Protein Requirements of Oncorhynchus mykiss Cultured in the Convection-Water Cages by Evaluating Growth, Body Composition and Liver Health
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Diets
2.2. Feeding Trial
2.3. Sampling
2.4. Chemical Analysis
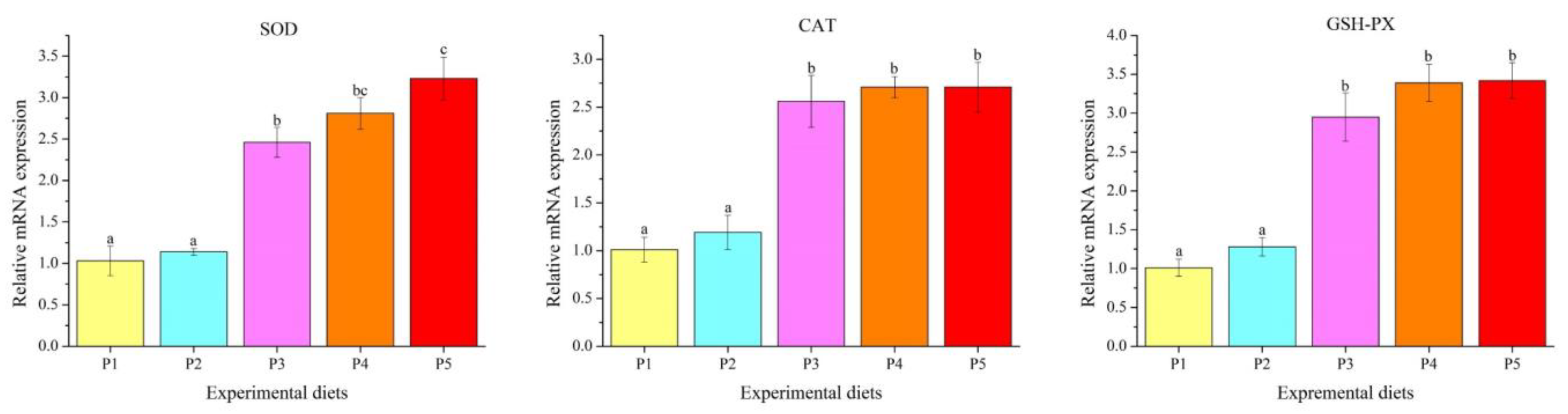
2.5. Anti-Oxidative Parameters Analysis
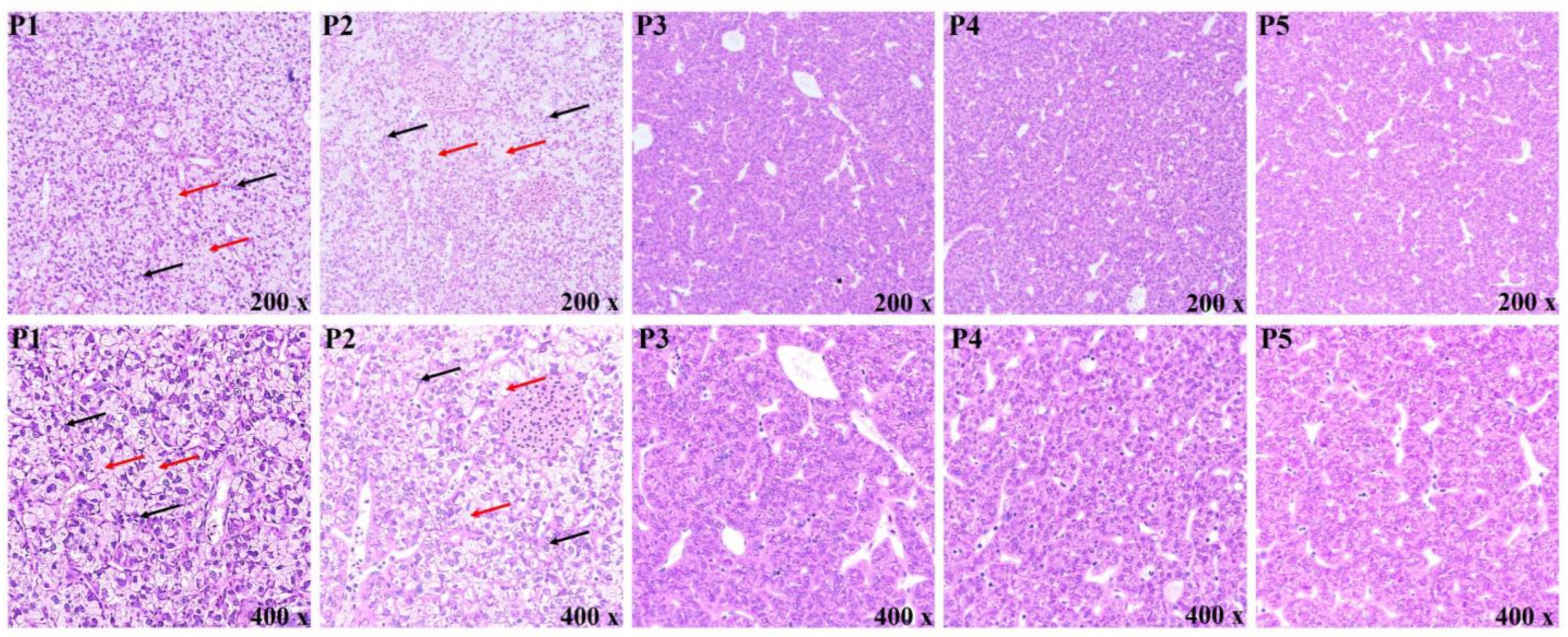
2.6. Liver Morphology Analysis
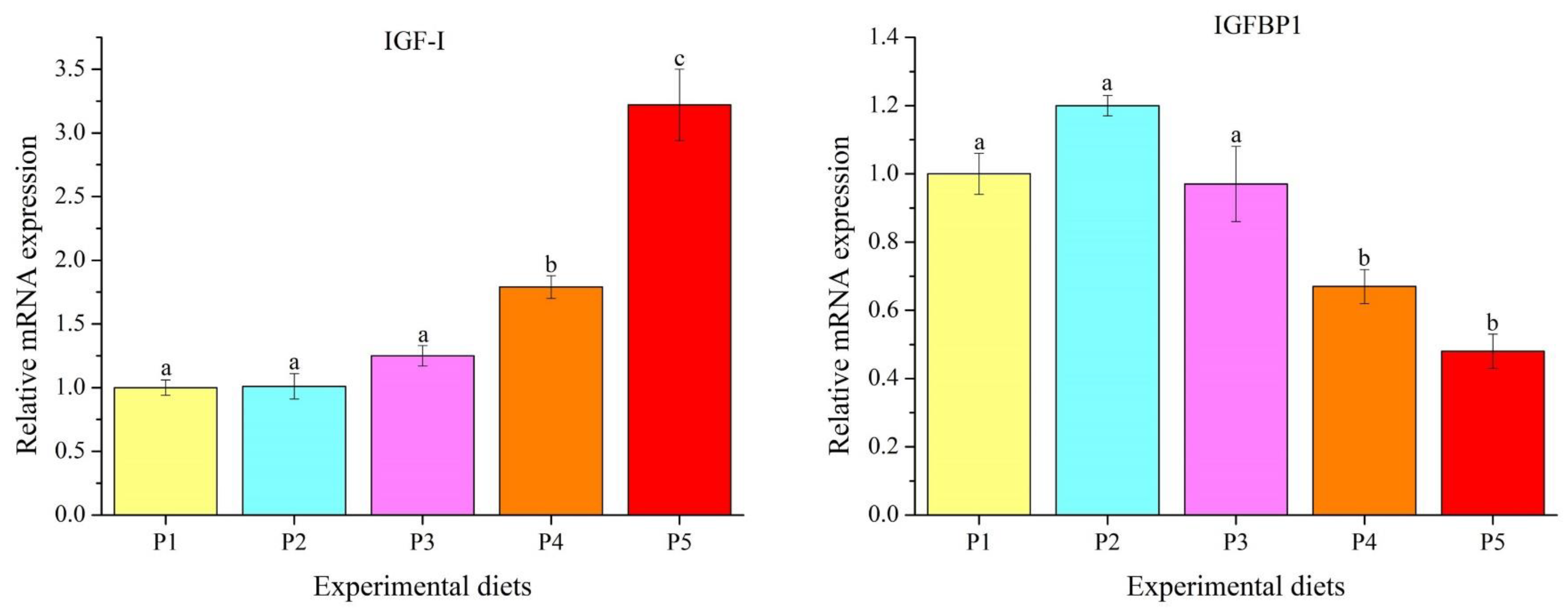
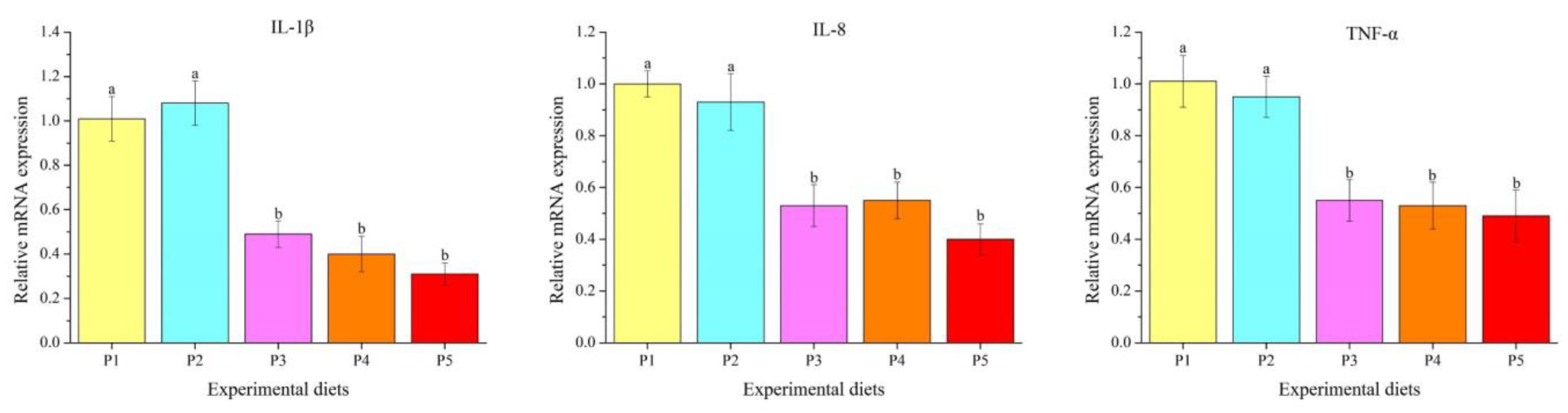
2.7. Gene Expression Analysis
2.8. Calculations and Statistical Analysis
3. Results
3.1. Biological Performance
3.2. Proximate Compositions of the Whole Body
3.3. Antioxidant Parameters
3.4. Morphological Observation of the Liver
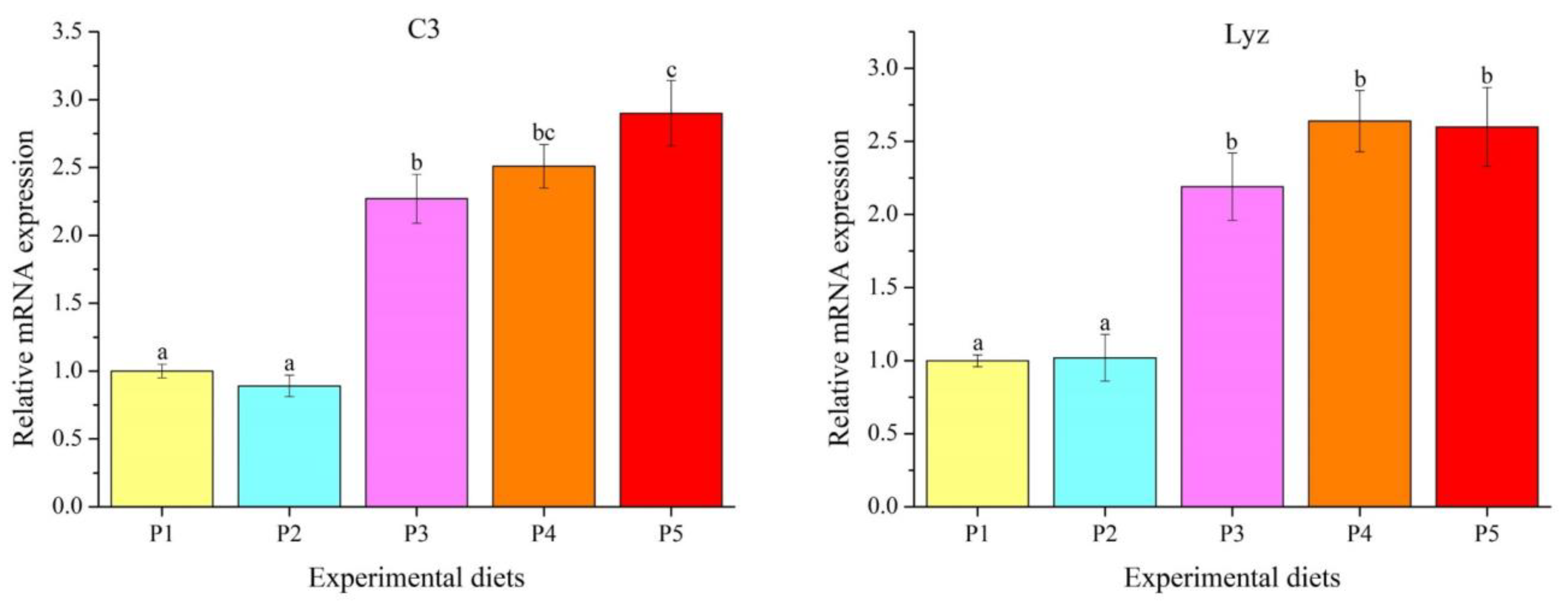
3.5. Genes Transcription Levels
4. Discussion
4.1. Growth and Feed Utilization
4.2. Proximate Composition of the Whole Body
4.3. Liver Morphology and Inflammatory Response
4.4. Antioxidation Property
4.5. Innate Immune Response
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Content Statement
Data Availability Statement
Conflicts of Interest
References
- Mohanta, K.N.; Rath, S.C.; Nayak, K.C.; Pradhan, C.; Mohanty, T.K.; Giri, S.S. Effect of restricted feeding and refeeding on compensatory growth, nutrient utilization and gain, production performance and whole body composition of carp cultured in earthen pond. Aquac. Nutr. 2017, 23, 460–469. [Google Scholar] [CrossRef]
- Liu, G.; Wang, L.; Yu, H.; Pan, M.; Yang, M.; Zhang, W.; Mai, K. Effects of dietary protein levels on growth performance, digestibility, anti-oxidative responses and expressions of growth-related genes in triploid rainbow trout Oncorhynchus mykiss farmed in seawater. Aquac. Nutr. 2021, 27, 998–1008. [Google Scholar] [CrossRef]
- Singha, K.P.; Shamna, N.; Sahu, N.P.; Sardar, P.; Harikrishna, V.; Thirunavukkarasar, R.; Kumar, M.; Krishna, G. Feeding graded levels of protein to Genetically Improved Farmed Tilapia (GIFT) juveniles reared in inland saline water: Effects on growth and gene expression of IGFeI, IGF-IR and IGF-BPI. Aquaculture 2020, 525, 735306. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, L.; Jiang, W.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.; Tang, L.; Tang, W.; Zhang, Y.; et al. Effects of dietary protein levels on the disease resistance, immune function and physical barrier function in the gill of grass carp (Ctenopharyngodon idella) after challenged with Flavobacterium columnare. Fish Shellfish Immunol. 2016, 57, 1–16. [Google Scholar] [CrossRef]
- Yadata, G.W.; Ji, K.; Liang, H.; Ren, M.; Ge, X.; Yang, Q. Effects of dietary protein levels with various stocking density on growth performance, whole body composition, plasma parameters, nitrogen emission and gene expression related to TOR signaling of juvenile blunt snout bream (Megalobrama ambylcephala). Aquaculture 2020, 519, 734730. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation, Rome. Available online: https://doi.org/10.4060/cc0461en (accessed on 1 October 2022).
- NRC (National Research Council). Nutrient Requirements of Fish and Shrimp; National Academies Press: Washington, DC, USA, 2011. [Google Scholar] [CrossRef]
- Eliason, E.J.; Higgs, D.A.; Farrell, A. Effect of isoenergetic diets with different protein and lipid content on the growth performance and heat increment of rainbow trout. Aquaculture 2007, 272, 723–736. [Google Scholar] [CrossRef]
- Ma, R.; Liu, X.; Meng, Y.; Wu, J.; Zhang, L.; Han, B.; Qian, K.; Luo, Z.; Wei, Y.; Li, C. Protein nutrition on sub-adult triploid rainbow trout (1): Dietary requirement and effect on anti-oxidative capacity, protein digestion and absorption. Aquaculture 2019, 507, 428–434. [Google Scholar] [CrossRef]
- Alami-Durante, H.; Cluzeaud, M.; Duval, C.; Maunas, P.; Girod-David, V.; Medale, F. Early decrease in dietary protein:energy ratio by fat addition and ontogenetic changes in muscle growth mechanisms of rainbow trout: Short- and long-term effects. Brit. J. Nutr. 2014, 112, 674–687. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists, Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1995. [Google Scholar]
- Plummer, P. Glycogen determination in animal tissues. In An Introduction to Practical Biochemistry, 3rd ed.; McGraw Hill Book: Maidenhead, UK, 1987; p. 332. [Google Scholar]
- Garling, J.D.L.; Wilson, R.P. Optimum dietary protein to energy ratio for channel catfish fingerlingsl, Ictalurus punctatus. J. Nutr. 1976, 106, 1368–1375. [Google Scholar] [CrossRef]
- Zhao, W.; Fang, H.H.; Gao, B.Y.; Dai, C.M.; Liu, Z.Z.; Zhang, C.W.; Niu, J. Dietary Tribonema sp. supplementation increased growth performance, antioxidant capacity, immunity and improved hepatic health in golden pompano (Trachinotus ovatus). Aquaculture 2020, 529, 735667. [Google Scholar] [CrossRef]
- Zhao, W.; Wei, H.; Chen, M.; Yao, R.; Wang, Z.; Niu, J. Effects of synthetic astaxanthin and Haematococcus pluvialis on growth, antioxidant capacity, immune response, and hepato-morphology of Oncorhynchus mykiss under cage culture with flowing freshwater. Aquaculture 2023, 562, 738860. [Google Scholar] [CrossRef]
- Lesiow, T.; Ockerman, H.W.; Dabrowski, K. Composition, properties and sensory quality of rainbow trout affected by feed formulations. J. World Aquacult. Soc. 2009, 40, 678–686. [Google Scholar] [CrossRef]
- Eya, J.C.; Yossa, R.; Ashame, M.F.; Pomeroy, C.F.; Gannam, A.L. Effects of dietary lipid levels on growth, feed utilization and mitochondrial function in low- and high-feed efficient families of rainbow trout (Oncorhynchus mykiss). Aquaculture 2013, 416, 119–128. [Google Scholar] [CrossRef]
- Ahmed, I.; Ahmad, I. Effect of dietary protein levels on growth performance, hematological profile and biochemical composition of fingerlings rainbow trout, Oncorhynchus mykiss reared in Indian himalayan region. Aquac. Rep. 2020, 16, 100268. [Google Scholar] [CrossRef]
- Yan, J.; Li, Y.; Liang, X.; Zhang, Y.; Dawood, M.A.O.; Matuli’c, D.; Gao, J. Effects of dietary protein and lipid levels on growth performance, fatty acid composition and antioxidant-related gene expressions in juvenile loach Misgurnus anguillicaudatus. Aquac. Res. 2017, 48, 5385–5393. [Google Scholar] [CrossRef]
- Velazco-vargas, J.; Tomás-vidal, A.; Hamdan, M.; López, F.J.M.; Cerda, M.J.; Martínez-llorens, S. Influence of digestible protein levels on growth and feed utilization of juvenile meagre Argyrosomus regius. Aquac. Nutr. 2014, 20, 520–531. [Google Scholar] [CrossRef]
- Tanaka, H.; Oishi, G.; Nakano, Y.; Mizuta, H.; Nagano, Y.; Hiramatsu, N.; Ando, H.; Shimizu, M. Production of recombinant salmon insulin-like growth factor binding protein-1 subtypes. Gen. Comp. Endocr. 2018, 257, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Pierce, A.L.; Breves, J.P.; Moriyama, S.; Hirano, T.; Grau, E.G. Differential regulation of Igf1 and Igf2 mRNA levels in tilapia hepatocytes: Effects of insulin and cortisol on GH sensitivity. J. Endocrinol. 2011, 211, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Wheatcroft, S.B.; Kearney, M.T. IGF-dependent and IGF-independent actions of IGF-binding protein-1 and -2: Implications for metabolic homeostasis. Trends Endocr. Met. 2009, 20, 153–162. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, S.; Cleveland, B.M.; Romano, N.; Lalgudi, R.S.; Benito, M.R.; Hardy, R.W. Comparative evaluation of processed soybean meal (EnzoMealTM) vs. regular soybean meal as a fishmeal replacement in diets of rainbow trout (Oncorhynchus mykiss): Effects on growth performance and growth-related genes. Aquaculture 2020, 516, 734652. [Google Scholar] [CrossRef]
- Madison, B.N.; Tavakoli, S.; Kramer, S.; Bernier, N.J. Chronic cortisol and the regulation of food intake and the endocrine growth axis in rainbow trout. J. Endocr. 2015, 226, 103–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Lu, S.; Wu, M.; Yao, W.; Jin, Z.; Wu, X. Effects of dietary protein levels on growth, feed utilization and expression of growth related genes of juvenile giant grouper (Epinephelus lanceolatus). Aquaculture 2019, 504, 369–374. [Google Scholar] [CrossRef]
- Wilson, R.P. Amino acids and proteins. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: Cambridge, MA, USA, 2002; pp. 143–179. [Google Scholar]
- Nguyen, M.C.; Fotedar, R.; Pham, H.D. Effects of dietary protein and lipid levels on growth performance, feed utilization and body composition of juvenile giant trevally (Caranx ignobilis Forsskal, 1775). Aquac. Res. 2022, 53, 6254–6263. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K. Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes). Aquaculture 2009, 287, 219–222. [Google Scholar] [CrossRef]
- Liu, W.; Wen, H.; Luo, Z. Effect of dietary protein levels and feeding rates on the growth and health status of juvenile genetically improved farmed tilapia (Oreochromis niloticus). Aquacult. Int. 2018, 26, 153–167. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Yu, H.; Su, Y.; Ren, M.; Zhu, J.; Ge, X. Serum biochemistry, liver histology and transcriptome profiling of bighead carp Aristichthys nobilis following different dietary protein levels. Fish Shellfish Immunol. 2019, 86, 832–839. [Google Scholar] [CrossRef]
- Camargo, M.M.P.; Martinez, C.B.R. Histopathology of gills, kidney and liver of a Neotropical fish caged in an urban stream. Neotrop. Ichthyol. 2007, 5, 327–336. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, W.S.; Alshiraihi, I.; Al-karim, S. Effect of maternal low protein diet during pregnancy on the fetal liver of rats. Ann. Anat. 2013, 195, 68–76. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, S.K.; Kim, M.S.; Cho, E.Y.; Lee, J.H.; Lee, K.U.; Pak, Y.K.; Lee, H.K. Fetal and early postnatal protein malnutrition cause long-term changes in rat liver and muscle mitochondria. J. Nutr. 2003, 133, 3085–3090. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, D.; Topczewski, F.; Pagliassotti, M.J. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. Am. J. Physiol. Endocrinol. Metab. 2006, 291, 275–281. [Google Scholar] [CrossRef]
- Zhao, W.; Cui, X.; Wang, Z.; Yao, R.; Xie, S.; Gao, B.; Zhang, C.; Niu, J. Beneficial changes in growth performance, antioxidant capacity, immune response, hepatic health, and flesh quality of Trachinotus ovatus fed with Oedocladium carolinianum. Front. Immunol. 2022, 13, 940929. [Google Scholar] [CrossRef] [PubMed]
- Ni, P.; Jiang, W.D.; Wu, P.; Liu, Y.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; Feng, L. Dietary low or excess levels of lipids reduced growth performance, and impaired immune function and structure of head kidney, spleen and skin in young grass carp (Ctenopharyngodon idella) under the infection of Aeromonas hydrophila. Fish Shellfish Immunol. 2016, 55, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Yousefi, S.; Doan, H.V.; Ashouri, G.; Gioacchini, G.; Maradonna, F.; Carnevali, O. Oxidative stress and antioxidant defense in fish: The implications of probiotic, prebiotic, and synbiotics. Rev. Fish. Sci. Aquac. 2021, 29, 198–217. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fisher. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Jiang, J.; Wu, P.; Chen, G.; Jiang, W.; Li, S.; Tang, L.; Kuang, S.; Feng, L.; et al. Effects of dietary isoleucine on growth, the digestion and absorption capacity and gene expression in hepatopancreas and intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture 2012, 368, 117–128. [Google Scholar] [CrossRef]
- Xu, J.; Wu, P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; Zhou, X.Q.; et al. Optimal dietary protein level improved growth, disease resistance, intestinal immune and physical barrier function of young grass carp (Ctenopharyngodon idella). Fish and Shellfish Immunol. 2016, 55, 64–87. [Google Scholar] [CrossRef]
- Yengkokpam, S.; Debnath, D.; Sahu, N.P.; Pal, A.K.; Jain, K.K.; Baruah, K. Dietary protein enhances non-specific immunity, anti-oxidative capability and resistance to Aeromonas hydrophila in Labeo rohita fingerlings pre-exposed to short feed deprivation stress. Fish Shellfish Immunol. 2016, 59, 439–446. [Google Scholar] [CrossRef]
- Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, 1006512. [Google Scholar] [CrossRef] [Green Version]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Jiang, W.D.; Xu, J.; Zhou, X.Q.; Wu, P.; Liu, Y.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary protein levels regulated antibacterial activity, inflammatory response and structural integrity in the head kidney, spleen and skin of grass carp (Ctenopharyngodon idella) after challenged with Aeromonas hydrophila. Fish Shellfish Immunol. 2017, 68, 154–172. [Google Scholar] [CrossRef]

| Ingredients | P1 | P2 | P3 | P4 | P5 |
|---|---|---|---|---|---|
| Fish meal | 180 | 220 | 260 | 300 | 340 |
| Soybean meal | 170 | 170 | 170 | 170 | 170 |
| Hermetia illucens meal | 100 | 100 | 100 | 100 | 100 |
| Soy protein concentrate | 40 | 40 | 40 | 40 | 40 |
| Wheat flour | 110 | 110 | 110 | 110 | 110 |
| Bone meal a | 156.9 | 119.7 | 82.6 | 45.3 | 8.1 |
| Krill meal | 30 | 30 | 30 | 30 | 30 |
| Chicken meal | 35 | 40 | 45 | 50 | 55 |
| Fish oil | 76.5 | 73.0 | 69.4 | 65.9 | 62.4 |
| Soybean lecithin | 20 | 20 | 20 | 20 | 20 |
| Ca(H2PO4)2 | 10 | 10 | 10 | 10 | 10 |
| Vitamin premix b | 10 | 10 | 10 | 10 | 10 |
| Mineral premix c | 10 | 10 | 10 | 10 | 10 |
| Choline | 5 | 5 | 5 | 5 | 5 |
| Vitamin C | 5 | 5 | 5 | 5 | 5 |
| DL-Met | 9.1 | 8.3 | 7.5 | 6.7 | 5.9 |
| Lys-HCL (99%) | 15.5 | 13.2 | 11.0 | 8.8 | 6.6 |
| Thr | 6.9 | 5.7 | 4.4 | 3.2 | 1.9 |
| Inositol | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Sodium alginate | 10 | 10 | 10 | 10 | 10 |
| Total | 1000 | 1000 | 1000 | 1000 | 1000 |
| Nutrient levels d | |||||
| Crude lipid | 125.1 | 123.7 | 125.4 | 124.8 | 126.6 |
| Crude protein | 356.3 | 383.9 | 411.5 | 439.2 | 466.8 |
| Moisture | 93.2 | 94.9 | 93.8 | 95.1 | 95.2 |
| Carbohydrate | 98.5 | 97.9 | 97.3 | 99.8 | 96.8 |
| Estimated energy (KJ/g) | 12.30 | 12.70 | 13.21 | 13.69 | 14.17 |
| Gene | Primer Sequence (5′ to 3′) | Genbank No. |
|---|---|---|
| SOD-F | TGAAGGCTGTTTGCGTGCTGAC | NM_001160614.1 |
| SOD-R | CCGTTGGTGTTGTCTCCGAAGG | |
| CAT-F | CCGTCCTTCGTCCACTCTCAGA | XM_021564302.2 |
| CAT-R | CTCGGCATCCTCAGGCTTCAAG | |
| GSH-PX-F | TCATCATGTGGAGCCCTGTCTG | AF281338.1 |
| GSH-PX-R | TCTGCCTCAATGTCACTGGTCA | |
| IGFBP1-F | GGAGAAGCTGGATGAATGCC | NM_001124561.1 |
| IGFBP1-R | GGTCTAGGATCCCCAGCTCTTG | |
| IGF-I-F | TGCGTCCTAACCCTGACTTCG | M95183.1 |
| IGF-I-R | GCAGCACTCGTCCACAATACC | |
| IL-1β-F | ACGGTTCGCTTCCTCTTCTACA | AJ245925.2 |
| IL-1β-R | GCTCCAGTGAGGTGCTGATGAA | |
| IL-8-F | GTCAGCCAGCCTTGTCGTTGT | NM_001124362.1 |
| IL-8-R | CGTCTGCTTTCCGTCTCAATGC | |
| TNF-α-F | GGCGAGCATACCACTCCTCTGA | NM_001124362.1 |
| TNF-α-R | AGCTGGAACACTGCACCAAGGT | |
| Lyz-F Lyz-R | GAAACAGCCTGCCCAACT GTCCAACACCACACGCTT | AF452171.1 |
| C3-F | GGCCAGTCCCTGGTGGTTA | XM_036955530.1 |
| C3-R | GGTGGACTGTGTGGATCCGTA | |
| β-actin-F | TACAACGAGCTGAGGGTGGC | AJ438158.1 |
| β-actin-R | GGCAGGGGTGTTGAAGGTCT |
| Dietary Protein Levels | ||||||||
|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | ANOVA | Linear | Quadratic | |
| IBW (g) | 15.88 ± 0.36 | 15.58 ± 0.37 | 15.67 ± 0.24 | 15.88 ± 0.18 | 16.09 ± 0.21 | 0.743 | 0.416 | 0.390 |
| FBW (g) | 55.66 ± 1.18 a | 56.11 ± 0.66 a | 55.57 ± 0.64 a | 60.72 ± 1.27 b | 64.50 ± 1.52 c | 0.001 | 0.000 | 0.000 |
| WGR (%) | 253.12 ± 8.07 a | 259.04 ± 14.38 a | 255.13 ± 9.99 a | 286.14 ± 4.51 bc | 306.11 ± 12.48 c | 0.018 | 0.002 | 0.003 |
| SGR (%/d) | 2.25 ± 0.04 a | 2.28 ± 0.08 a | 2.26 ± 0.05 a | 2.41 ± 0.02 bc | 2.50 ± 0.05 c | 0.020 | 0.002 | 0.003 |
| SR (%) | 98.33 ± 1.67 | 99.17 ± 0.83 | 97.50 ± 2.50 | 98.33 ± 0.96 | 98.33 ± 0.96 | 0.791 | 0.741 | 0.935 |
| FCR | 1.31 ± 0.03 a | 1.31 ± 0.08 a | 1.31 ± 0.02 a | 1.19 ± 0.04 bc | 1.08 ± 0.03 c | 0.012 | 0.002 | 0.001 |
| Item | Moisture | Crude Protein | Crude Lipid |
|---|---|---|---|
| P1 | 65.79 ± 0.44 | 47.90 ± 0.89 a | 31.07 ± 0.22 |
| P2 | 64.82 ± 0.52 | 48.18 ± 1.12 a | 31.28 ± 0.43 |
| P3 | 66.55 ± 1.14 | 48.86 ± 0.28 a | 31.84 ± 0.31 |
| P4 | 66.15 ± 0.71 | 50.02 ± 0.28 a | 31.78 ± 0.58 |
| P5 | 65.53 ± 1.67 | 52.64 ± 0.44 b | 32.03 ± 0.13 |
| ANOVA | 0.790 | 0.004 | 0.369 |
| Linear | 0.792 | 0.000 | 0.039 |
| Quadratic | 0.897 | 0.000 | 0.119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Guo, Y.-C.; Yao, R.; Chen, A.-Q.; Chen, B.-Y.; Niu, J. Protein Requirements of Oncorhynchus mykiss Cultured in the Convection-Water Cages by Evaluating Growth, Body Composition and Liver Health. Foods 2023, 12, 175. https://doi.org/10.3390/foods12010175
Zhao W, Guo Y-C, Yao R, Chen A-Q, Chen B-Y, Niu J. Protein Requirements of Oncorhynchus mykiss Cultured in the Convection-Water Cages by Evaluating Growth, Body Composition and Liver Health. Foods. 2023; 12(1):175. https://doi.org/10.3390/foods12010175
Chicago/Turabian StyleZhao, Wei, Yu-Cai Guo, Rong Yao, An-Qi Chen, Bao-Yang Chen, and Jin Niu. 2023. "Protein Requirements of Oncorhynchus mykiss Cultured in the Convection-Water Cages by Evaluating Growth, Body Composition and Liver Health" Foods 12, no. 1: 175. https://doi.org/10.3390/foods12010175
APA StyleZhao, W., Guo, Y.-C., Yao, R., Chen, A.-Q., Chen, B.-Y., & Niu, J. (2023). Protein Requirements of Oncorhynchus mykiss Cultured in the Convection-Water Cages by Evaluating Growth, Body Composition and Liver Health. Foods, 12(1), 175. https://doi.org/10.3390/foods12010175







