Bioaccessibility and Bioavailability of (-)-Epigallocatechin Gallate in the Bread Matrix with Glycemic Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. The Making of Bread Samples
2.3. EGCG Extraction and Detection
2.4. Quantitative Detection of EGCG Using HPLC/DAD
2.5. Simulated Digestion In Vitro and Dialysis of Bread Samples
2.6. Detection of Released Reducing Sugars
2.7. Calculation of the EGCG Bioaccessibility and Bioavailability
2.8. EGCG Recovery Rate from Digesta
2.9. Mathematical Simulation of the Starch Digestion Kinetics
2.10. Assessment of Total Available Carbohydrates (TAC)
2.11. Calculation of pGI and pGL
2.12. Computational Molecular Docking
2.13. Statistical Analysis
3. Results and Discussion
3.1. Bread Starch In Vitro Digestibility
3.2. Mathematical Modeling of Starch Digestion Curves
3.3. Calculation of pGI and pGL
3.4. Bioaccessibility and Bioavailability of EGCG after Digestion
3.5. Computational Simulation of EGCG on α-Amylase and α-Glucosidase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ludwig, D.S. The Glycemic IndexPhysiological Mechanisms Relating to Obesity, Diabetes, and Cardiovascular Disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Koh, A.H.S.; Zhou, W. Enhancing health benefits of bakery products using phytochemicals. Adv. Food Nutr. Res. 2022, 99, 239–281. [Google Scholar] [PubMed]
- Van Loo-Bouwman, C.A.; Naber, T.H.; Minekus, M.; Van Breemen, R.B.; Hulshof, P.J.; Schaafsma, G. Food Matrix Effects on Bioaccessibility of β-Carotene Can be Measured in an in Vitro Gastrointestinal Model. J. Agric. Food Chem. 2014, 62, 950–955. [Google Scholar] [CrossRef]
- Sui, X.; Zhang, Y.; Zhou, W. Bread fortified with anthocyanin-rich extract from black rice as nutraceutical sources: Its quality attributes and in vitro digestibility. Food Chem. 2016, 196, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhou, W. Role of quercetin in the physicochemical properties, antioxidant and antiglycation activities of bread. J. Funct. Foods 2018, 40, 299–306. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.H.; David, B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods; Agricultural Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2011; Volume 3, pp. 98–103. [Google Scholar]
- Namal Senanayake, S.P.J. Green tea extract: Chemistry, antioxidant properties and food applications—A review. J. Funct. Foods 2013, 5, 1529–1541. [Google Scholar] [CrossRef]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Nutraceuticals for body-weight management: The role of green tea catechins. Physiol. Behav. 2016, 162, 83–87. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Lin, J.; Teo, L.M.; Leong, L.P.; Zhou, W. In vitro bioaccessibility and bioavailability of quercetin from the quercetin-fortified bread products with reduced glycemic potential. Food Chem. 2019, 286, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Goh, R.; Gao, J.; Ananingsih, V.K.; Ranawana, V.; Henry, C.J.; Zhou, W. Green tea catechins reduced the glycaemic potential of bread: An in vitro digestibility study. Food Chem. 2015, 180, 203–210. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Isabelle, M. Comparison study of the effect of green tea extract (GTE) on the quality of bread by instrumental analysis and sensory evaluation. Food Res. Int. 2007, 40, 470–479. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.; Jiang, X. Mathematical modeling of the stability of green tea catechin epigallocatechin gallate (EGCG) during bread baking. J. Food Eng. 2008, 87, 505–513. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; He, Q.; Wu, Y.; Chen, X.; Ning, Y.; Chen, Y. Investigating the Bioaccessibility and Bioavailability of Cadmium in a Cooked Rice Food Matrix by Using an 11-Day Rapid Caco-2/HT-29 Co-culture Cell Model Combined with an In Vitro Digestion Model. Biol. Trace Elem. Res. 2019, 190, 336–348. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Gao, J.; Tay, S.L.; Koh, A.H.-S.; Zhou, W. Dough and bread making from high- and low-protein flours by vacuum mixing: Part 3. Oral processing of bread. J. Cereal Sci. 2018, 79, 408–417. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioaccessibility, bioactivity and cell metabolism of dark chocolate phenolic compounds after in vitro gastro-intestinal digestion. J. Funct. Foods 2018, 49, 424–436. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Chong, J.E.L.; Lu, J.; Zhou, W. Fucoidan Regulates Starch Digestion: In Vitro and Mechanistic Study. Foods 2022, 11, 427. [Google Scholar] [CrossRef]
- Lau, E.; Soong, Y.Y.; Zhou, W.; Henry, J. Can bread processing conditions alter glycaemic response? Food Chem. 2015, 173, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A starch hydrolysis procedure to estimate glycemic index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Gomez, M. Gluten-free bakery products: Ingredients and processes. Adv. Food Nutr. Res. 2022, 99, 189–238. [Google Scholar] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yeo, I.K.X.; Guo, C.; Kai, Y.; Lu, Y.; Yang, H. Elucidating the inhibitory mechanism on polyphenol oxidase from mushroom and melanosis formation by slightly acid electrolysed water. Food Chem. 2022, 404 Pt A, 134580. [Google Scholar] [CrossRef]
- Forester, S.C.; Gu, Y.; Lambert, J.D. Inhibition of starch digestion by the green tea polyphenol, (-)-epigallocatechin-3-gallate. Mol. Nutr. Food Res. 2012, 56, 1647–1654. [Google Scholar] [CrossRef]
- Zhan, W.; Liu, Y.; Li, D.; Liu, Y. Advancing insights on the anti-obesity biochemical mechanism of (−)-epigallocatechin gallate (EGCG) by inhibiting α-amylase activity. RSC Adv. 2016, 6, 96918–96927. [Google Scholar] [CrossRef]
- Culetu, A.; Duta, D.E.; Andlauer, W. Influence of black tea fractions addition on dough characteristics, textural properties and shelf life of wheat bread. Eur. Food Res. Technol. 2018, 244, 1133–1145. [Google Scholar] [CrossRef]
- Naumovski, N.; Blades, B.L.; Roach, P.D. Food Inhibits the Oral Bioavailability of the Major Green Tea Antioxidant Epigallocatechin Gallate in Humans. Antioxidants 2015, 4, 373–393. [Google Scholar] [CrossRef]
- Yao, Y.; Tan, P.; Kim, J.E. Effects of dietary fats on the bioaccessibility and bioavailability of carotenoids: A systematic review and meta-analysis of in vitro studies and randomized controlled trials. Nutr. Rev. 2022, 80, 741–761. [Google Scholar] [CrossRef]
- Mizukami, Y.; Sawai, Y.; Yamaguchi, Y. Changes in the Concentrations of Acrylamide, Selected Odorants, and Catechins Caused by Roasting of Green Tea. J. Agric. Food Chem. 2008, 56, 2154–2159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Cao, C.; Cao, J.; Chen, W.; Zhang, Y.; Wang, C.; Wang, J.; Zhang, X.; Zhao, X. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutr. Cancer 2010, 62, 1120–1127. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape seed and tea extracts and catechin 3-gallates are potent inhibitors of alpha-amylase and alpha-glucosidase activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Toy, J.Y.H.; Seta, C.; Yeo, T.C.; Huang, D. Inhibition Effect of Extract of Psychotria viridiflora Stem on alpha-Amylase and alpha-Glucosidase and Its Application in Lowering the Digestibility of Noodles. Front. Nutr. 2021, 8, 701114. [Google Scholar] [CrossRef] [PubMed]

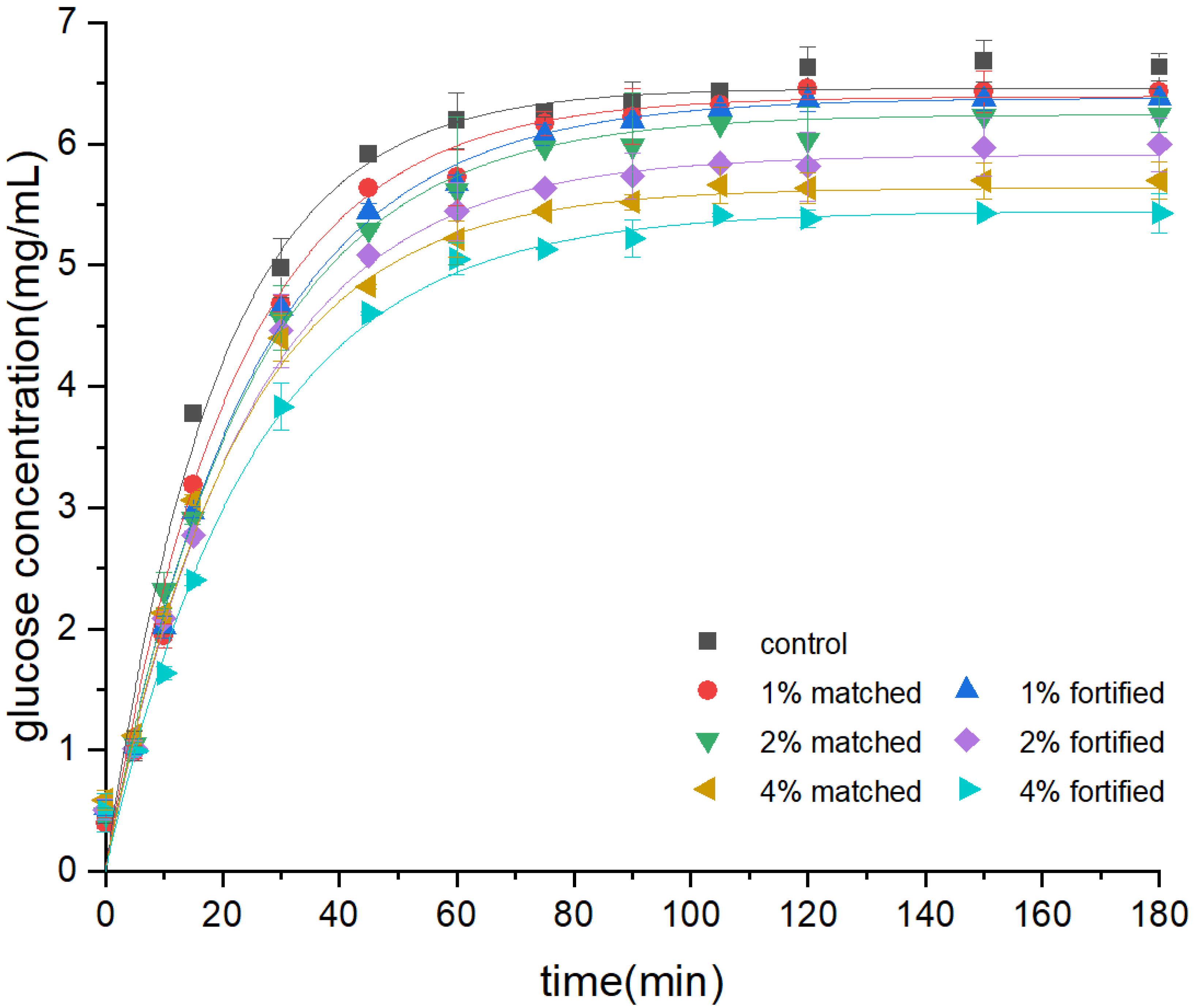

| Model | BoxLucas1 | Equation | |||||
|---|---|---|---|---|---|---|---|
| Plot | Control | 1% Matched | 1% Fortified | 2% Matched | 2% Fortified | 4% Matched | 4% Fortified |
| 6.4610 ± 0.0080 a | 6.3926 ± 0.0208 b | 6.3798 ± 0.0066 b | 6.2450 ± 0.0031 b | 5.9121 ± 0.0075 c | 5.6387 ± 0.0292 c | 5.4466 ± 0.0069 d | |
| 0.0524 ± 0.0027 a | 0.0459 ± 0.0013 b | 0.0410 ± 0.0007 b | 0.0419 ± 0.0003 b | 0.0417 ± 0.0007 c | 0.0449 ± 0.0017 c | 0.0396 ± 0.0009 d | |
| Reduced Chi2 | 2.3182 | 1.6722 | 1.3716 | 0.4497 | 0.7962 | 1.4566 | 3.2006 |
| R2 | 0.9996 | 0.9997 | 0.9998 | 0.9999 | 0.9998 | 0.9997 | 0.9996 |
| RMSE | 1.5225 | 1.2931 | 1.1711 | 0.6706 | 0.8923 | 1.2069 | 1.7890 |
| Control | 1% Matched | 1% Fortified | 2% Matched | 2% Fortified | 4% Matched | 4% Fortified | |
|---|---|---|---|---|---|---|---|
| AUC/g min 100 g−1 | 1039.68 ± 13.23 a | 1011.54 ± 15.08 b | 992.87 ± 16.89 b | 974.98 ± 16.55 b | 922.58 ± 16.60 c | 889.46 ± 15.43 c | 842.97 ± 17.48 d |
| HI/% | 100.00 ± 1.27 a | 97.29 ± 1.45 b | 95.50 ± 1.62 b | 93.78 ± 1.59 b | 88.74 ± 1.60 c | 85.55 ± 1.48 c | 81.08 ± 1.68 d |
| pGI bread/% | 100.00 ± 1.27 a | 98.43 ± 1.47 b | 97.39 ± 1.66 b | 96.39 ± 1.64 b | 93.46 ± 1.68 c | 91.62 ± 1.59 c | 86.83 ± 1.85 d |
| pGI glucose/% | 70.00 ± 0.89 a | 68.90 ± 1.03 b | 68.17 ± 1.16 b | 67.47 ± 1.15 b | 65.42 ± 1.18 c | 64.13 ± 1.11 c | 60.78 ± 1.29 d |
| pGL/% | 17.74 ± 0.23 a | 17.12 ± 0.26 b | 16.56 ± 0.28 b | 15.96 ± 0.27 b | 15.14 ± 0.27 c | 14.84 ± 0.26 c | 14.07 ± 0.29 d |
| Bread Type | EGCG Dosage | Retention Level of EGCG before the In Vitro Digestion/% | EGCG in the Dialysate/mg per 5 g Bread | EGCG in the Digestate/mg per 5 g Bread | Bioaccessibility/% | Bioavailability/% |
|---|---|---|---|---|---|---|
| fortified | 1% | 70.068 ± 0.459 c | 2.947 ± 0.002 c | 6.469 ± 0.040 c | 26.877 ± 1.725 c | 8.412 ± 0.068 c |
| 2% | 75.496 ± 0.378 b | 8.311 ± 0.025 b | 18.075 ± 0.028 b | 34.949 ± 0.740 b | 11.008 ± 0.651 b | |
| 4% | 79.314 ± 1.213 a | 22.715 ± 0.631 a | 49.306 ± 1.296 a | 45.402 ± 5.344 a | 14.320 ± 2.602 a | |
| matched | 1% | N/A | 2.703 ± 0.018 c | 6.540 ± 0.141 c | 18.486 ± 2.812 d | 5.406 ± 0.367 d |
| 2% | N/A | 8.997 ± 0.003 b | 17.880 ± 0.173 b | 26.877 ± 1.725 c | 8.997 ± 0.031 c | |
| 4% | N/A | 19.467 ± 0.107 a | 45.218 ± 0.736 a | 32.342 ± 3.681 b | 9.734 ± 0.535 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gao, J.; Koh, H.S.A.; Zhou, W. Bioaccessibility and Bioavailability of (-)-Epigallocatechin Gallate in the Bread Matrix with Glycemic Reduction. Foods 2023, 12, 30. https://doi.org/10.3390/foods12010030
Li L, Gao J, Koh HSA, Zhou W. Bioaccessibility and Bioavailability of (-)-Epigallocatechin Gallate in the Bread Matrix with Glycemic Reduction. Foods. 2023; 12(1):30. https://doi.org/10.3390/foods12010030
Chicago/Turabian StyleLi, Lanqi, Jing Gao, Hui Si Audrey Koh, and Weibiao Zhou. 2023. "Bioaccessibility and Bioavailability of (-)-Epigallocatechin Gallate in the Bread Matrix with Glycemic Reduction" Foods 12, no. 1: 30. https://doi.org/10.3390/foods12010030
APA StyleLi, L., Gao, J., Koh, H. S. A., & Zhou, W. (2023). Bioaccessibility and Bioavailability of (-)-Epigallocatechin Gallate in the Bread Matrix with Glycemic Reduction. Foods, 12(1), 30. https://doi.org/10.3390/foods12010030





