2.2632 | up | FA |
| FA 22:5 | 4734.286 | 18,623.7508 | 3.9338 | <0.0001 | 2.2632 | up | FA |
| MS2 Metabolites | WT | MSTN | FC (MSTN/WT) | t.Test_p.Value | VIP | Regulated | MS2class |
|---|---|---|---|---|---|---|---|
| DG 30:2; DG(12:0/18:2) | 8560.459 | 20,125.29867 | 2.3510 | 0.0055 | 2.1145 | up | DG |
| DG 33:0; DG(16:0/17:0) | 5994.942 | 12,379.94687 | 2.0651 | 0.0033 | 1.8401 | up | DG |
| DG 33:2; DG(15:0/18:2) | 5044.778 | 12,492.03431 | 2.4762 | 0.0054 | 1.9723 | up | DG |
| DG 34:2; DG(16:0/18:2) | 170,198.4 | 355,028.0342 | 2.0860 | 0.0060 | 1.8450 | up | DG |
| DG 34:3; DG(16:1/18:2) | 103,739.4 | 233,561.7196 | 2.2514 | 0.0040 | 1.9747 | up | DG |
| DG 34:4; DG(16:1/18:3) | 6334.194 | 13,508.78926 | 2.1327 | 0.0005 | 2.1073 | up | DG |
| DG 35:0; DG(17:0/18:0) | 9121.664 | 24,772.23257 | 2.7158 | 0.0105 | 2.2335 | up | DG |
| DG 35:1; DG(17:0/18:1) | 14,536.48 | 34,174.0373 | 2.3509 | 0.0003 | 2.1624 | up | DG |
| DG 35:2; DG(17:0/18:2) | 22,347.37 | 49,492.0652 | 2.2147 | 0.0058 | 1.8518 | up | DG |
| DG 35:3; DG(17:1/18:2) | 8888.985 | 18,309.88358 | 2.0598 | 0.0152 | 1.6628 | up | DG |
| DG 36:2; DG(18:1/18:1) | 62,498.74 | 151,512.7417 | 2.4243 | 0.0038 | 2.0144 | up | DG |
| DG 36:3; DG(18:1/18:2) | 34,011.48 | 115,883.6619 | 3.4072 | 0.0026 | 2.3690 | up | DG |
| DG 36:4; DG(18:2/18:2) | 32,631.76 | 1,002,795.866 | 3.8613 | 0.0017 | 2.5261 | up | DG |
| DG 36:5; DG(18:2/18:3) | 32,631.76 | 115,389.4501 | 3.5361 | 0.0010 | 2.5030 | up | DG |
| DG 37:3; DG(19:1/18:2) | 4571.692 | 10,533.324 | 2.3040 | 0.0012 | 2.1446 | up | DG |
| DG 38:4; DG(19:2/19:2) | 18,561.4 | 58,653.825 | 3.1600 | 0.0016 | 2.3852 | up | DG |
| DG 40:4; DG(18:0/22:4) | 5632.562 | 12,793.07064 | 2.2713 | 0.0023 | 2.0061 | up | DG |
| DG 40:5; DG(18:1/22:4) | 10,866.66 | 22,187.87537 | 2.0418 | 0.0111 | 1.9783 | up | DG |
| TG 42:4; TG(12:1/12:1/18:2) | 7388.643 | 15,922.36588 | 2.1550 | 0.0190 | 1.9034 | up | TG |
| TG 50:0; TG(16:0/16:0/18:0) | 349,899.6 | 169,207.3744 | 0.4836 | 0.0084 | 1.9507 | down | TG |
| MS2 Metabolites | WT | MSTN | FC (MSTN/WT) | t.Test_p.Value | VIP | Regulated | MS2class |
|---|---|---|---|---|---|---|---|
| Cer[NS] 32:1; Cer[NS](d18:1/14:0) | 3861.602 | 9693.892561 | 2.5103 | 0.0282 | 2.0953 | up | Cer_NS |
| Cer[NS] 33:1; Cer[NS](d17:1/16:0) | 1068.501 | 2993.02488 | 2.8011 | 0.0044 | 2.1338 | up | Cer_NS |
| Cer[NS] 34:1; Cer[NS](d18:1/16:0) | 31,982.4 | 100,204.3959 | 3.1331 | 0.0117 | 2.3630 | up | Cer_NS |
| Cer[NS] 34:2; Cer[NS](d18:1/16:1) | 4002.504 | 17,169.607 | 4.2897 | 0.0029 | 2.7466 | up | Cer_NS |
| Cer[NS] 35:1; Cer[NS](d18:1/17:0) | 1267.395 | 5045.999952 | 3.9814 | 0.0011 | 2.8133 | up | Cer_NS |
| Cer[NS] 36:1; Cer[NS](d18:1/18:0) | 14,143.47 | 39,845.93159 | 2.8173 | 0.0184 | 2.2561 | up | Cer_NS |
| Cer[NS] 36:2; Cer[NS](d18:2/18:0) | 2028.951 | 6282.032455 | 3.0962 | 0.0170 | 2.0525 | up | Cer_NS |
| Cer[NS] 36:4; Cer[NS](d17:3/19:1) | 57,570.47 | 142,091.5412 | 2.4681 | 0.0200 | 1.9485 | up | Cer_NS |
| Cer[NS] 38:1; Cer[NS](d18:1/20:0) | 14,266.6 | 41,073.31911 | 2.8797 | 0.0186 | 2.1327 | up | Cer_NS |
| Cer[NS] 38:2; Cer[NS](d18:2/20:0) | 1844.387 | 6254.25343 | 3.3910 | 0.0051 | 2.6342 | up | Cer_NS |
| Cer[NS] 42:1; Cer[NS](d18:1/24:0) | 16,619.03 | 43,973.47298 | 2.6460 | 0.0412 | 1.9252 | up | Cer_NS |
| Cer[NS] 42:2; Cer[NS](d18:1/24:1) | 20,262.9 | 48,511.80825 | 2.3941 | 0.0399 | 1.9521 | up | Cer_NS |
| Cer[NS] 42:3; Cer[NS](d18:1/24:2) | 10,174.64 | 26,046.28408 | 2.5599 | 0.0162 | 2.1515 | up | Cer_NS |
| SM 36:1; SM(d14:0/22:1) | 14,790.72 | 30,015.73873 | 2.0294 | 0.0172 | 1.8125 | up | SM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pei, Y.; Song, Y.; Feng, Z.; Li, H.; Mu, Y.; Rehman, S.u.; Liu, Q.; Li, K. Myostatin Alteration in Pigs Enhances the Deposition of Long-Chain Unsaturated Fatty Acids in Subcutaneous Fat. Foods 2022, 11, 1286. https://doi.org/10.3390/foods11091286
Pei Y, Song Y, Feng Z, Li H, Mu Y, Rehman Su, Liu Q, Li K. Myostatin Alteration in Pigs Enhances the Deposition of Long-Chain Unsaturated Fatty Acids in Subcutaneous Fat. Foods. 2022; 11(9):1286. https://doi.org/10.3390/foods11091286
Chicago/Turabian StylePei, Yangli, Yuxin Song, Zheng Feng, Hua Li, Yulian Mu, Saif ur Rehman, Qingyou Liu, and Kui Li. 2022. "Myostatin Alteration in Pigs Enhances the Deposition of Long-Chain Unsaturated Fatty Acids in Subcutaneous Fat" Foods 11, no. 9: 1286. https://doi.org/10.3390/foods11091286
APA StylePei, Y., Song, Y., Feng, Z., Li, H., Mu, Y., Rehman, S. u., Liu, Q., & Li, K. (2022). Myostatin Alteration in Pigs Enhances the Deposition of Long-Chain Unsaturated Fatty Acids in Subcutaneous Fat. Foods, 11(9), 1286. https://doi.org/10.3390/foods11091286









