Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Cultivation of Carrot
2.3. Analysis of Carotenoids
2.4. Analysis of Sugar Content and Total Sweetness Index (TSI)
2.5. Analysis of Ascorbic Acid
2.6. Analysis of Vitamin E and Phytosterols
2.7. Analysis of Fatty Acid Composition
2.8. Measurement of Total Phenol, Total Flavonoid, and Total Anthocyanin Content
2.9. Measurement of Antioxidant Activities
2.10. Statistical Analyses
3. Results
3.1. Variation in Carotenoid Content
3.2. Variation in Free Sugar Content and Total Sweetness Index (TSI)
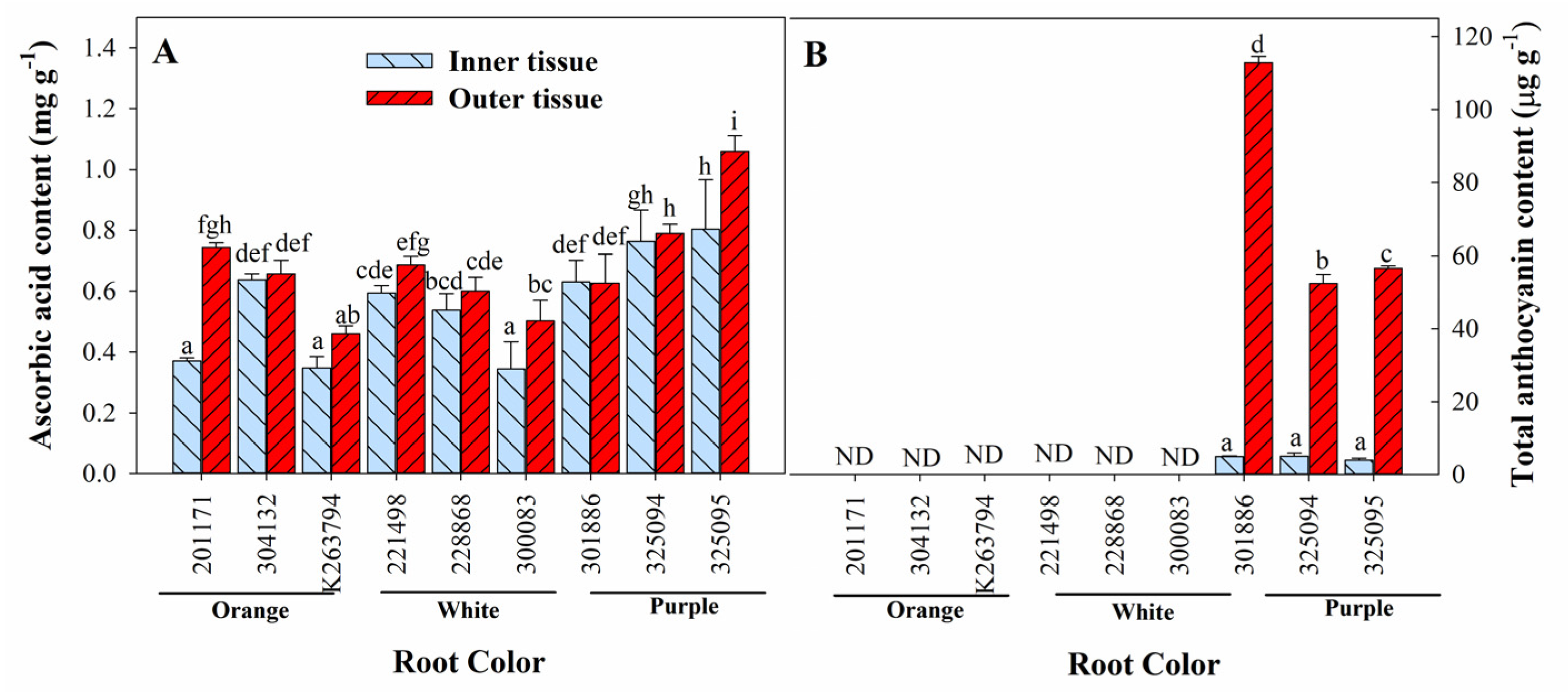
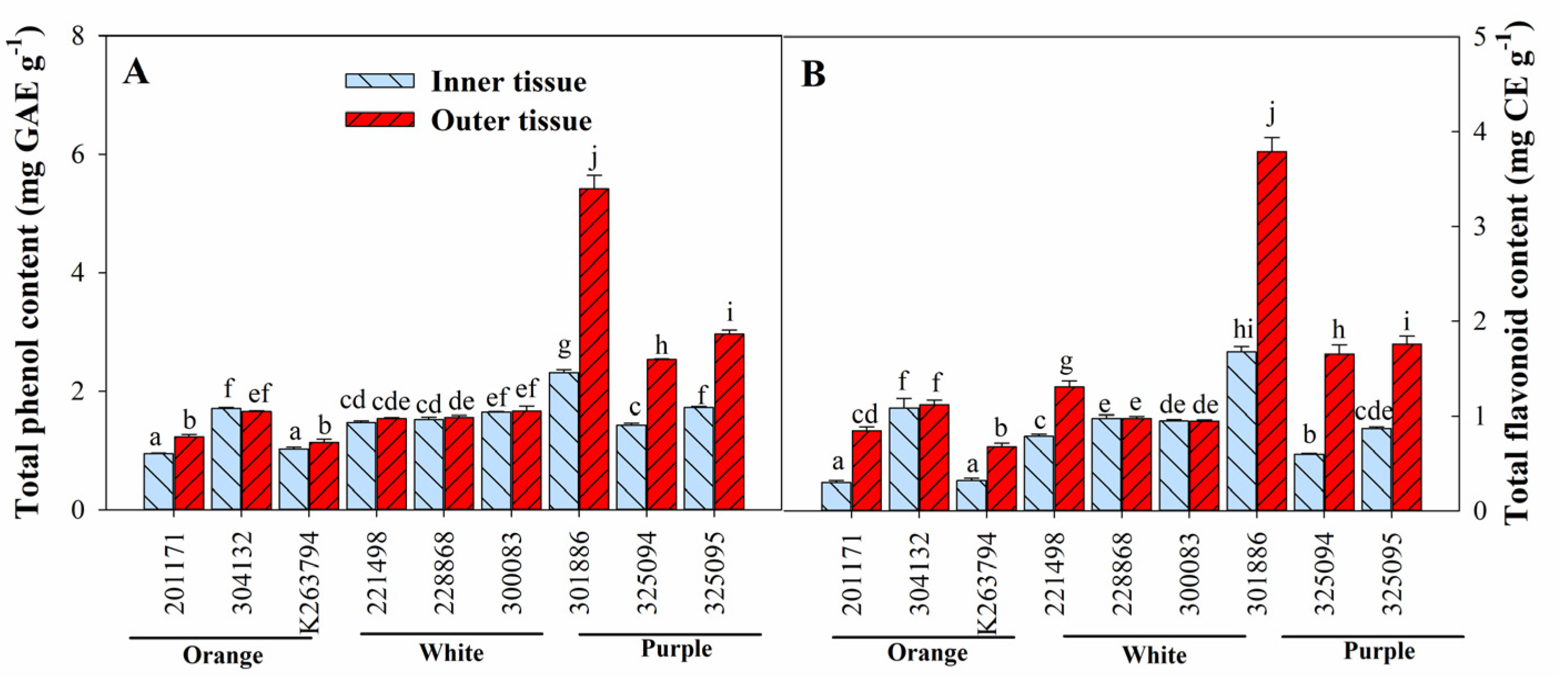
3.3. Variation in Ascorbic Acid, Total Anthocyanin, Total Phenol, and Total Flavonoid Content
3.4. Variation in Vitamin E and Phytosterols Content
3.5. Variation in Fatty Acid Composition
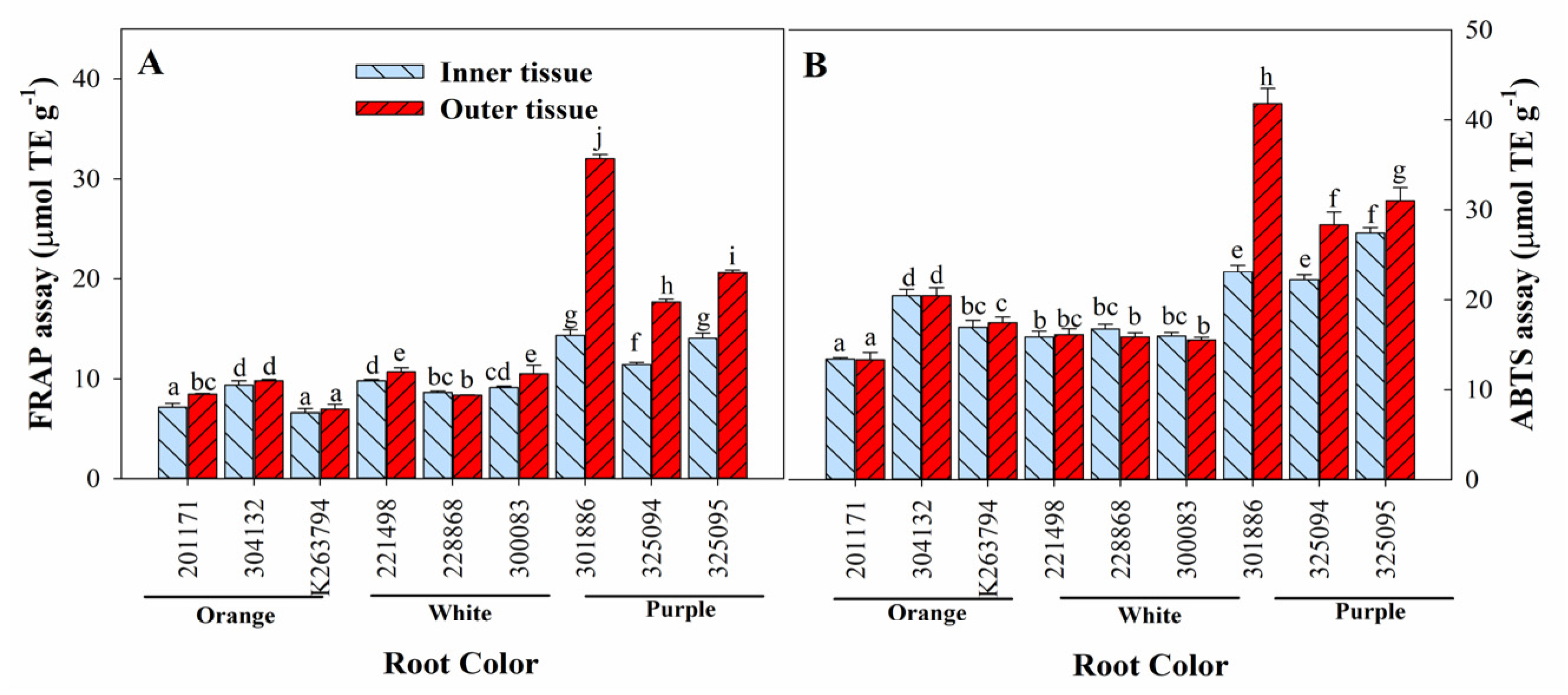
3.6. Variation in Antioxidant Activities
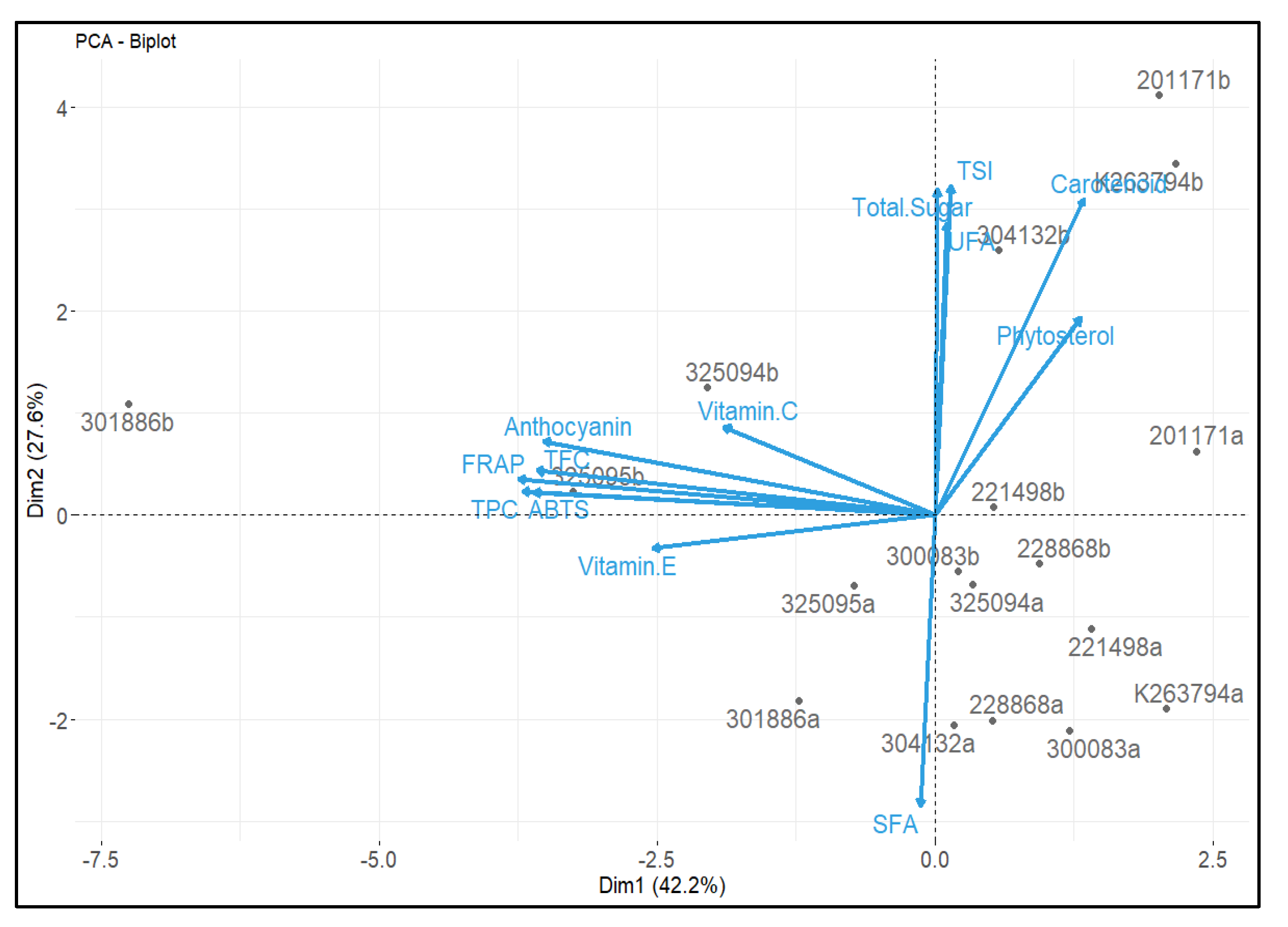
3.7. Correlation Analysis between Chemical Composition and Antioxidant Activities, and Principal Component Analysis (PCA)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO (Food and Agriculture Organization). Agricultural Statistical Database for 2020. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 6 September 2022).
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Arino, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and their health benefits-review article. Foods 2019, 8, 424. [Google Scholar] [CrossRef]
- Kulczynski, B.; Gramza-Michalowska, A.; Kobus-Cisowska, J.; Kmiecik, D. The role of carotenoids in the prevention and treatment of cardiovascular disease-current state of knowledge. J. Funct. Foods 2017, 38, 45–65. [Google Scholar] [CrossRef]
- Smolen, S.; Sady, W. The effect of various nitrogen fertilization and foliar nutrition regimes on the concentrations of sugar, carotenoids and phenolic compounds in carrot (Daucus carota L.). Sci. Hortic. 2009, 120, 315–324. [Google Scholar] [CrossRef]
- Yoo, K.S.; Bang, H.; Pike, L.; Patil, B.S.; Lee, E.J. Comparing carotene, anthocyanins, and terpenoid concentrations in selected carrot lines of different colors. Hortic. Environ. Biotechnol. 2020, 61, 385–393. [Google Scholar] [CrossRef]
- Soltoft, M.; Bysted, A.; Madsen, K.H.; Mark, A.B.; Bugel, S.G.; Nielsen, J.; Knuthsen, P. Effects of organic and conventional growth systems on the content of carotenoids in carrot roots, and on intake and plasma status of carotenoids in humans. J. Sci. Food Agric. 2011, 91, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.R.; Rhee, J.; Choi, C.S.; Jo, J.S.; Shin, Y.K.; Song, J.W.; Kim, S.-H.; Lee, J.G. Morphological and biochemical variation in carrot genetic resources grown under open field conditions: The selection of functional genotypes for a breeding program. Agronomy 2022, 12, 553. [Google Scholar] [CrossRef]
- Jourdan, M.; Gagne, S.; Dubois-Laurent, C.; Maghraoui, M.; Huet, S.; Suel, A.; Hamama, L.; Briard, M.; Peltier, D.; Geoffriau, E. Carotenoid content and root color of cultivated carrot: A candidate-gene association study using an original broad unstructured population. PLoS ONE 2015, 10, e0116674. [Google Scholar] [CrossRef][Green Version]
- Britton, G. Carotenoid research: History and new perspectives for chemistry in biological systems. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158699. [Google Scholar] [CrossRef]
- Christensen, K.; Lawler, T. Dietary carotenoids and non-alcoholic fatty liver disease among US adults, NHANES 2003(−) 2014. Nutrients 2019, 11, 1101. [Google Scholar] [CrossRef]
- Clugston, R.D. Carotenoids and fatty liver disease: Current knowledge and research gaps. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158597. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Elvira-Torales, L.I.; García-Alonso, J.; Periago-Caston, M.J. Nutritional importance of carotenoids and their effect on liver health: A review. Antioxidants 2019, 8, 229. [Google Scholar] [CrossRef]
- Rowles, J.L., III; Erdman, J.W., Jr. Carotenoids and their role in cancer prevention. BBA-Mol. Cell Biol. Lipids 2020, 1865, 158613. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Yanai, H.; Ito, K.; Tomono, Y.; Koikeda, T.; Tsukahara, H.; Tada, N. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis 2010, 209, 520–523. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Quaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesti-cated carrot (D. carota L. var. sativa). Hortic. Res. 2014, 1, 15. [Google Scholar] [CrossRef]
- Kaloustian, J.; Alhanout, K.; Amiot-Carlin, M.-J.; Lairon, D.; Portugal, H.; Nicolay, A.; Collaboration, T. Effect of water cooking on free phytosterol levels in beans and vegetables. Food Chem. 2008, 107, 1379–1386. [Google Scholar] [CrossRef]
- Auerswald, H.; Schwarz, D.; Kornelson, C.; Krumbein, A.; Bruckner, B. Sensory analysis, sugar and acid content of tomato at different EC values of the nutrient solution. Sci. Hortic. 1999, 82, 227–242. [Google Scholar] [CrossRef]
- VandenLangenberg, K.M.; Bethke, P.C.; Nienhuis, J. Patterns of fructose, glucose, and sucrose accumulation in snap and dry bean (Phaseolus vulgaris) pods. HortScience 2012, 47, 874–878. [Google Scholar] [CrossRef]
- Li, B.W.; Andrews, K.W.; Pehrsson, P.R. Individual sugars, soluble, and insoluble dietary fiber contents of 70 high consumption foods. J. Food Compos. Anal. 2002, 15, 715–723. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morpho-biometrical, nutritional and phytochemical characterization of carrot landraces from Puglia region (Southern Italy). Sustainability 2021, 13, 3940. [Google Scholar] [CrossRef]
- Tsukakoshi, Y.; Naito, S.; Ishida, N.; Yasui, A. Variation in moisture, total sugar, and carotene content of Japanese carrots: Use in sample size determination. J. Food Compos. Anal. 2009, 22, 373–380. [Google Scholar] [CrossRef]
- Lin, B.-W.; Gong, C.-C.; Song, H.-F.; Cui, Y.-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br. J. Pharmacol. 2017, 174, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Baranski, R.; Allender, C.; Klimek-Chodacka, M. Towards better testing and more nutritious carrots: Carotenoid and sugar content variation in carrot genetic resources. Food Res. Int. 2012, 47, 182–187. [Google Scholar] [CrossRef]
- Bolton, A.; Simon, P. Variation for salinity tolerance during seed germination in diverse carrot [Daucus carota (L.)] germplasm. HortScience 2019, 54, 38–44. [Google Scholar] [CrossRef]
- Leja, M.; Kaminska, I.; Kramer, M.; Maksylewicz-Kaul, A.; Kammerer, D.; Carle, R.; Baranski, R. The content of phenolic compounds and radical scavenging activity varies with carrot origin and root color. Plant Foods Hum. Nutr. 2013, 68, 163–170. [Google Scholar] [CrossRef]
- Stinco, C.M.; Szczepanska, J.; Marszalek, K.; Pinto, C.A.; Inacio, R.S.; Mapelli-Brahm, P.; Barba, F.J.; Lorenzo, J.M.; Saraiva, J.A.; Meléndez-Martínez, A.J. Effect of high-pressure processing on carotenoids profile, colour, microbial and enzymatic stability of cloudy carrot juice. Food Chem. 2019, 299, 125112. [Google Scholar] [CrossRef]
- Magwaza, L.S.; Opara, U.L. Analytical methods for determination of sugars and sweetness of horticultural crops-A review. Sci. Hortic. 2015, 184, 179–192. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Lee, J.G. Ripening-dependent changes in antioxidants, color attributes, and antioxidant activities of seven tomato (Solanum lycopersisum L.) cultivars. J. Anal. Method Chem. 2016, 2016, 5498618. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jung, B.-D.; Baek, H.Y.; Lee, Y.S. Ripening-dependent changes in phytonutrients and antioxidant activity of red pepper (Capsicum annuum L.) fruits cultivated under open field conditions. HortScience 2013, 48, 1275–1283. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Cho, M.-C.; Lee, J.G. Genotypic variation in carotenoid, ascorbic acid, total phenolic, and flavonoid contents, and antioxidant activity in selected tomato breeding lines. Hortic. Environ. Biotechnol. 2016, 57, 440452. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Bashyal, U.; Lee, Y.-S. Variation in proximate nutrients, phytochemicals, and antioxidant activity of field-cultivated red pepper fruits at different harvest times. Hortic. Environ. Biotechnol. 2016, 57, 493–503. [Google Scholar] [CrossRef]
- Lazcano, C.A.; Yoo, K.S.; Pike, L.M. A method for measuring anthocyanins after removing carotenes in purple colored carrots. Sci. Horic. 2001, 90, 321–324. [Google Scholar] [CrossRef]
- Perrin, F.; Hartmann, L.; Dubois-Laurent, C.; Welsch, R.; Huet, S.; Hamama, L.; Briard, M.; Peltier, D.; Gange, S.; Geoffriau, E. Carotenoid gene expression explains the difference of carotenoid accumulation in carrot root tissues. Planta 2017, 245, 737–747. [Google Scholar] [CrossRef]
- Iorizzo, M.; Ellison, S.; Senalik, D.; Zeng, P.; Satapoomin, P.; Huang, J.; Bowman, M.; Iovene, M.; Sanseverino, W.; Cavagnaro, P.; et al. A high-quality carrot genome assembly provides new insights into carotenoid accumulation and asterid genome evolution. Nat. Genet. 2016, 48, 657–666. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Koch, T.C.; Goldman, I.L. Relationship of carotenoids and tocopherols in a sample of carrto root-color accessions and carrot germplasm carrying Rp and rp alleles. J. Agric. Food Chem. 2005, 53, 325–331. [Google Scholar] [CrossRef]
- Bufler, G. Accumulation and degradation of starch in carrot roots. Sci. Hortic. 2013, 150, 251–258. [Google Scholar] [CrossRef]
- Clausen, M.R.; Edelenbos, M.; Bertram, H.C. Mapping the variation of the carrot metabolome using 1H NMR spectroscopy and consensus PCA. J. Agric. Food Chem. 2014, 62, 4392–4398. [Google Scholar] [CrossRef]
- Yusuf, E.; Tkacz, K.; Turkiewics, I.P.; Wojdylo, A.; Nowicka, P. Analysis of chemical compounds’ content in different varieties of carrots, including qualification and quantification of sugars, organic acids, minerals, and bioactive compounds by UPLC. Eur. Food Res. Technol. 2021, 247, 3053–3062. [Google Scholar] [CrossRef]
- Bannoud, F.; Carvajal, S.; Ellison, S.; Senalik, D.; Gomez Talquenca, S.; Iorizzo, M.; Simon, P.W.; Cavagnaro, P.F. Genetic and transcription profile analysis of tissue-specific anthocyanin pigmentation in carrot root phloem. Genes 2021, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Munne-Bosch, S.; Falk, J. New insights into the function of tocopherols in plants. Planta 2004, 218, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Ombodi, A.; Daood, H.G.; Helyes, L. Carotenoid and tocopherol composition of an orange-colored carrot as affected by water supply. HortScience 2014, 49, 729–733. [Google Scholar] [CrossRef]
- DellaPenna, D.; Mene-Saffranne, L. Biosynthesis of vitamins in plants part B; vitamin E. Adv. Botanical Res. 2011, 59, 179–227. [Google Scholar]
- Cedo, L.; Farras, M.; Lee-Rueckert, M.; Escola-Gil, J.C. Molecular insights into the mechanisms underlying the cholesterol-lowering effects of phytosterols. Curr. Med. Chem. 2019, 26, 6704–6723. [Google Scholar] [CrossRef]
- Li, X.; Xin, Y.; Mo, Y.; Marozik, P.; He, T.; Guo, H. The bioavailability and biological activities of phytosterols as modulators of cholesterol metabolism. Molecules 2022, 27, 523. [Google Scholar] [CrossRef]
- Marangoni, F.; Poli, A. Phytosterols and cardiovascular health. Pharmacol. Res. 2010, 61, 193–199. [Google Scholar] [CrossRef]
- Lesma, G.; Luraghi, A.; Bavaro, T.; Bortolozzi, R.; Rainoldi, G.; Roda, G.; Viola, G.; Ubiali, D.; Silvani, A. Phytosterol and γ-oryzanol conjugates: Synthesis and evaluation of their antioxidant, antiproliferative, and anticholesterol activities. J. Nat. Prod. 2018, 81, 2212–2221. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padro, T. Phytosterols and inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Gao, F.; Yang, S.; Birch, J. Physicochemical characteristics, fatty acid positional distribution and triglyceride composition of oil extracted from carrot seeds using supercritical CO2. J. Food Comp. Anal. 2016, 45, 26–33. [Google Scholar] [CrossRef]
- Willett, W.C. The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J. Cardiovasc. Med. 2007, 8, S42–S45. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Cho, M.C.; Yang, E.Y.; Lee, J.G. Response to salt stress in lettuce: Changes in chlorophyll fluorescence parameters, phytochemical contents, and antioxidant activities. Agronomy 2020, 10, 1627. [Google Scholar] [CrossRef]
| Root Color | IT Number | Tissue | Lutein | α-Carotene | β-Carotene | Total Carotenoid |
|---|---|---|---|---|---|---|
| Orange | 201171 | Inner | 0.24 ± 0.00 fg | 0.16 ± 0.00 b | 0.30 ± 0.01 e | 0.69 ± 0.01 i |
| Outer | 0.26 ± 0.01 h | 0.48 ± 0.03 e | 0.91 ± 0.01 g | 1.64 ± 0.02 l | ||
| 304132 | Inner | 0.21 ± 0.01 e | 0.08 ± 0.00 a | 0.25 ± 0.01 d | 0.54 ± 0.02 h | |
| Outer | 0.30 ± 0.01 i | 0.33 ± 0.01 d | 0.94 ± 0.02 h | 1.57 ± 0.03 k | ||
| K263794 | Inner | 0.14 ± 0.01 b | 0.23 ± 0.01 c | 0.36 ± 0.02 f | 0.72 ± 0.04 j | |
| Outer | 0.12 ± 0.00 a | 0.65 ± 0.01 f | 0.94 ± 0.02 h | 1.70 ± 0.04 m | ||
| White | 221498 | Inner | 0.20 ± 0.01 d | ND | ND | 0.20 ± 0.01 b |
| Outer | 0.25 ± 0.01 gh | ND | ND | 0.25 ± 0.01 c | ||
| 228868 | Inner | 0.22 ± 0.01 e | ND | ND | 0.22 ± 0.01 b | |
| Outer | 0.31 ± 0.01 i | ND | ND | 0.31 ± 0.01 de | ||
| 300083 | Inner | 0.16 ± 0.00 c | ND | ND | 0.16 ± 0.00 a | |
| Outer | 0.26 ± 0.01 h | ND | ND | 0.26 ± 0.01 c | ||
| Purple | 301886 | Inner | 0.23 ± 0.01 f | ND | 0.03 ± 0.00 a | 0.26 ± 0.01 c |
| Outer | 0.31 ± 0.00 i | ND | 0.04 ± 0.00 a | 0.35 ± 0.00 f | ||
| 325094 | Inner | 0.26 ± 0.02 h | ND | 0.07 ± 0.01 b | 0.33 ± 0.03 ef | |
| Outer | 0.26 ± 0.02 h | ND | 0.24 ± 0.02 d | 0.50 ± 0.02 g | ||
| 325095 | Inner | 0.17 ± 0.01 c | ND | 0.04 ± 0.00 a | 0.21 ± 0.00 b | |
| Outer | 0.17 ± 0.02 c | ND | 0.13 ± 0.00 c | 0.29 ± 0.02 d |
| Root Color | IT Number | Tissue | Fructose | Glucose | Sucrose | Total Sugar | TSI |
|---|---|---|---|---|---|---|---|
| Orange | 201171 | Inner | 86.7 ± 3.1 g | 110.3 ± 8.8 k | 173.7 ± 10.4 de | 370.6 ± 15.4 gh | 387.5 ± 14.3 g |
| Outer | 93.7 ± 4.6 h | 97.3 ± 2.6 j | 186.8 ± 3.8 ef | 377.9 ± 2.5 h | 401.4 ± 4.0 g | ||
| 304132 | Inner | 79.5 ± 4.4 f | 64.6 ± 1.7 gh | 145.2 ± 9.9 ab | 289.2 ± 10.9 bc | 313.4 ± 12.3 cd | |
| Outer | 85.7 ± 5.1 g | 143.0 ± 6.9 l | 155.3 ± 5.0 bc | 383.9 ± 9.8 h | 392.5 ± 10.6 g | ||
| K263794 | Inner | 67.1 ± 2.9 de | 106.7 ± 10.6 k | 166.2 ± 10.7 cd | 340.0 ± 18.6 ef | 347.9 ± 14.7 ef | |
| Outer | 68.8 ± 2.2 e | 83.3 ± 1.6 i | 281.7 ± 5.6 h | 433.8 ± 6.9 i | 448.2 ± 6.7 g | ||
| White | 221498 | Inner | 62.1 ± 5.4 d | 60.7 ± 2.4 fg | 150.3 ± 5.0 abc | 273.0 ± 11.0 b | 289.5 ± 13.0 b |
| Outer | 67.2 ± 1.9 de | 71.5 ± 0.8 h | 192.1 ± 7.7 ef | 330.8 ± 5.3 ef | 347.2 ± 4.6 ef | ||
| 228868 | Inner | 70.4 ± 4.8 e | 93.1 ± 7.1 j | 135.5 ± 9.8 a | 299.0 ± 19.4 a | 311.9 ± 19.5 cd | |
| Outer | 85.3 ± 5.0 g | 99.4 ± 3.5 j | 146.9 ± 7.7 ab | 331.7 ± 14.0 cd | 350.4 ± 15.1 ef | ||
| 300083 | Inner | 36.9 ± 0.7 b | 36.2 ± 1.4 bcd | 178.6 ± 4.2 de | 251.7 ± 6.2 de | 261.4 ± 6.2 a | |
| Outer | 45.0 ± 1.6 c | 49.7 ± 0.6 e | 200.6 ± 5.1 f | 295.3 ± 5.7 fg | 305.9 ± 6.2 bc | ||
| Purple | 301886 | Inner | 50.8 ± 1.2 c | 33.7 ± 1.1 abc | 231.8 ± 3.3 g | 316.3 ± 1.5 ef | 333.6 ± 1.3 de |
| Outer | 48.8 ± 2.9 c | 54.3 ± 2.5 ef | 245.3 ± 5.1 g | 348.5 ± 4.5 gh | 359.9 ± 4.2 f | ||
| 325094 | Inner | 48.3 ± 0.6 c | 40.7 ± 2.2 cd | 246.5 ± 6.2 g | 335.5 ± 7.7 ef | 349.9 ± 7.0 ef | |
| Outer | 51.0 ± 4.6 c | 42.1 ± 2.5 d | 276.7 ± 30.9 h | 369.9 ± 29.7 gh | 385.3 ± 29.7 g | ||
| 325095 | Inner | 26.1 ± 2.5 a | 26.6 ± 1.7 a | 277.6 ± 11.8 h | 330.3 ± 14.9 ef | 336.9 ± 15.9 ef | |
| Outer | 27.2 ± 0.4 a | 29.3 ± 2.4 ab | 291.0 ± 6.9 h | 347.5 ± 9.3 f | 354.1 ± 8.9 ef |
| Root Color | IT Number | Tissue | Vitamin E (µg g−1 DW) | ||||
|---|---|---|---|---|---|---|---|
| γ-T | α-T | γ-T3 | α-T3 | Total | |||
| Orange | 201171 | Inner | ND | 15.99 ± 0.36 a | ND | 6.35 ± 0.05 i | 22.34 ± 0.40 b |
| Outer | ND | 24.22 ± 1.84 b | ND | 4.66 ± 0.05 f | 28.88 ± 1.89 c | ||
| 304132 | Inner | ND | 37.16 ± 1.90 ef | ND | 4.26 ± 0.05 e | 41.41 ± 1.95 f | |
| Outer | ND | 41.06 ± 0.83 gh | ND | 6.25 ± 0.10 i | 47.31 ± 0.93 g | ||
| K263794 | Inner | ND | 16.63 ± 0.96 a | ND | 6.53 ± 0.12 j | 23.16 ± 0.84 b | |
| Outer | ND | 28.74 ± 1.24 cd | ND | 6.82 ± 0.02 k | 35.55 ± 1.23 d | ||
| White | 221498 | Inner | 1.31 ± 0.03 a | 17.89 ± 1.11 a | ND | ND | 19.19 ± 1.08 a |
| Outer | 2.53 ± 0.02 c | 26.65 ± 1.02 c | ND | ND | 29.18 ± 1.04 c | ||
| 228868 | Inner | 5.15 ± 0.05 d | 37.26 ± 0.70 ef | 4.90 ± 0.11 d | 5.18 ± 0.04 g | 52.49 ± 0.82 h | |
| Outer | 1.21 ± 0.03 a | 39.28 ± 1.41 fg | 2.99 ± 0.22 b | 2.95 ± 0.06 c | 46.44 ± 1.17 g | ||
| 300083 | Inner | 1.93 ± 0.06 b | 27.66 ± 2.20 c | 3.33 ± 0.08 c | 2.92 ± 0.07 c | 35.85 ± 2.11 d | |
| Outer | 9.71 ± 0.34 e | 49.83 ± 0.29 j | 2.41 ± 0.04 a | 4.00 ± 0.05 d | 65.95 ± 0.54 j | ||
| Purple | 301886 | Inner | ND | 48.78 ± 2.20 j | ND | 8.36 ± 0.22 l | 57.14 ± 2.42 i |
| Outer | ND | 61.17 ± 0.93 k | ND | 6.34 ± 0.13 i | 67.51 ± 1.06 j | ||
| 325094 | Inner | ND | 30.07 ± 0.62 d | ND | 5.94 ± 0.16 h | 36.01 ± 0.78 d | |
| Outer | ND | 36.46 ± 0.82 e | ND | 2.04 ± 0.09 b | 38.50 ± 0.87e | ||
| 325095 | Inner | ND | 42.21 ± 1.03 h | ND | 4.59 ± 0.04 f | 46.79 ± 1.07 g | |
| Outer | ND | 45.44 ± 0.95 i | ND | 1.81 ± 0.02 a | 47.25 ± 0.96 g | ||
| Root Color | IT Number | Tissue | Phytosterols (µg g−1 DW) | |||
|---|---|---|---|---|---|---|
| β-Sitosterol | Stigmasterol | Campesterol | Total Phytosterol | |||
| Orange | 201171 | Inner | 432.7 ± 7.3 d | 52.8 ± 2.4 a | 44.7 ± 1.4 a | 530.2 ± 3.5 bc |
| Outer | 541.2 ± 5.8 g | 106.4 ± 5.1 h | 59.7 ± 2.7 fg | 707.3 ± 8.3 g | ||
| 304132 | Inner | 373.5 ± 7.1 a | 53.3 ± 2.1 a | 44.3 ± 1.4 a | 471.0 ± 3.7 a | |
| Outer | 410.5 ± 8.2 bc | 78.7 ± 3.0 ef | 60.6 ± 1.9 g | 549.8 ± 13.1 d | ||
| K263794 | Inner | 410.4 ± 5.0 bc | 59.6 ± 0.7 b | 44.0 ± 0.2 a | 513.9 ± 4.2 b | |
| Outer | 538.4 ± 7.3 g | 90.0 ± 1.3 g | 55.3 ± 3.0 de | 683.7 ± 5.5 f | ||
| White | 221498 | Inner | 498.8 ± 8.5 f | 66.8 ± 1.6 cd | 58.7 ± 1.0 efg | 624.2 ± 11.0 e |
| Outer | 413.3 ± 7.5 bc | 55.2 ± 2.8 ab | 45.8 ± 2.1 a | 514.3 ± 12.4 b | ||
| 228868 | Inner | 411.6 ± 6.0 bc | 58.8 ± 3.4 b | 79.6 ± 0.6 j | 550.0 ± 8.8 d | |
| Outer | 504.8 ± 17.7 f | 78.0 ± 2.4 ef | 86.1 ± 1.9 k | 668.9 ± 17.0 f | ||
| 300083 | Inner | 466.8 ± 6.6 e | 89.9 ± 4.6 g | 69.3 ± 1.0 i | 626.1 ± 10.3 e | |
| Outer | 383.9 ± 8.5 a | 70.5 ± 1.3 cd | 65.1 ± 2.7 h | 519.5 ± 12.6 b | ||
| Purple | 301886 | Inner | 401.4 ± 5.9 b | 58.4 ± 2.9 b | 55.8 ± 3.5 de | 515.5 ± 12.4 b |
| Outer | 410.4 ± 7.2 bc | 75.8 ± 2.5 e | 57.2 ± 1.7 ef | 543.4 ± 6.4 cd | ||
| 325094 | Inner | 419.4 ± 9.3 cd | 78.1 ± 2.6 ef | 53.6 ± 2.0 cd | 551.2 ± 9.9 d | |
| Outer | 422.6 ± 7.0 cd | 65.9 ± 3.5 c | 49.7 ± 1.2 b | 538.3 ± 11.6 cd | ||
| 325095 | Inner | 398.7 ± 4.1 b | 71.1 ± 1.9 d | 50.6 ± 0.6 bc | 520.4 ± 5.3 b | |
| Outer | 409.9 ± 9.8 bc | 81.6 ± 2.3 f | 49.1 ± 0.7 b | 540.6 ± 12.9 cd | ||
| Root Color | IT Number | Tissue | Fatty Acid Composition (%) | SFA | UFA | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C12:0 | C14:0 | C15:0 | C16:0 | C17:0 | C18:0 | C20:0 | C22:0 | C16:1 | C18:1 | C18:2 | C18:3 | C20:1 | C22:1 | |||||
| Orange | 201171 | Inner | 0.06 ± 0.00 | 0.21 ± 0.01 | 0.34 ± 0.02 | 21.35 ± 0.23 | 0.32 ± 0.01 | 0.70 ± 0.02 | 0.37 ± 0.02 | 0.71 ± 0.02 | 0.88 ± 0.04 | 1.17 ± 0.02 | 66.60 ± 0.40 | 6.88 ± 0.06 | 0.22 ± 0.00 | 0.19 ± 0.02 | 25.03 ± 0.29 | 75.00 ± 0.30 |
| Outer | 0.06 ± 0.01 | 0.19 ± 0.01 | 0.32 ± 0.01 | 18.57 ± 0.44 | 0.28 ± 0.01 | 0.66 ± 0.02 | 0.47 ± 0.02 | 0.60 ± 0.02 | 1.07 ± 0.03 | 1.89 ± 0.02 | 70.70 ± 0.49 | 4.80 ± 0.03 | 0.18 ± 0.01 | 0.20 ± 0.01 | 23.07 ± 0.45 | 76.93 ± 0.45 | ||
| 304132 | Inner | 0.10 ± 0.02 | 0.27 ± 0.02 | 0.40 ± 0.02 | 22.66 ± 0.13 | 0.55 ± 0.02 | 0.87 ± 0.01 | 0.48 ± 0.01 | 0.69 ± 0.02 | 0.44 ± 0.01 | 0.54 ± 0.01 | 63.49 ± 0.10 | 8.93 ± 0.02 | 0.45 ± 0.01 | 0.13 ± 0.02 | 25.80 ± 0.10 | 74.20 ± 0.10 | |
| Outer | 0.06 ± 0.01 | 0.16 ± 0.01 | 0.33 ± 0.01 | 19.39 ± 0.13 | 0.33 ± 0.01 | 0.84 ± 0.01 | 0.69 ± 0.04 | 0.82 ± 0.02 | 1.06 ± 0.04 | 1.32 ± 0.04 | 69.57 ± 0.02 | 5.16 ± 0.04 | 0.17 ± 0.01 | 0.09 ± 0.01 | 23.50 ± 0.10 | 76.50 ± 0.10 | ||
| K263794 | Inner | 0.08 ± 0.01 | 0.20 ± 0.01 | 0.59 ± 0.01 | 23.60 ± 0.06 | 0.67 ± 0.01 | 0.87 ± 0.03 | 0.50 ± 0.06 | 0.99 ± 0.01 | 0.91 ± 0.01 | 1.11 ± 0.02 | 62.83 ± 0.10 | 6.91 ± 0.01 | 0.55 ± 0.01 | 0.20 ± 0.01 | 28.03 ± 0.06 | 71.97 ± 0.06 | |
| Outer | 0.09 ± 0.01 | 0.19 ± 0.01 | 0.48 ± 0.01 | 20.06 ± 0.19 | 0.46 ± 0.01 | 1.11 ± 0.06 | 0.73 ± 0.01 | 0.85 ± 0.04 | 1.45 ± 0.01 | 1.39 ± 0.02 | 67.35 ± 0.10 | 5.46 ± 0.09 | 0.20 ± 0.01 | 0.18 ± 0.02 | 25.23 ± 0.25 | 74.77 ± 0.25 | ||
| White | 221498 | Inner | 0.07 ± 0.00 | 0.17 ± 0.01 | 0.40 ± 0.02 | 21.78 ± 0.18 | 0.37 ± 0.01 | 0.34 ± 0.03 | 0.36 ± 0.03 | 0.54 ± 0.02 | 0.60 ± 0.06 | 1.97 ± 0.02 | 65.60 ± 0.35 | 7.51 ± 0.08 | 0.22 ± 0.02 | 0.07 ± 0.01 | 25.67 ± 0.25 | 74.33 ± 0.25 |
| Outer | 0.07 ± 0.00 | 0.17 ± 0.01 | 0.32 ± 0.01 | 20.69 ± 0.13 | 0.31 ± 0.03 | 0.51 ± 0.03 | 0.61 ± 0.02 | 0.68 ± 0.01 | 1.04 ± 0.06 | 3.26 ± 0.13 | 66.82 ± 0.37 | 5.19 ± 0.02 | 0.25 ± 0.02 | 0.06 ± 0.01 | 26.37 ± 0.35 | 73.63 ± 0.35 | ||
| 228868 | Inner | 0.07 ± 0.01 | 0.17 ± 0.01 | 0.43 ± 0.02 | 23.50 ± 1.29 | 0.38 ± 0.02 | 0.51 ± 0.04 | 0.39 ± 0.02 | 0.46 ± 0.02 | 0.93 ± 0.02 | 1.09 ± 0.03 | 63.26 ± 1.20 | 8.55 ± 0.10 | 0.18 ± 0.01 | 0.08 ± 0.01 | 27.10 ± 1.30 | 72.90 ± 1.30 | |
| Outer | 0.10 ± 0.02 | 0.24 ± 0.02 | 0.40 ± 0.02 | 22.07 ± 0.92 | 0.39 ± 0.03 | 1.07 ± 0.02 | 0.58 ± 0.02 | 0.65 ± 0.00 | 1.19 ± 0.03 | 1.17 ± 0.02 | 63.16 ± 0.92 | 8.68 ± 0.02 | 0.16 ± 0.02 | 0.15 ± 0.01 | 26.63 ± 0.95 | 73.37 ± 0.95 | ||
| 300083 | Inner | 0.07 ± 0.00 | 0.21 ± 0.01 | 0.40 ± 0.03 | 21.81 ± 1.02 | 0.42 ± 0.03 | 0.64 ± 0.01 | 0.41 ± 0.04 | 0.61 ± 0.03 | 0.79 ± 0.07 | 1.28 ± 0.03 | 64.44 ± 0.95 | 8.58 ± 0.04 | 0.16 ± 0.01 | 0.18 ± 0.02 | 25.63 ± 0.95 | 74.40 ± 1.00 | |
| Outer | 0.06 ± 0.01 | 0.25 ± 0.02 | 0.32 ± 0.03 | 19.61 ± 0.88 | 0.30 ± 0.02 | 0.91 ± 0.02 | 0.53 ± 0.02 | 0.62 ± 0.01 | 1.12 ± 0.02 | 2.54 ± 0.04 | 67.61 ± 0.97 | 5.86 ± 0.11 | 0.20 ± 0.02 | 0.06 ± 0.01 | 25.13 ± 0.85 | 74.87 ± 0.86 | ||
| Purple | 301886 | Inner | 0.06 ± 0.00 | 0.21 ± 0.03 | 0.43 ± 0.01 | 23.65 ± 0.70 | 0.46 ± 0.03 | 0.64 ± 0.02 | 0.41 ± 0.03 | 0.67 ± 0.02 | 0.48 ± 0.02 | 0.89 ± 0.05 | 60.45 ± 0.94 | 10.76 ± 0.21 | 0.75 ± 0.03 | 0.15 ± 0.01 | 26.80 ± 0.80 | 73.20 ± 0.80 |
| Outer | 0.07 ± 0.01 | 0.19 ± 0.01 | 0.33 ± 0.01 | 20.98 ± 0.26 | 0.33 ± 0.03 | 0.64 ± 0.02 | 0.56 ± 0.11 | 0.84 ± 0.03 | 0.93 ± 0.02 | 1.88 ± 0.08 | 62.25 ± 0.24 | 10.74 ± 0.25 | 0.18 ± 0.01 | 0.09 ± 0.01 | 25.33 ± 0.15 | 74.67 ± 0.14 | ||
| 325094 | Inner | 0.09 ± 0.00 | 0.15 ± 0.01 | 0.36 ± 0.03 | 22.25 ± 0.79 | 0.50 ± 0.01 | 0.79 ± 0.02 | 0.43 ± 0.02 | 0.85 ± 0.02 | 0.65 ± 0.02 | 1.08 ± 0.03 | 64.95 ± 0.95 | 7.39 ± 0.17 | 0.33 ± 0.02 | 0.17 ± 0.01 | 25.87 ± 0.75 | 74.13 ± 0.74 | |
| Outer | 0.08 ± 0.00 | 0.13 ± 0.01 | 0.29 ± 0.01 | 19.68 ± 0.68 | 0.36 ± 0.03 | 1.47 ± 0.06 | 0.84 ± 0.05 | 1.05 ± 0.05 | 1.19 ± 0.04 | 1.94 ± 0.02 | 67.81 ± 0.81 | 4.83 ± 0.02 | 0.21 ± 0.01 | 0.13 ± 0.02 | 25.13 ± 0.64 | 74.90 ± 0.70 | ||
| 325095 | Inner | 0.07 ± 0.01 | 0.18 ± 0.01 | 0.42 ± 0.01 | 21.92 ± 0.48 | 0.42 ± 0.02 | 0.70 ± 0.03 | 0.46 ± 0.01 | 0.61 ± 0.07 | 0.88 ± 0.06 | 1.00 ± 0.05 | 64.68 ± 0.71 | 8.28 ± 0.09 | 0.21 ± 0.01 | 0.17 ± 0.01 | 25.57 ± 0.55 | 74.43 ± 0.56 | |
| Outer | 0.06 ± 0.00 | 0.17 ± 0.01 | 0.29 ± 0.02 | 21.17 ± 0.93 | 0.32 ± 0.01 | 1.25 ± 0.03 | 0.81 ± 0.02 | 0.88 ± 0.02 | 1.30 ± 0.02 | 1.77 ± 0.01 | 66.22 ± 0.99 | 5.47 ± 0.10 | 0.17 ± 0.01 | 0.12 ± 0.02 | 26.33 ± 0.85 | 73.67 ± 0.83 | ||
| F-Value | 9.19 | 25.85 | 65.26 | 16.47 | 79.43 | 306.8 | 46.71 | 105.02 | 182.89 | 678.99 | 43.56 | 1033.08 | 476.25 | 53.15 | 10.06 | 9.86 | ||
| Significance | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ||
| Antioxidants | FRAP | ABTS |
|---|---|---|
| Ascorbic acid | 0.472 ** | 0.504 ** |
| Total anthocyanin | 0.952 ** | 0.893 ** |
| Total phenol | 0.972 ** | 0.899 ** |
| Total flavonoid | 0.930 ** | 0.833 ** |
| Total carotenoid | −0.300 * | −0.234 |
| Total phytosterol | −0.261 | −0.337 * |
| Total vitamin E | 0.565 ** | 0.541 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, S.R.; Choi, C.S.; Rhee, J.; Shin, Y.K.; Song, J.W.; Kim, S.-H.; Kang, S.; Lee, J.G. Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes. Foods 2023, 12, 120. https://doi.org/10.3390/foods12010120
Bhandari SR, Choi CS, Rhee J, Shin YK, Song JW, Kim S-H, Kang S, Lee JG. Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes. Foods. 2023; 12(1):120. https://doi.org/10.3390/foods12010120
Chicago/Turabian StyleBhandari, Shiva Ram, Chang Sun Choi, Juhee Rhee, Yu Kyeong Shin, Jae Woo Song, Seong-Hoon Kim, Solly Kang, and Jun Gu Lee. 2023. "Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes" Foods 12, no. 1: 120. https://doi.org/10.3390/foods12010120
APA StyleBhandari, S. R., Choi, C. S., Rhee, J., Shin, Y. K., Song, J. W., Kim, S.-H., Kang, S., & Lee, J. G. (2023). Influence of Root Color and Tissue on Phytochemical Contents and Antioxidant Activities in Carrot Genotypes. Foods, 12(1), 120. https://doi.org/10.3390/foods12010120








