Oleogels—Innovative Technological Solution for the Nutritional Improvement of Meat Products
Abstract
:1. Introduction
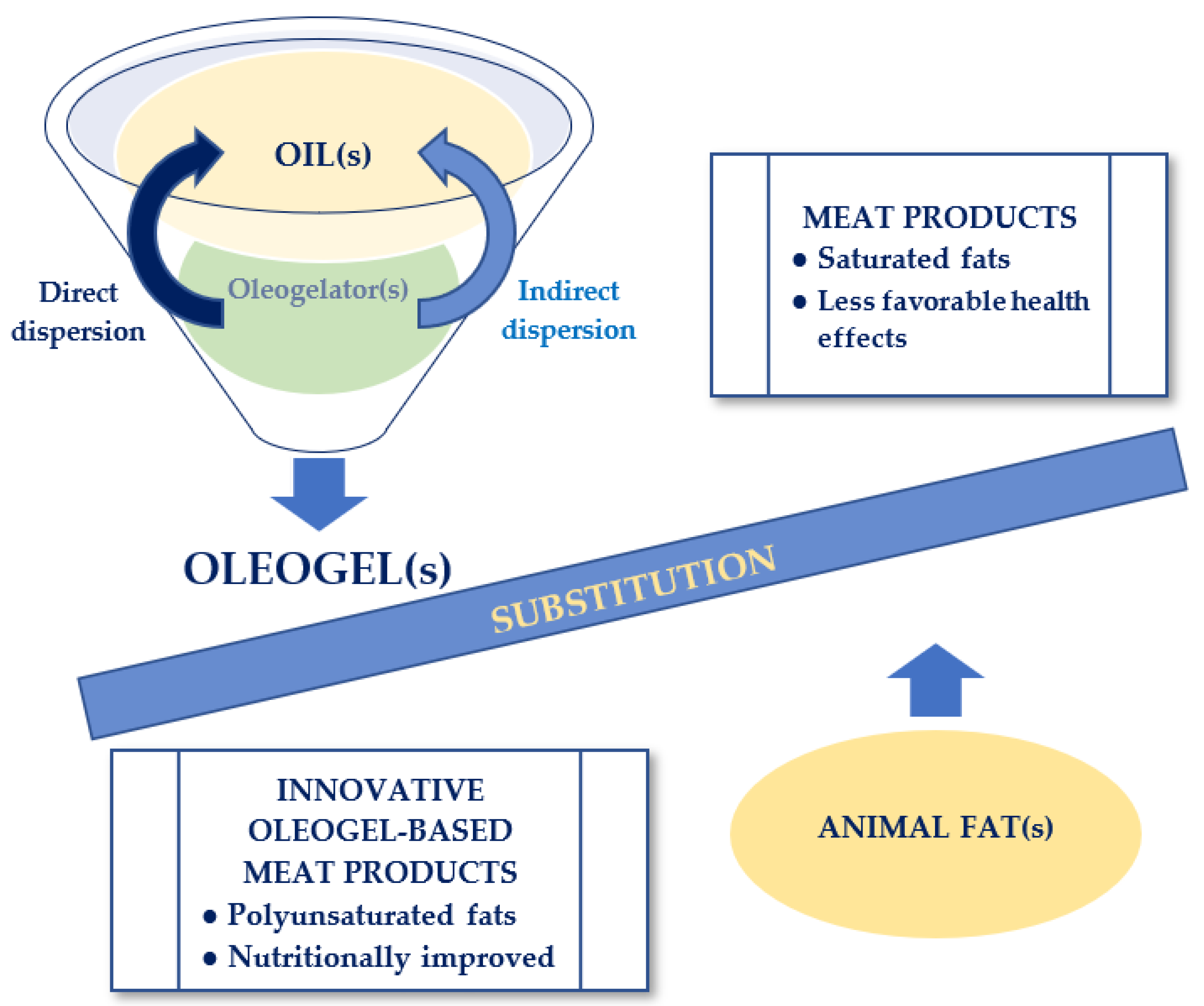
2. Oleogels—Novel Suitable Substitutes for Animal Fats from Foods
2.1. Definition and Description
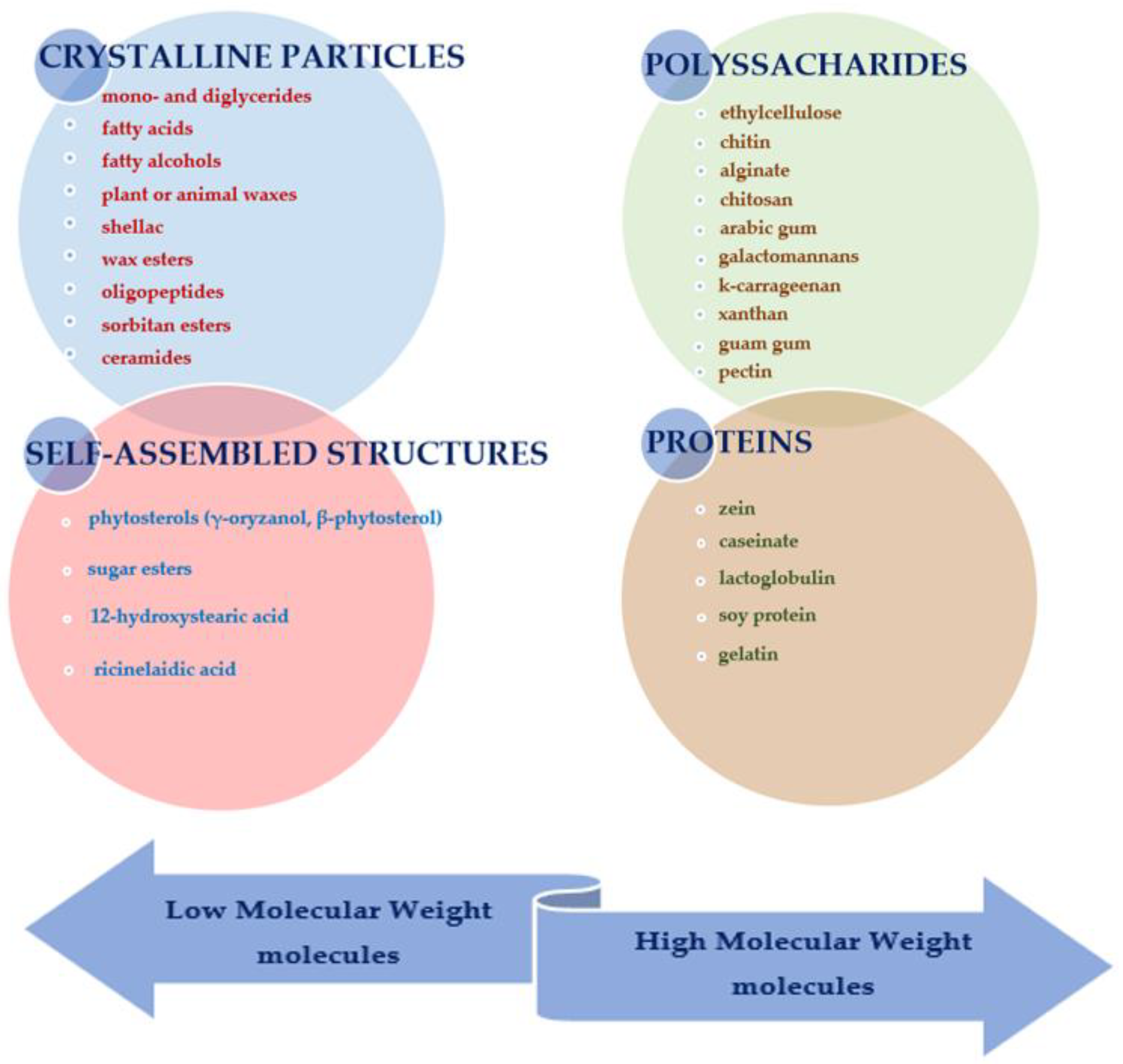
2.2. Oleogelators: Properties and Classification
2.2.1. Crystalline Particles
2.2.2. Self-Assembled Structures
2.3. Methods of Obtaining Oleogels
2.3.1. Direct Dispersion
2.3.2. Indirect Dispersion
- (i)
- Biphasic emulsion method
- (ii)
- Solvent exchange
- (iii)
- Foam-templated method
| Oleagelator | Oil | Gelation Conditions | Application | Reference |
|---|---|---|---|---|
| Hydroxypropyl methylcellulose (HPMC) | Sunflower oil (SFO) | 2 wt% aqueous HPMC solution was aerated, frozen at −23 °C, and freeze-dried. The resulting cryogel was submerged in SFO and sheared by centrifugation (11,000 rpm). | Comparative evaluation of different structured oil systems. | [75] |
| Hydroxypropyl methylcellulose (HPMC) 0.2, 0.4, 0.6, 0.8, 1.0 wt% Xanthan gum (XG) 0.3% | Soybean oil (SO) | SO (60 wt%) was dispersed (13,000 rpm) in an aqueous HPMC solution, followed by adding of XG solution under high-speed shearing. The mixture was dried at 90 °C and resulting product was smashed by a grinder, followed by shearing at 10,000 rpm. | Evaluation and optimization of soybean oil, HPMC and XG oleogel characteristics. | [92] |
| Gelatin (G) (3%, 5%) Xanthan gum (XG) (0.1%, 0.2%) | Canola oil (CO) | G and XG were dissolved in water, aerated by homogenization (13,000 rpm, 5 min), frozen at −20 °C overnight, and freeze-dried (24 h). Cryogel samples were saturated with CO and sheared by homogenization (0.5–2 min, 10,000 rpm). | Study about the ability of G and XG to produce oleogel through foam- templated method. | [93] |
| Pork skin (PS) | High oleic sunflower oil (HOSO) | PS, cooked for 40 min at 80 °C and comminuted in a blender, water and HOSO were mixed in the ratio of 1.5:1.5:1. | Replacement of 50% pork backfat in bologna sausages. | [94] |
| Canola protein isolate (CPI) | Canola oil (CO) | 50% CO in water emulsion stabilized by high-pressure homogenization with 4% CPI was heated at 90 °C for 30 min and dried at a 0.4 atm vacuum and 60 °C, followed by shearing. | Replacing 50% of traditional shortening in the cake batter. | [95] |
| Xanthan gum (XG) 0.3 wt% Hydroxypropyl methylcellulose (HPMC) 0.6 wt% | Soybean oil (SO) | 60% SO was dispersed (13,000 rpm) in HPMC aqueous solution and mixed with XG solution under high-speed shearing. The mixture was dried (90 °C) and smashed by a grinder (10,000 rpm). | Characterization of structure and molecular properties of polysaccharide oleogels. | [92] |
| Methylcellulose (MC) Hydroxypropyl methylcellulose (HPMC) | Sunflower oil (SFO) | Emulsion template approach (ETA): MC/HPMC aqueous solution (1.5 w/w%) and SFO (18%, 33%, 47% w/w) mixture was homogenized with water (16,500 rpm/1 min). The emulsion was dried (60 °C, 48 h) and sheared. Foam template approach (FTA): MC/HPMC aqueous solution (2 w/w%) was homogenized (16,500 rpm/2 min) and lyophilized. The sample was minced and saturated with SFO. | Comparison between ETA and FTA for designing edible oleogels based on cellulose ethers. | [96] |
| Regenerated keratin (RCh) | Sunflower oil (SFO) | 2 g of SFO was added to 6.5 g of RCh aqueous suspension (0.4–1.4 %) and emulsified by ultrasonication (2 min, 60% sonication amplitude). The emulsion was freeze-dried and sheared. | Characterization of SFO/RCh oleogel in terms of morphology, thermal behavior and viscoelastic properties. | [97]) |
| Citrus pectin (CP) Tea polyphenol-palmitate (TP) | Camellia oil (CO) | TP (2.5%) was melted in CO and cooled, followed by dispersion of CP (1.5–4.5% m/v) at room temperature. The mixture was emulsified by high-speed shearing (20,000 rpm/2 min), freeze-dried (48 h) and sheared (10,000 rpm/2 min). | Characterization of CO oleogel structured with TP and CP. | [63] |
2.4. Advantages and Benefits of the Use of Oleogels in Foods
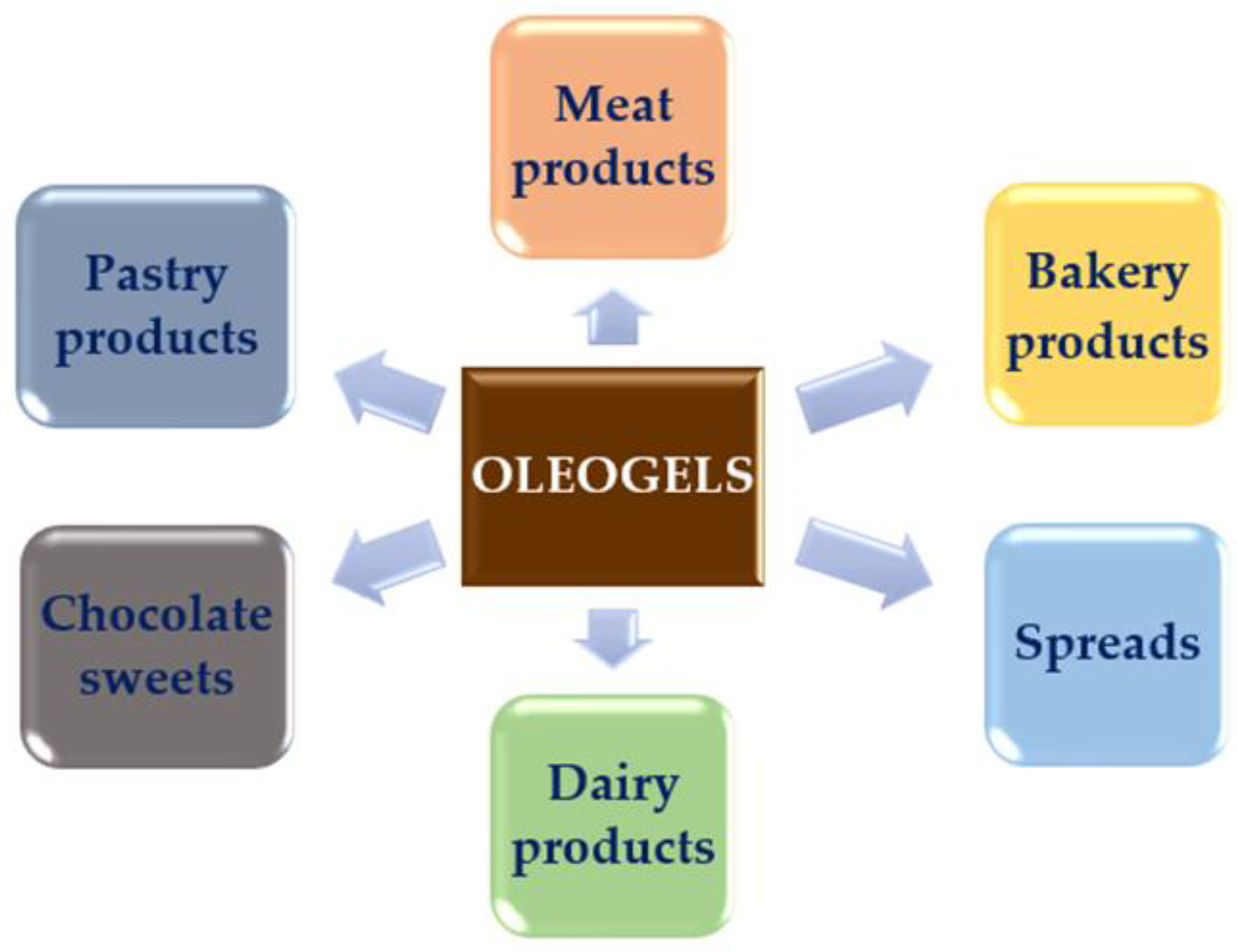
3. Trends in Improving Nutritional Profile of Meat Products by Using Oleogels
4. Designing of Innovative Oleogel-Based Meat Products
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; de Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- López-Pedrouso, M.; Lorenzo, J.M.; Gullón, B.; Campagnol, P.C.B.; Franco, D. Novel strategy for developing healthy meat products replacing saturated fat with oleogels. Curr. Opin. Food Sci. 2021, 40, 40–45. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Heseker, H.; Kiesswetter, E.; Koletzko, B. Reprint of: Dietary fat and fatty foods in the prevention of non-communicable diseases: A review of the evidence. Trends Food Sci. Technol. 2022, 130, 20–31. [Google Scholar] [CrossRef]
- FAO. Fats and fatty acids in human nutrition. Report of an expert consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Chowdhury, R.; Warnakula, S.; Kunutsor, S.; Crowe, F.; Ward, H.A.; Johnson, L.; Franco, O.H.; Butterworth, A.S.; Forouhi, N.G.; Thompson, S.G.; et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: A systematic review and meta-analysis. Ann. Intern. Med. 2014, 160, 398–406. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schunemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef] [Green Version]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2015, CD011737. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 8, CD011737. [Google Scholar] [CrossRef]
- Schwab, U.; Lauritzen, L.; Tholstrup, T.; Haldorssoni, T.; Riserus, U.; Uusitupa, M.; Becker, W. Effect of the amount and type of dietary fat on cardiometabolic risk factors and risk of developing type 2 diabetes, cardiovascular diseases, and cancer: A systematic review. Food Nutr. Res. 2014, 58. [Google Scholar] [CrossRef] [Green Version]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 535–546. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, X.; Fan, C.; Wu, W.; Zhang, W.; Wang, Y. Health Benefits, Food Applications, and Sustainability of Microalgae-Derived N-3 PUFA. Foods 2022, 11, 1883. [Google Scholar] [CrossRef] [PubMed]
- Feichtinger, A.; Scholten, E. Preparation of Protein Oleogels: Effect on Structure and Functionality. Foods 2020, 9, 1745. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cui, L.; Meng, Z. Oleogels/emulsion gels as novel saturated fat replacers in meat products: A review. Food Hydrocoll. 2022, 108313. [Google Scholar] [CrossRef]
- Patel, A.R.; Rajarethinem, P.S.; Gredowska, A.; Turhan, O.; Lesaffer, A.; De Vos, W.H.; Van de Walle, D.; Dewettinck, K. Edible applications of shellac oleogels: Spreads, chocolate paste and cakes. Food Funct. 2014, 5, 645–652. [Google Scholar] [CrossRef]
- Patel, A.R.; Cludts, N.; Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Edible oleogels based on water soluble food polymers: Preparation, characterization and potential application. Food Funct. 2014, 5, 2833–2841. [Google Scholar] [CrossRef] [Green Version]
- Marangoni, A.G.; Garti, N. Edible Oleogels: Structure and Health Implications; AOCS Press: Urbana, IL, USA, 2018. [Google Scholar]
- De Luca, L. Fats: Nutritional and Physiological Importance. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Oxford, UK, 2019; pp. 302–306. [Google Scholar]
- Ablao, C.J.N.; Sagum, R.S.; Maddela, A.K.M.; Macapagal, J. Road to trans-fat free Philippines: An emerging milestone amidst COVID-19 pandemic. Lancet Reg. Health West. Pac. 2021, 10, 100148. [Google Scholar] [CrossRef]
- Prasad, R.B.N.; Yadav, K.D. 17—Trans Fats Replacement Solutions in India. In Trans Fats Replacement Solutions; Kodali, D.R., Ed.; AOCS Press: Urbana, IL, USA, 2014; pp. 365–383. [Google Scholar]
- Wesdorp, L.H.; Melnikov, S.M.; Gaudier, E.A. 13—Trans Fats Replacement Solutions in Europe. In Trans Fats Replacement Solutions; Kodali, D.R., Ed.; AOCS Press: Urbana, IL, USA, 2014; pp. 287–312. [Google Scholar]
- Zhang, J.; Adhikari, P.; Yang, T.; Xia, S.; Hu, P.; Jiang, Y.; Xu, X. 15—Trans Fats Replacement Solutions in China. In Trans Fats Replacement Solutions; Kodali, D.R., Ed.; AOCS Press: Urbana, IL, USA, 2014; pp. 337–354. [Google Scholar]
- U. S. Food and Drug Administration. Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat); U. S. Food and Drug Administration: Montgomery, MD, USA, 2018. [Google Scholar]
- World Health Organization. Healthy Diet; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- European Food Safety Authority. Scientific and technical assistance on trans fatty acids. Sci. Tech. Assist. Trans Fat. Acids 2018, 15, 1433E. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zou, Y.; Que, F.; Zhang, H. Recent advances in fabrication of edible polymer oleogels for food applications. Curr. Opin. Food Sci. 2022, 43, 114–119. [Google Scholar] [CrossRef]
- Alongi, M.; Lucci, P.; Clodoveo, M.L.; Schena, F.P.; Calligaris, S. Oleogelation of extra virgin olive oil by different oleogelators affects the physical properties and the stability of bioactive compounds. Food Chem. 2022, 368, 130779. [Google Scholar] [CrossRef]
- Puscas, A.; Muresan, V.; Muste, S. Application of Analytical Methods for the Comprehensive Analysis of Oleogels—A Review. Polymers 2021, 13, 1934. [Google Scholar] [CrossRef] [PubMed]
- Floter, E.; Wettlaufer, T.; Conty, V.; Scharfe, M. Oleogels-Their Applicability and Methods of Characterization. Molecules 2021, 26, 1673. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Auzanneau, F.I.; Rogers, M.A. Advances in edible oleogel technologies—A decade in review. Food Res. Int. 2017, 97, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Lupi, F.R.; Gabriele, D.; Facciolo, D.; Baldino, N.; Seta, L.; de Cindio, B. Effect of organogelator and fat source on rheological properties of olive oil-based organogels. Food Res. Int. 2012, 46, 177–184. [Google Scholar] [CrossRef]
- Davidovich-Pinhas, M. Oleogels: A promising tool for delivery of hydrophobic bioactive molecules. Ther. Deliv. 2016, 7, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.W.; Fu, S.Y.; Hou, J.J.; Guo, J.; Wang, J.M.; Yang, X.Q. Zein based oil-in-glycerol emulgels enriched with beta-carotene as margarine alternatives. Food Chem. 2016, 211, 836–844. [Google Scholar] [CrossRef]
- Stortz, T.A.; Zetzl, A.K.; Barbut, S.; Cattaruzza, A.; Marangoni, A.G. Edible oleogels in food products to help maximize health benefits and improve nutritional profiles. Lipid Technol. 2012, 24, 151–154. [Google Scholar] [CrossRef]
- Yu, H.; Shi, K.; Liu, D.; Huang, Q. Development of a food-grade organogel with high bioaccessibility and loading of curcuminoids. Food Chem. 2012, 131, 48–54. [Google Scholar] [CrossRef]
- Pinto, T.C.; Martins, A.J.; Pastrana, L.; Pereira, M.C.; Cerqueira, M.A. Oleogel-Based Systems for the Delivery of Bioactive Compounds in Foods. Gels 2021, 7, 86. [Google Scholar] [CrossRef]
- Kanelaki, A.; Zampouni, K.; Mourtzinos, I.; Katsanidis, E. Hydrogels, Oleogels and Bigels as Edible Coatings of Sardine Fillets and Delivery Systems of Rosemary Extract. Gels 2022, 8, 660. [Google Scholar] [CrossRef]
- Zhuang, X.; Gaudino, N.; Clark, S.; Acevedo, N.C. Novel lecithin-based oleogels and oleogel emulsions delay lipid oxidation and extend probiotic bacteria survival. LWT 2021, 136. [Google Scholar] [CrossRef]
- Bei, W.; Zhou, Y.; Xing, X.; Zahi, M.R.; Li, Y.; Yuan, Q.; Liang, H. Organogel-nanoemulsion containing nisin and D-limonene and its antimicrobial activity. Front. Microbiol. 2015, 6, 1010. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Guo, X.L.; Huang, H.B.; Li, X.; Xu, Y.; Hu, J.N. Structural characterization of modified whey protein isolates using cold plasma treatment and its applications in emulsion oleogels. Food Chem. 2021, 356, 129703. [Google Scholar] [CrossRef]
- Yadav, I.; Kasiviswanathan, U.; Soni, C.; Paul, S.R.; Nayak, S.K.; Sagiri, S.S.; Anis, A.; Pal, K. Stearic Acid Modified Stearyl Alcohol Oleogel: Analysis of the Thermal, Mechanical and Drug Release Properties. J. Surfactants Deterg. 2017, 20, 851–861. [Google Scholar] [CrossRef]
- Okuro, P.K.; Martins, A.J.; Vicente, A.A.; Cunha, R.L. Perspective on oleogelator mixtures, structure design and behaviour towards digestibility of oleogels. Curr. Opin. Food Sci. 2020, 35, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Cornara, L.; Biagi, M.; Xiao, J.; Burlando, B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front. Pharm. 2017, 8, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratini, F.; Cilia, G.; Turchi, B.; Felicioli, A. Beeswax: A minireview of its antimicrobial activity and its application in medicine. Asian Pac. J. Trop Med. 2016, 9, 839–843. [Google Scholar] [CrossRef]
- Szulc, J.; Machnowski, W.; Kowalska, S.; Jachowicz, A.; Ruman, T.; Steglinska, A.; Gutarowska, B. Beeswax-Modified Textiles: Method of Preparation and Assessment of Antimicrobial Properties. Polymers 2020, 12, 344. [Google Scholar] [CrossRef] [Green Version]
- Pintado, T.; Cofrades, S. Quality Characteristics of Healthy Dry Fermented Sausages Formulated with a Mixture of Olive and Chia Oil Structured in Oleogel or Emulsion Gel as Animal Fat Replacer. Foods 2020, 9, 830. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Dimakopoulou-Papazoglou, D.; Moschakis, T.; Biliaderis, C.G.; Katsanidis, E. Modified fermented sausages with olive oil oleogel and NaCl–KCl substitution for improved nutritional quality. LWT 2022, 158. [Google Scholar] [CrossRef]
- Pehlivanoglu, H.; Demirci, M.; Toker, O.S.; Konar, N.; Karasu, S.; Sagdic, O. Oleogels, a promising structured oil for decreasing saturated fatty acid concentrations: Production and food-based applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- da Silva, T.L.T.; Arellano, D.B.; Martini, S. Interactions between candelilla wax and saturated triacylglycerols in oleogels. Food Res. Int. 2019, 121, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Banas, K.; Harasym, J. Natural Gums as Oleogelators. Int. J. Mol. Sci. 2021, 22, 12977. [Google Scholar] [CrossRef]
- Zetzl, A.K.; Gravelle, A.J.; Kurylowicz, M.; Dutcher, J.; Barbut, S.; Marangoni, A.G. Microstructure of ethylcellulose oleogels and its relationship to mechanical properties. Food Struct. 2014, 2, 27–40. [Google Scholar] [CrossRef]
- Fayaz, G.; Calligaris, S.; Nicoli, M.C. Comparative Study on the Ability of Different Oleogelators to Structure Sunflower Oil. Food Biophys. 2019, 15, 42–49. [Google Scholar] [CrossRef]
- Co, E.D.; Marangoni, A.G. Organogels: An Alternative Edible Oil-Structuring Method. J. Am. Oil Chem. Soc. 2012, 89, 749–780. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Fawzia, S.; Madhujith, T.; Karim, A. Synergistic effects of oleogelators in tailoring the properties of oleogels: A review. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3507–3539. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.M.; Barbut, S.; Marangoni, A.G. Edible oleogels for the oral delivery of lipid soluble molecules: Composition and structural design considerations. Trends Food Sci. Technol. 2016, 57, 59–73. [Google Scholar] [CrossRef] [Green Version]
- Bharti, D.; Kim, D.; Banerjee, I.; Rousseau, D.; Pal, K. Effects of Sorbitan Monostearate and Stearyl Alcohol on the Physicochemical Parameters of Sunflower-Wax-Based Oleogels. Gels 2022, 8, 520. [Google Scholar] [CrossRef]
- Lim, J.; Hwang, H.-S.; Lee, S. Oil-structuring characterization of natural waxes in canola oil oleogels: Rheological, thermal, and oxidative properties. Appl. Biol. Chem. 2017, 60, 17–22. [Google Scholar] [CrossRef]
- Sahu, D.; Bharti, D.; Kim, D.; Sarkar, P.; Pal, K. Variations in Microstructural and Physicochemical Properties of Candelilla Wax/Rice Bran Oil-Derived Oleogels Using Sunflower Lecithin and Soya Lecithin. Gels 2021, 7, 226. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.J.; Barrera-Arellano, D.; Ribeiro, A.P.B. Oleogel-based emulsions: Concepts, structuring agents, and applications in food. J. Food Sci. 2021, 86, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.; Wesseling, A.; van der Linden, E.; Scholten, E. Protein oleogels from heat-set whey protein aggregates. J. Colloid Interface Sci. 2017, 486, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Plazzotta, S.; Calligaris, S.; Manzocco, L. Structure of oleogels from κ-carrageenan templates as affected by supercritical-CO2-drying, freeze-drying and lettuce-filler addition. Food Hydrocoll. 2019, 96, 1–10. [Google Scholar] [CrossRef]
- Abdolmaleki, K.; Alizadeh, L.; Nayebzadeh, K.; Hosseini, S.M.; Shahin, R. Oleogel production based on binary and ternary mixtures of sodium caseinate, xanthan gum, and guar gum: Optimization of hydrocolloids concentration and drying method. J. Texture Stud. 2020, 51, 290–299. [Google Scholar] [CrossRef]
- Luo, S.-Z.; Hu, X.-F.; Jia, Y.-J.; Pan, L.-H.; Zheng, Z.; Zhao, Y.-Y.; Mu, D.-D.; Zhong, X.-Y.; Jiang, S.-T. Camellia oil-based oleogels structuring with tea polyphenol-palmitate particles and citrus pectin by emulsion-templated method: Preparation, characterization and potential application. Food Hydrocoll. 2019, 95, 76–87. [Google Scholar] [CrossRef]
- Wang, Q.; Rao, Z.; Chen, Y.; Lei, X.; Zhao, J.; Li, F.; Lei, L.; Zeng, K.; Ming, J. Characterization of responsive zein-based oleogels with tunable properties fabricated from emulsion-templated approach. Food Hydrocoll. 2022, 133. [Google Scholar] [CrossRef]
- Martins, A.J.; Vicente, A.A.; Pastrana, L.M.; Cerqueira, M.A. Oleogels for development of health-promoting food products. Food Sci. Hum. Wellness 2020, 9, 31–39. [Google Scholar] [CrossRef]
- Gravelle, A.J.; Davidovich-Pinhas, M.; Barbut, S.; Marangoni, A.G. Influencing the crystallization behavior of binary mixtures of stearyl alcohol and stearic acid (SOSA) using ethylcellulose. Food Res. Int. 2017, 91, 1–10. [Google Scholar] [CrossRef]
- Kouzounis, D.; Lazaridou, A.; Katsanidis, E. Partial replacement of animal fat by oleogels structured with monoglycerides and phytosterols in frankfurter sausages. Meat Sci. 2017, 130, 38–46. [Google Scholar] [CrossRef]
- Adili, L.; Roufegarinejad, L.; Tabibiazar, M.; Hamishehkar, H.; Alizadeh, A. Development and characterization of reinforced ethyl cellulose based oleogel with adipic acid: Its application in cake and beef burger. LWT 2020, 126. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, S.; Oh, I. Development of Antioxidant-Fortified Oleogel and Its Application as a Solid Fat Replacer to Muffin. Foods 2021, 10, 3059. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.-S. A critical review on structures, health effects, oxidative stability, and sensory properties of oleogels. Biocatal. Agric. Biotechnol. 2020, 26. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, F.; Vicente, A.A.; Cunha, R.L.; Pastrana, L.M.; Cerqueira, M.A. Gelation Behavior and Stability of Multicomponent Sterol-Based Oleogels. Gels 2022, 8, 37. [Google Scholar] [CrossRef]
- Gaudino, N.; Ghazani, S.M.; Clark, S.; Marangoni, A.G.; Acevedo, N.C. Development of lecithin and stearic acid based oleogels and oleogel emulsions for edible semisolid applications. Food Res. Int. 2019, 116, 79–89. [Google Scholar] [CrossRef]
- Mert, B.; Demirkesen, I. Reducing saturated fat with oleogel/shortening blends in a baked product. Food Chem. 2016, 199, 809–816. [Google Scholar] [CrossRef]
- Öğütcü, M.; Yılmaz, E. Characterization of Hazelnut Oil Oleogels Prepared with Sunflower and Carnauba Waxes. Int. J. Food Prop. 2014, 18, 1741–1755. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.R.; Dewettinck, K. Comparative evaluation of structured oil systems: Shellac oleogel, HPMC oleogel, and HIPE gel. Eur. J. Lipid Sci. Technol. 2015, 117, 1772–1781. [Google Scholar] [CrossRef]
- Puscas, A.; Tanislav, A.E.; Muresan, A.E.; Farcas, A.C.; Muresan, V. Walnut Oil Oleogels as Milk Fat Replacing System for Commercially Available Chocolate Butter. Gels 2022, 8, 613. [Google Scholar] [CrossRef]
- Sena, B.; Dhal, S.; Sahu, D.; Sarkar, P.; Mohanty, B.; Jarzebski, M.; Wieruszewski, M.; Behera, H.; Pal, K. Variations in Microstructural and Physicochemical Properties of Soy Wax/Soybean Oil-Derived Oleogels Using Soy Lecithin. Polymers 2022, 14, 3928. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Saleh, A.S.M.; Yang, H.; Wang, N.; Wang, P.; Yue, X.; Xiao, Z. Functional Characteristics of Oleogel Prepared from Sunflower Oil with β-Sitosterol and Stearic Acid. J. Am. Oil Chem. Soc. 2017, 94, 1153–1164. [Google Scholar] [CrossRef]
- Pan, J.; Tang, L.; Dong, Q.; Li, Y.; Zhang, H. Effect of oleogelation on physical properties and oxidative stability of camellia oil-based oleogels and oleogel emulsions. Food Res. Int. 2021, 140, 110057. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hwang, H.-S.; Jeong, S.; Lee, S. Utilization of oleogels with binary oleogelator blends for filling creams low in saturated fat. LWT 2022, 155. [Google Scholar] [CrossRef]
- Aliasl Khiabani, A.; Tabibiazar, M.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Preparation and characterization of carnauba wax/adipic acid oleogel: A new reinforced oleogel for application in cake and beef burger. Food Chem. 2020, 333, 127446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhang, R.; Yu, J.; Gao, Y.; Mao, L. Tuning the rheological and tribological properties to simulate oral processing of novel high internal phase oleogel-in-water emulsions. Food Hydrocoll. 2022, 131. [Google Scholar] [CrossRef]
- Borriello, A.; Masi, P.; Cavella, S. Novel pumpkin seed oil-based oleogels: Development and physical characterization. LWT 2021, 152. [Google Scholar] [CrossRef]
- Callau, M.; Sow-Kebe, K.; Nicolas-Morgantini, L.; Fameau, A.L. Effect of the ratio between behenyl alcohol and behenic acid on the oleogel properties. J. Colloid Interface Sci. 2020, 560, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, S.; Mirolo, G.; Da Pieve, S.; Arrighetti, G.; Nicoli, M.C. Effect of Oil Type on Formation, Structure and Thermal Properties of γ-oryzanol and β-sitosterol-Based Organogels. Food Biophysics 2013, 9, 69–75. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; Lopez-Pedrouso, M.; Purrinos, L.; Cerqueira, M.A.; Vicente, A.A.; Pastrana, L.M.; Zapata, C.; Lorenzo, J.M. Strategy towards Replacing Pork Backfat with a Linseed Oleogel in Frankfurter Sausages and its Evaluation on Physicochemical, Nutritional, and Sensory Characteristics. Foods 2019, 8, 366. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Lo, Y.M.; Yan, M.; Li, P.; Cao, Y. Characterization of thermo-oxidative behavior of ethylcellulose oleogels. Food Chem. 2020, 305, 125470. [Google Scholar] [CrossRef]
- Alvarez, M.D.; Cofrades, S.; Espert, M.; Salvador, A.; Sanz, T. Thermorheological Characterization of Healthier Reduced-Fat Cocoa Butter Formulated by Substitution with a Hydroxypropyl Methylcellulose (HPMC)-Based Oleogel. Foods 2021, 10, 793. [Google Scholar] [CrossRef] [PubMed]
- Scholten, E. Edible oleogels: How suitable are proteins as a structurant? Curr. Opin. Food Sci. 2019, 27, 36–42. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Naqash, F.; Rashid, R. Oleogels: Promising alternatives to solid fats for food applications. Food Hydrocoll. Health 2022, 2. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Bosqui, K.; Rabelo, R.S.; Kurozawa, L.E.; Hubinger, M.D. High internal phase emulsions (HIPE) using pea protein and different polysaccharides as stabilizers. Food Hydrocoll. 2020, 105. [Google Scholar] [CrossRef]
- Meng, Z.; Qi, K.; Guo, Y.; Wang, Y.; Liu, Y. Macro-micro structure characterization and molecular properties of emulsion-templated polysaccharide oleogels. Food Hydrocoll. 2018, 77, 17–29. [Google Scholar] [CrossRef]
- Abdollahi, M.; Goli, S.A.H.; Soltanizadeh, N. Physicochemical Properties of Foam-Templated Oleogel Based on Gelatin and Xanthan Gum. Eur. J. Lipid Sci. Technol. 2019, 122. [Google Scholar] [CrossRef]
- da Silva, S.L.; Amaral, J.T.; Ribeiro, M.; Sebastiao, E.E.; Vargas, C.; de Lima Franzen, F.; Schneider, G.; Lorenzo, J.M.; Fries, L.L.M.; Cichoski, A.J.; et al. Fat replacement by oleogel rich in oleic acid and its impact on the technological, nutritional, oxidative, and sensory properties of Bologna-type sausages. Meat Sci. 2019, 149, 141–148. [Google Scholar] [CrossRef]
- Tang, Y.R.; Ghosh, S. Canola protein thermal denaturation improved emulsion-templated oleogelation and its cake-baking application. RSC Adv. 2021, 11, 25141–25157. [Google Scholar] [CrossRef]
- Wang, Q.; Espert, M.; Larrea, V.; Quiles, A.; Salvador, A.; Sanz, T. Comparison of different indirect approaches to design edible oleogels based on cellulose ethers. Food Hydrocoll. 2023, 134. [Google Scholar] [CrossRef]
- Baraki, S.Y.; Jiang, Y.; Li, X.; Debeli, D.K.; Wang, B.; Feng, X.; Mao, Z.; Sui, X. Stable sunflower oil oleogel from oil/water pickering emulsion with regenerated chitin. LWT 2021, 146. [Google Scholar] [CrossRef]
- Hoti, G.; Matencio, A.; Rubin Pedrazzo, A.; Cecone, C.; Appleton, S.L.; Khazaei Monfared, Y.; Caldera, F.; Trotta, F. Nutraceutical Concepts and Dextrin-Based Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4102. [Google Scholar] [CrossRef] [PubMed]
- Puscas, A.; Muresan, V.; Socaciu, C.; Muste, S. Oleogels in Food: A Review of Current and Potential Applications. Foods 2020, 9, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Otto, M.C.; Mozaarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez-Colmenero, F.; Salcedo-Sandoval, L.; Bou, R.; Cofrades, S.; Herrero, A.M.; Ruiz-Capillas, C. Novel applications of oil-structuring methods as a strategy to improve the fat content of meat products. Trends Food Sci. Technol. 2015, 44, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Jeong, S.; Oh, I.K.; Lee, S. Evaluation of soybean oil-carnauba wax oleogels as an alternative to high saturated fat frying media for instant fried noodles. LWT 2017, 84, 788–794. [Google Scholar] [CrossRef]
- Patel, A.R. A colloidal gel perspective for understanding oleogelation. Curr. Opin. Food Sci. 2017, 15, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Liu, G. Corn oil-based oleogels with different gelation mechanisms as novel cocoa butter alternatives in dark chocolate. J. Food Eng. 2019, 263, 114–122. [Google Scholar] [CrossRef]
- Bemer, H.L.; Limbaugh, M.; Cramer, E.D.; Harper, W.J.; Maleky, F. Vegetable organogels incorporation in cream cheese products. Food Res. Int. 2016, 85, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Moriano, M.E.; Alamprese, C. Organogels as novel ingredients for low saturated fat ice creams. LWT 2017, 86, 371–376. [Google Scholar] [CrossRef]
- Jang, A.; Bae, W.; Hwang, H.S.; Lee, H.G.; Lee, S. Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods. Food Chem. 2015, 187, 525–529. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lim, J.; Lee, J.; Hwang, H.S.; Lee, S. Utilization of Oleogels as a Replacement for Solid Fat in Aerated Baked Goods: Physicochemical, Rheological, and Tomographic Characterization. J. Food Sci. 2017, 82, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Singh, M.; Winkler-Moser, J.K.; Bakota, E.L.; Liu, S.X. Preparation of margarines from organogels of sunflower wax and vegetable oils. J. Food Sci. 2014, 79, C1926–C1932. [Google Scholar] [CrossRef]
- Manzoor, S.; Masoodi, F.A.; Rashid, R.; Naqash, F.; Ahmad, M. Oleogels for the development of healthy meat products: A review. Appl. Food Res. 2022, 2. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Herrero, A.M.; Herranz, B.; Álvarez, M.D.; Jiménez-Colmenero, F.; Cofrades, S. Characterization of ethyl cellulose and beeswax oleogels and their suitability as fat replacers in healthier lipid pâtés development. Food Hydrocoll. 2019, 87, 960–969. [Google Scholar] [CrossRef]
- Martins, A.J.; Cerqueira, M.A.; Fasolin, L.H.; Cunha, R.L.; Vicente, A.A. Beeswax organogels: Influence of gelator concentration and oil type in the gelation process. Food Res. Int. 2016, 84, 170–179. [Google Scholar] [CrossRef] [Green Version]
- Agregan, R.; Barba, F.J.; Gavahian, M.; Franco, D.; Khaneghah, A.M.; Carballo, J.; Ferreira, I.C.; da Silva Barretto, A.C.; Lorenzo, J.M. Fucus vesiculosus extracts as natural antioxidants for improvement of physicochemical properties and shelf life of pork patties formulated with oleogels. J. Sci. Food Agric. 2019, 99, 4561–4570. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Yong, H.I.; Jung, S.; Kim, Y.B.; Choi, Y.S. Effects of replacing pork fat with grape seed oil and gelatine/alginate for meat emulsions. Meat Sci 2020, 163, 108079. [Google Scholar] [CrossRef]
- Moghtadaei, M.; Soltanizadeh, N.; Goli, S.A.H. Production of sesame oil oleogels based on beeswax and application as partial substitutes of animal fat in beef burger. Food Res. Int. 2018, 108, 368–377. [Google Scholar] [CrossRef]
- Lopes, R.; Costa, V.; Costa, M.; Paiva-Martins, F. Olive oil oleogels as strategy to confer nutritional advantages to burgers. Food Chem. 2022, 397, 133724. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Pintado, T.; Jiménez-Colmenero, F.; Cofrades, S. The effect of household storage and cooking practices on quality attributes of pork burgers formulated with PUFA- and curcumin-loaded oleogels as healthy fat substitutes. LWT 2020, 119. [Google Scholar] [CrossRef]
- Ferrer-González, B.M.; García-Martínez, I.; Totosaus, A. Textural properties, sensory acceptance and fatty acid profile of cooked meat batters employing pumpkin seed paste or soybean oil oleogel as fat replacers. Grasas Aceites 2019, 70. [Google Scholar] [CrossRef]
- Oh, I.; Lee, J.; Lee, H.G.; Lee, S. Feasibility of hydroxypropyl methylcellulose oleogel as an animal fat replacer for meat patties. Food Res. Int. 2019, 122, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, M.; Zhang, L.; Wang, Z.; Yu, Q.; Han, L. Preparation of rapeseed oil oleogels based on beeswax and its application in beef heart patties to replace animal fat. LWT 2021, 149. [Google Scholar] [CrossRef]
- Martins, A.J.; Lorenzo, J.M.; Franco, D.; Pateiro, M.; Dominguez, R.; Munekata, P.E.S.; Pastrana, L.M.; Vicente, A.A.; Cunha, R.L.; Cerqueira, M.A. Characterization of Enriched Meat-Based Pate Manufactured with Oleogels as Fat Substitutes. Gels 2020, 6, 17. [Google Scholar] [CrossRef]
- Wolfer, T.L.; Acevedo, N.C.; Prusa, K.J.; Sebranek, J.G.; Tarte, R. Replacement of pork fat in frankfurter-type sausages by soybean oil oleogels structured with rice bran wax. Meat Sci. 2018, 145, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Tarté, R.; Paulus, J.S.; Acevedo, N.C.; Prusa, K.J.; Lee, S.-L. High-oleic and conventional soybean oil oleogels structured with rice bran wax as alternatives to pork fat in mechanically separated chicken-based bologna sausage. LWT 2020, 131. [Google Scholar] [CrossRef]
- Ferro, A.C.; de Souza Paglarini, C.; Rodrigues Pollonio, M.A.; Lopes Cunha, R. Glyceryl monostearate-based oleogels as a new fat substitute in meat emulsion. Meat Sci. 2021, 174, 108424. [Google Scholar] [CrossRef]
- Issara, U. Improvement of Thai Sweet Sausage (Goon Chiang) Properties by Oleogel Made of Rice Bran Wax and Rice Bran Oil: A Textural, Sensorial, and Nutritional Aspect. IOP Conf. Ser. Earth Environ. Sci. 2022, 995. [Google Scholar] [CrossRef]
- Franco, D.; Martins, A.J.; Lopez-Pedrouso, M.; Cerqueira, M.A.; Purrinos, L.; Pastrana, L.M.; Vicente, A.A.; Zapata, C.; Lorenzo, J.M. Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages. J. Sci. Food Agric. 2020, 100, 218–224. [Google Scholar] [CrossRef] [Green Version]
- Barbut, S.; Wood, J.; Marangoni, A. Potential use of organogels to replace animal fat in comminuted meat products. Meat Sci. 2016, 122, 155–162. [Google Scholar] [CrossRef]
- Barbut, S.; Wood, J.; Marangoni, A. Quality effects of using organogels in breakfast sausage. Meat Sci. 2016, 122, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S.; Wood, J.; Marangoni, A.G. Effects of Organogel Hardness and Formulation on Acceptance of Frankfurters. J. Food Sci. 2016, 81, C2183–C2188. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Toksoz, B. Flaxseed oil-wax oleogels replacement for tallowfat in sucuk samples provided higher concentrations of polyunsaturated fatty acids and aromatic volatiles. Meat Sci. 2022, 192, 108875. [Google Scholar] [CrossRef] [PubMed]
- Barbut, S.; Tiensa, B.E.; Marangoni, A.G. Partial fat replacement in liver pâté using canola oil organogel. LWT 2021, 139, 110428. [Google Scholar] [CrossRef]
| Oleagelator | Oil | Gelation Conditions | Application | Reference |
|---|---|---|---|---|
| Candelilla wax (CW) 3 and 6% | Canola oil (CO) | Heating to 150 °C, under gentle agitation for 15 min and cooling at room temperature. | Partial replacement of shortening in cookies. | [73] |
| Sunflower and Carnauba waxes (SW, CW) 3, 7 and 10% | Hazelnut oil (HO) | Heating to 90 °C, stirring vigorously for 5 min and cooling at ambient temperature. | Comparative study with a commercial shortening. | [74] |
| Shellac wax (SW) 0–6 wt% | Rapeseed oil (RO) | The oil and wax dispersion was heated at 90 °C, under mild agitation for 30 min. | Comparative evaluation of different structured oil systems. | [75] |
| Candelilla wax (CW) 10% | Walnut oil (WO) | Dispersion of oleogelator in the oil, followed by heating at the melting point of oleogelator under stirring (450 rot/min) and cooling down at 0–4 °C. | Nutritional improvement of chocolate spread. | [76] |
| Glyceryl monostearate (GMS) 10% | ||||
| Soy wax (SW) 11% Soy lecithin (SYL) 5 mg/30.6 g | Refined soybean oil (RSO) | Melting SW in RSO, at 65 °C under stirring (300 rpm) for 15 min, followed by cooling at 4 °C for 90 min. | Optimization of RSO-SW oleogel characteristics by adding SYL. | [77] |
| β-sitosterol / stearic acid mass ratio: 1:0, 4:1, 3:2, 2:3, 1:4, 0:1 | Sunflower oil (SFO) | The gelator/oil mixtures (20 g/100 g) were heated at 90 °C under stirring (400 rpm) and cooled at ambient temperature. | Evaluation and optimization of the characteristics of sunflower oil, β-sitosterol and stearic acid oleogel. | [78] |
| Glycerol monolaurate (GML) 1, 3, 5 and 10 wt% | Camellia oil (CO) | Camellia oil (CO) and GML mixtures were constantly stirred at 80 °C and cooled at room temperature. | Improving physical properties and oxidation stability of Camellia oil (CO) by oleogelation. | [79] |
| Candelilla wax (CDW) and glyceryl monostearate (GMS) mixture 80:20, 60:40, 40:60, 20:80 (w/w) | Canola oil (CO) | Melting CDW and GMS mixture in CO at 90 °C under stirring at a weight ratio of 9:1, until the complete dissolution, followed by cooling at 25 °C for 24 h. | Improving physicochemical properties of oleogels with binary blends of oleogelators. | [80] |
| Carnauba wax (CW)/adipic acid (AA) mixture 50:10, 40:20, 30:30, 20:40, 10:50 (w/w) | Soybean oil (SO) | CW/AA (6%) were dissolved in SO at 150 °C until complete dissolution and cooled down at ambient temperature (1 °C/min). | Carnauba wax/adipic acid oleogel characterization for fat replacement in cake and beef burger. | [81] |
| Ethyl-cellulose (EC) 5 wt%; 10 wt% | Corn oil (CO) | EC powder was mixed with heated CO (150 °C), stirred (15 min) and cooled at room temperature. | Oleogel rheological and tribological properties evaluation. | [82] |
| Sunflower wax (SW) (5%) sorbitan monostearate (SP); stearyl alcohol (SA) (0.05% w/w) | Sunflower oil (SFO) | Direct dispersion of SP and SA in heated mixture (80 °C) of SW and SFO, followed by cooling at room temperature. | Improving oleogels physicochemical properties by addition of SP and SA emulsifiers. | [56] |
| Beeswax (BW) 4, 5, 6, 8% w/w Carnauba wax (CW) 4, 5, 6, 8% w/w | Pumpkin seed oil (PSO) | Direct dispersion of oleogelators in heated oil (80 °C for BW and 90 °C for CW) under stirring (200 rpm), followed by cooling at 25 °C. | Physical characterization of pumpkin seed oil oleogel. | [83] |
| Behenyl alcohol (BO) and behenic acid (BA) mixture, in different weight ratio | Sunflower oil (SFO) | Dispersion of BO and BA mixture (10%) in a heated SO (85 °C) until complete dissolution and cooling at room temperature. | Improving oleogel properties by mixing BO and BA oleogelators. | [84] |
| β-sitosterol, γ-oryzanol (3:2 w/w mixture) | Flax-seed oil (FSO), sunflower oil (SFO), olive oil (OO), triolein, castor oil (CO) | 5, 10, 20% (w/w) oleogelators were mixed with oils, heated at 90 °C under stirring and cooled at 4 °C. | Study on the effect of oil type on the gelation process: gelling time, mechanical and thermal behavior. | [85] |
| Beeswax (BW) 8% | Linseed oil (LO) | 8% (w/w) BW was dispersed under stirring in heated LO (80 °C) for 30 min and cooled at room temperature. | Fat substitution with LO/BW oleogel in frankfurters. | [86] |
| Ethyl-cellulose (EC) (0–10% w/w) | Soybean oil (SO) | EC was added to the heated SO (140 °C) under stirring (14 min), followed by cooling at room temperature. | Characterization of thermo-oxidative behavior of EC oleogels. | [87] |
| Meat Product | Oleogel Components and Preparation | Level of Animal Fat Substitution | Effects of Oleogel Incorporation on the Formulated Meat Product | Reference | |
|---|---|---|---|---|---|
| Vegetable Oil(s) | Oleogelator(s) | ||||
| Beef burgers | Sesame oil (SO) | Beeswax (BW) | 25% and 50% by animal fat with 10% BW oleogel | Decrease of more than 50% in the hardness, chewiness, gumminess, and lightness of raw burgers. Reduction of 11% in cooking loss and 1.6% in fat absorption after processing; color insignificantly changed; lipid oxidation significantly increased. Cooked burgers could not mimic the control in terms of textural properties, but proved good acceptability. | [115] |
| 5%, 7.5%, 10% BW (w/w), dissolved in SO under vacuum at 70 °C | |||||
| Beef burgers | Soybean oil (SO) | Carnauba wax (CW) and | 50% by bovine fat | Acceptable texture profile, color and organoleptic characteristics, comparable with the un-substituted product. | [81] |
| 2% CW reinforced with 4% AA for improving the thermal behaviour and crystallinity, dissolved in SO at 150 °C | |||||
| Burgers | Olive oil (OO) | Pork skin (PS) | 100% by bovine backfat | Reduction of 80% in fat content, 35% decrease in energy value, 15% higher protein content and a better fatty acid profile. After processing at 180 °C, the hardness and chewiness, sensory characteristics and overall acceptability proved high and comparable to the control. Better oxidative stability than the control over 7 days at 4 °C. | [116] |
| PS in coarse powder form:deionized water:OO 1:3:1, mixed under heating at 100 °C | |||||
| Pork burgers | Mixture of olive oil (44.39%), linseed oil (37.87%) and fish oil (17.74%) | Ethyl cellulose (EC) Beeswax (BW) | 6% by pork backfat | Acceptable technological properties and good sensory acceptability. Decrease of lipid oxidation during storage or cooking due to the addition of curcumin, but reduced sensory acceptance. Regarding the use of EC in the present combination, further studies are needed to reduce lipid oxidation during refrigeration and cooking and to increase the sensory acceptability of burgers. | [117] |
| 11% EC or 11% BW, incorporated in the oil mixture while adding or unadding 0.2% curcumin as antioxidant | |||||
| Cooked meat batters | Soybean oil (SO) | Mixture of Ethyl cellulose, Avicel RC-591 and α-cellulose 67.0:16.5:16.5 | 100% by pork backfat | Considerably improved polyunsaturated fatty acids profile of meat batters, decreased lipid oxidation, with unaffected texture and acceptance. Darker and less red color than the control, but more yellow due to the presence of SO. | [118] |
| 11% celluloses mixture and 3.67% of Span® 60 as surfactant (w/w), dissolved in 85.33% SO at 120 °C | |||||
| Meat patties | Canola oil (CO) | Hydroxypropyl methylcellulose (HPMC) | 50% and 100% by beef tallow | Enhanced quality attributes, such as lower cooking loss, softer texture and reduced fatty acids levels from 42% to 15%, by replacing 50% of the beef tallow; lowered saturated to unsaturated fat ratio, from 0.73 to 0.18. | [119] |
| 2, 4, 6% (w/w) of 1% HPMC aqueous solution in the form of freeze-dried and grinded foam, added into CO and sheared | |||||
| Beef heart patties | Rapeseed oil (RO) | Beeswax (BW) | 100% by beef fat | Improved nutritional, fatty acid profile and cooking loss by incorporating the 10% BW oleogel; decreased hardness and oxidative stability during cold storage. | [120] |
| 2.5, 5, 7.5, 10, 12.5% BW (w/w), dissolved in RO at 90 °C | |||||
| Meat-based pâté | Linseed oil (LO) | Beeswax (BW) | 30% and 60% by pork subcutaneous fat | Improved nutritional value by the increased polyunsaturated fatty acids content and decreased omega-6/omega-3 ratio up to 90%. Decreased hardness and adhesivity. | [121] |
| 8% BW (w/w), dispersed in LO at 80 °C | |||||
| Frankfurters | Soybean oil (SO) | Rice bran wax (RBW) | 100% by pork backfat | Acceptable technological quality in terms of emulsion stability, cook/chill yields and oxidation stability. No substantial differences for adhesiveness, cohesiveness, and chewiness in relation to the control. Significantly reduced flavor. | [122] |
| 2.5% and 10% RBW (w/w), mixed with SO at 90 °C | |||||
| Chicken-based bologna sausages | High-oleic oil (HOSO) and conventional soybean oil (CSO) | Rice bran wax (RBW) | 100% by pork backfat | Similar quality and organoleptic properties between formulated sausages, when used HOSO and CSO oleogels. Higher nutritional value when used HOSO oleogel. | [123] |
| 10% and 2.5% RBW, mixed with 90% and 97.5% HOSO or CSO at 90 °C | |||||
| Bologna sausages | Conventional sunflower oil (SFO) or high oleic sunflower oil (HOSO) | Glyceryl monostearate (GM) | 25, 50, 75 and 100% by pork fat | Stable emulsions and good sensory acceptance of the sausages, with no significant differences between treatments. Higher level of unsaturated fatty acids and a more compact structure that affected the sliceability. Reducing the pork fat by 50% proved to be the best option, which not affected the hardness of the sausages. | [124] |
| 5% GM (w/w), mixed with SFO or HOSO at 90 °C | |||||
| Thai sweet sausages | Rice bran oil (RBO) | Rice bran wax (RBW) | 25%, 50%, and 75% by total fat | Reduction in total content of saturated fat and cholesterol, but increased softness of sausages. Replacing the 50% of fat with RBW oleogel showed the highest score of overall acceptance. | [125] |
| RBW and RBO, mixed at the ratio 2:100 g (w/w) at 80 °C | |||||
| Fermented sausages | Linseed oil (LO) | Mixture of γ-oryzanol and β-sitosterol (60:40 w/w) Beeswax (BW) | 20% and 40% by pork backfat | Substantial quality changes in terms of pH, color, texture. Improvement of polyunsaturated fatty acid/saturated fatty acid and n-6/n-3 ratios. The textural parameters of formulated sausages need to be improved for a better acceptability. | [126] |
| 8% oleogelators (w/w), dissolved in LO at 80 °C | |||||
| Fermented pork sausages | Olive oil (OO) | Monoglycerides (MG) | 50% by pork backfat (in addition, 50% of NaCl was replaced by KCl) | Significant changes in the physicochemical and microbiological properties of the sausages. Acceptable sensory attributes and a healthier nutritional profile than the control. Additional studies are needed for improving the sensory characteristics and consumer acceptability. | [47] |
| 15% MG, dissolved in OO at 90–95 °C | |||||
| Frankfurters | Canola oil (CO) | Ethyl cellulose (EC) | Substitution of beef fat so as to obtain 18.2% fat provided by oleogel in meat batters | Lower sensory hardness when adding 1.5% or 3.0% SMS than the sample with 0.0% SMS, at the low EC levels. Similarity between the oleogel sample with 8% EC and the control and significantly increased hardness at higher EC concentrations. The acceptance of the product unaltered by the replacements. | [127] |
| 8, 10, 12, 14% EC; 8, 10, 12, 14% EC and 1.5% SMS; 8, 10, 12, 14% EC and 3.0% SMS, mixed with CO at 140 °C | |||||
| Breakfast sausages | Canola oil (CO) | Ethyl cellulose (EC) | Substitution of pork fat so as to obtain 20.8% fat provided by oleogel in sausages. 8% rusk was added | Similar objective hardness of the most SMS oleogels compared with the pork fat control sample. No rancidity and chemical taste for the final product, prevented by the addition of BHT and rosemary oleoresin to CO. No changes in the water and fat contents during the heating of sausages, due to the presence of the rusk that contributed to their binding. | [128] |
| 8, 10, 12, 14% EC and 1.5, 3.0% SMS, mixed with CO added with 50 ppm BHT and 0.6% rosemary oleoresin, for preventing heat-induced oxidation of the oil, at 170 °C | |||||
| Frankfurters | Canola Oil (CO) | Ethyl cellulose (EC) | 20%, 40%, 60%, and 80% by beef fat | Diminished hardness of the frankfurters compared to the control, but similar shear forces for all samples. No differences detected by sensory analysis compared to the control. Decreased water loss and smokehouse yield. | [129] |
| 8% EC and 1.5% SMS; 8% EC and 3.0% SMS; 10% EC and 1.5% SMS, mixed with CO at 170 °C | |||||
| Sucuk | Flaxseed oil (FO) | Sunflower wax (SFW) Beeswax (BW) | Oleogels were included at 17.17% in the same recipe as the control containing tallow fat | Significantly higher concentrations of unsaturated fatty acids content, but lower texture and sensory attributes than the control, such as hardness, chewiness, juiciness, fattiness, aroma, and flavor. | [130] |
| 10% SFW or BW (w/w), dissolved in FO at 80 °C | |||||
| Liver pâté | Canola oil (CO) | Ethyl cellulose (EC) and Glycerol monostearate (GMS) | 20%, 40%, 60%, 80%, 100% by lard | The hardness, oiliness, cohesiveness, and perceived off-flavors undifferentiated in relation to the control for all samples. The replacement of 60% lard is recommended, in terms of oil retention, proper texture, color, and due to the reduced saturated fat content of about 40%. | [131] |
| 12% EC and 3% GMS, mixed with CO at 140 °C | |||||
| Pâtés | Mixture of olive (44.39%), linseed (37.87%), and fish (17.74%) oils | Ethyl cellulose (EC) Beeswax (BW) | Partial or total substitution of pork backfat so as to obtain 15% fat content pâtés | Optimal fatty acid profile (high PUFA/SFA and low n-6/n-3 ratios), but a significantly increased lipid oxidation. Insignificantly affected emulsion stability, color and texture, compared to the control. The sensory characteristics not significantly affected by using BW oleogel, but a negative effect on sensorial properties when using EC oleogel, in direct relation with the substitution level. | [111] |
| 11% EC and 3.67% SMS, mixed with oils at 160 °C 11% BW, mixed with oils at 65 °C | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perța-Crișan, S.; Ursachi, C.-Ș.; Chereji, B.-D.; Munteanu, F.-D. Oleogels—Innovative Technological Solution for the Nutritional Improvement of Meat Products. Foods 2023, 12, 131. https://doi.org/10.3390/foods12010131
Perța-Crișan S, Ursachi C-Ș, Chereji B-D, Munteanu F-D. Oleogels—Innovative Technological Solution for the Nutritional Improvement of Meat Products. Foods. 2023; 12(1):131. https://doi.org/10.3390/foods12010131
Chicago/Turabian StylePerța-Crișan, Simona, Claudiu-Ștefan Ursachi, Bianca-Denisa Chereji, and Florentina-Daniela Munteanu. 2023. "Oleogels—Innovative Technological Solution for the Nutritional Improvement of Meat Products" Foods 12, no. 1: 131. https://doi.org/10.3390/foods12010131





