Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SAEW Preparation and Characterization of Physical and Chemical Properties
2.3. Processing of Raw Material
2.4. Optimization of SAEW Treatment Conditions
2.5. Analysis of Sample Quality during Storage
2.6. Statistical Analyses
3. Results and Discussion
3.1. Optimization of SAEW Treatment Conditions
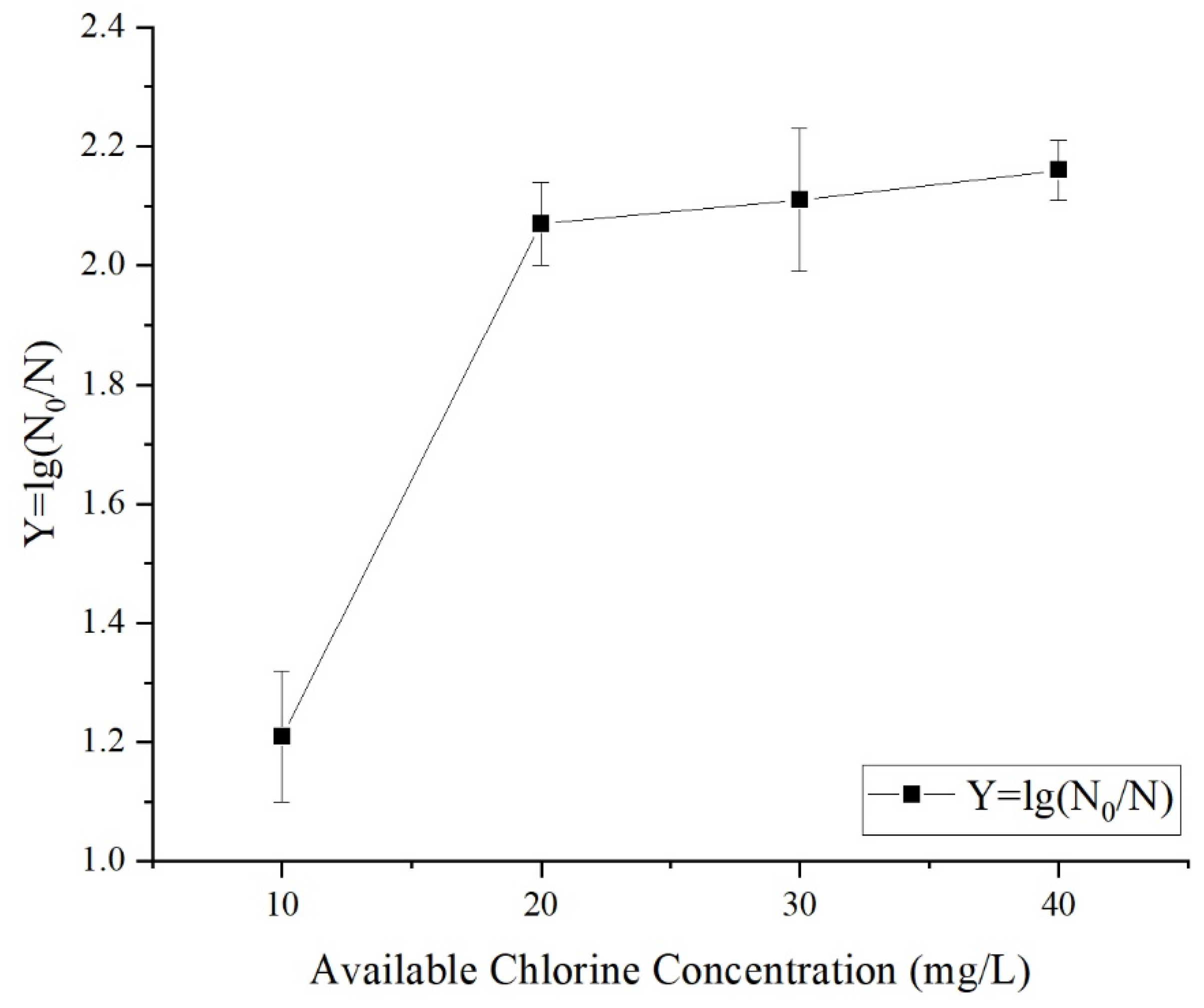
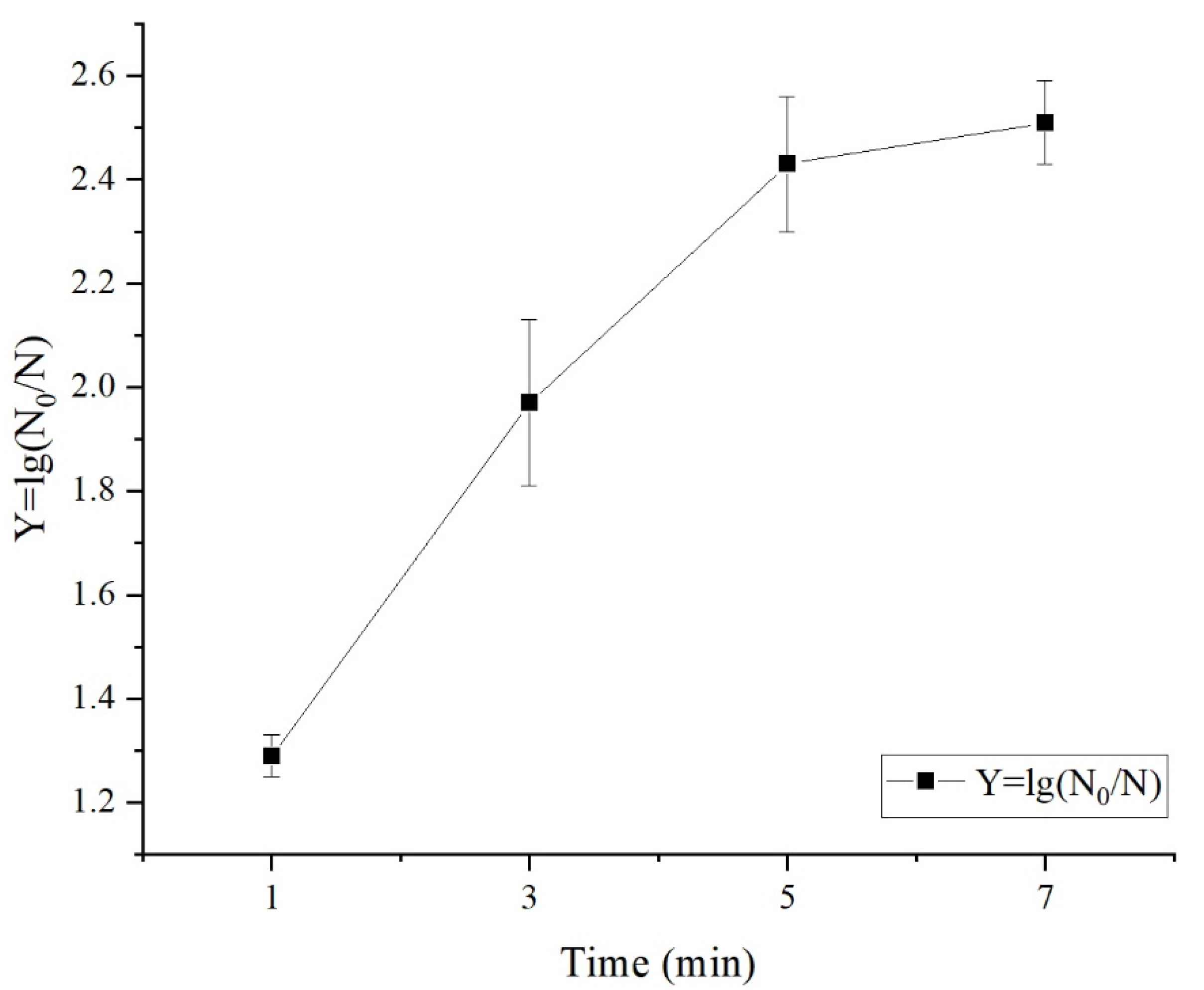
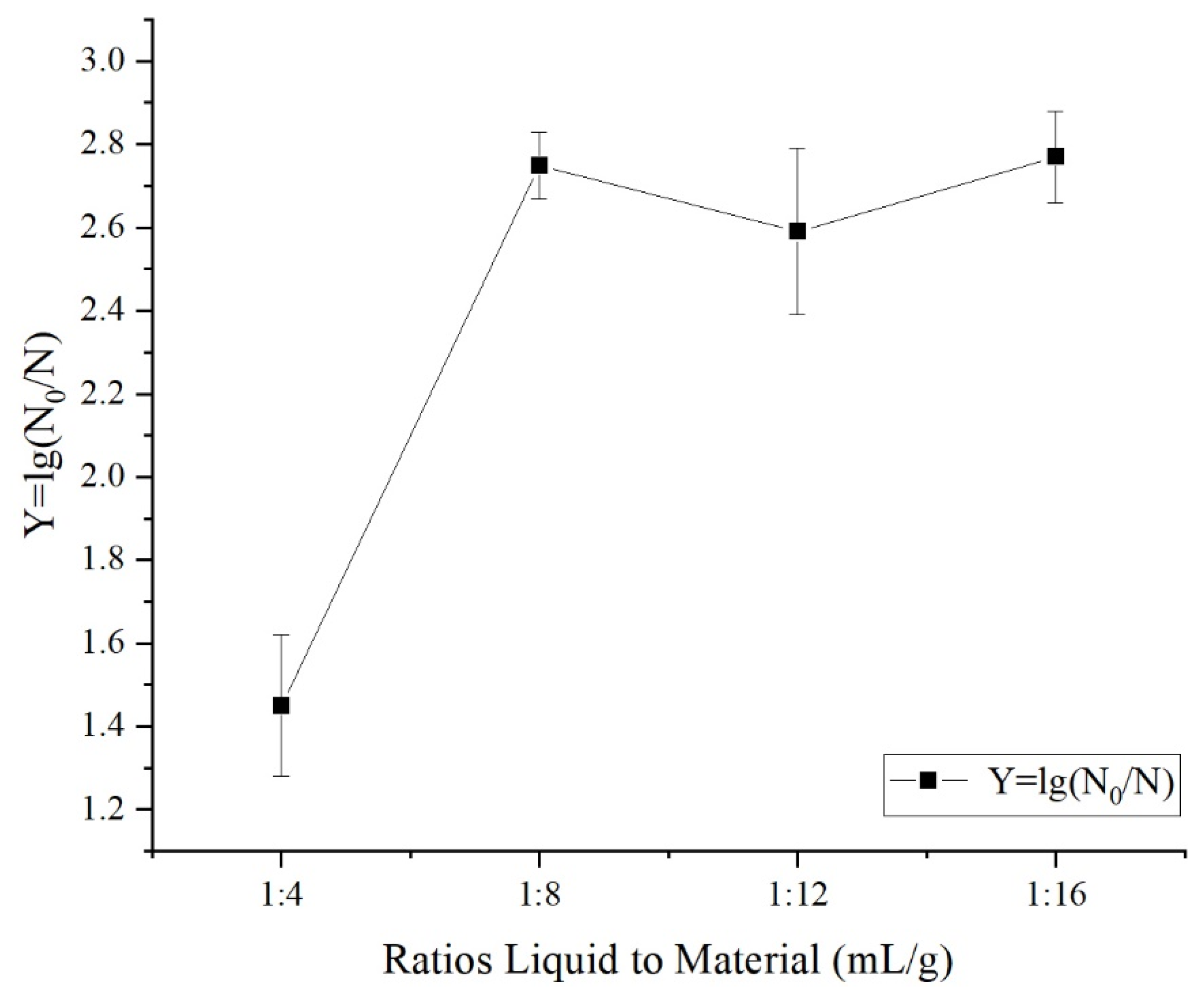
3.1.1. Effects of Different Treatment Variables
3.1.2. Establishment of the Regression Equation
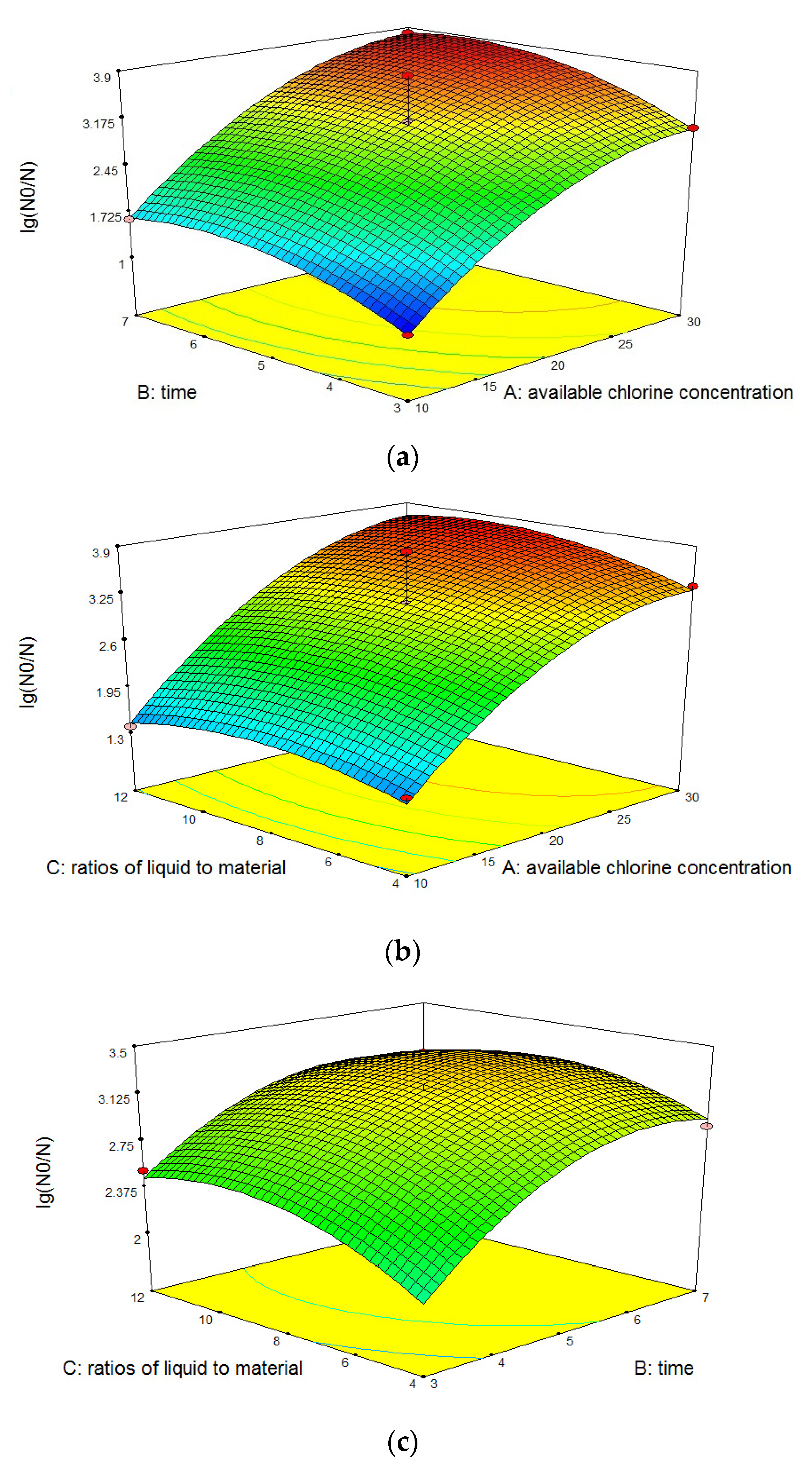
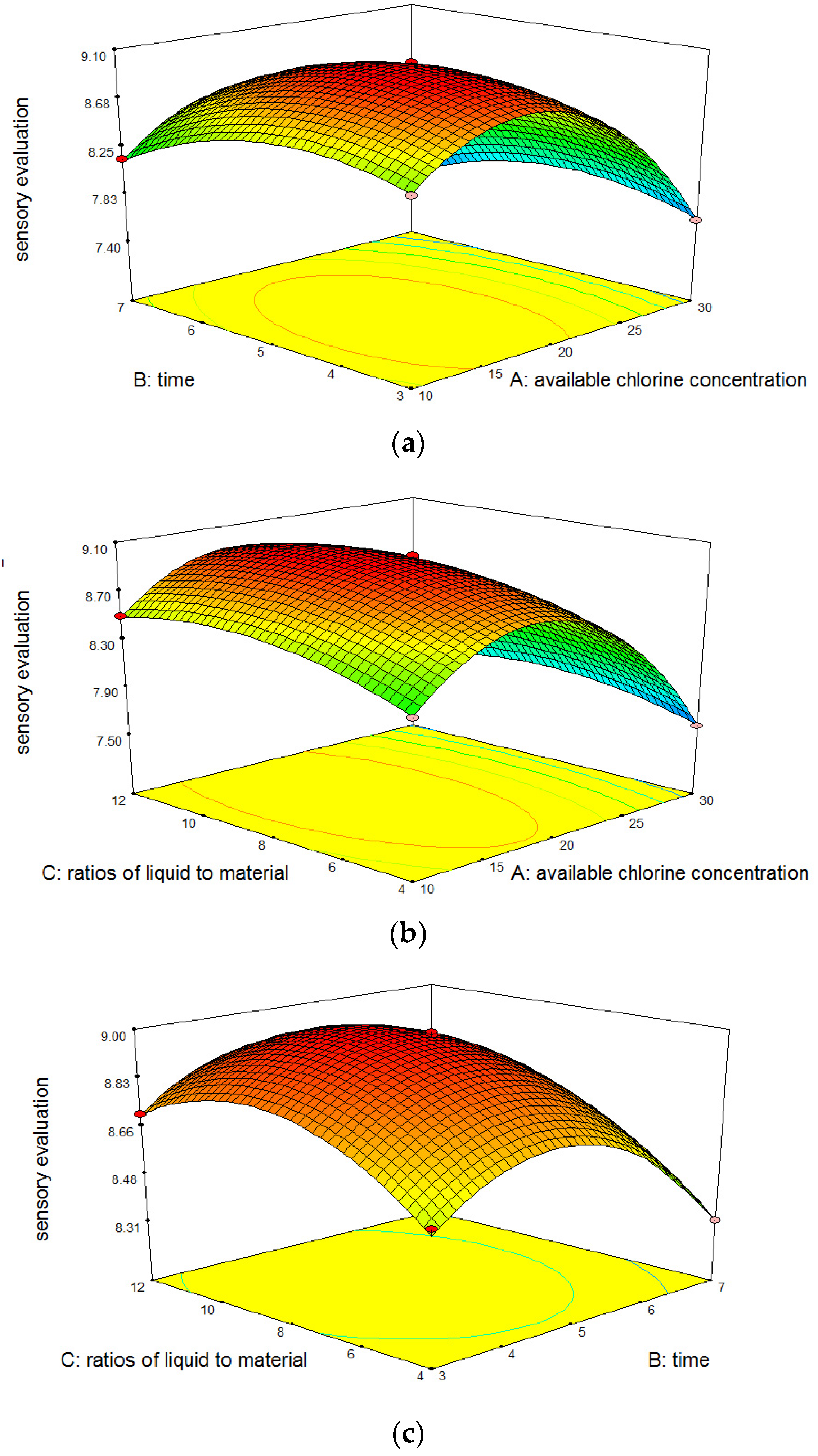
3.1.3. Response Surface Interaction Analysis
3.1.4. Determination and Verification of the Optimal Treatment Conditions
3.2. Analysis of Quality Changes during Storage
3.2.1. Effects of the SAEW Treatment on Total Number of Colonies
3.2.2. Effects of SAEW Treatments on the Vitamin C Content of Fresh-Cut Apple Samples
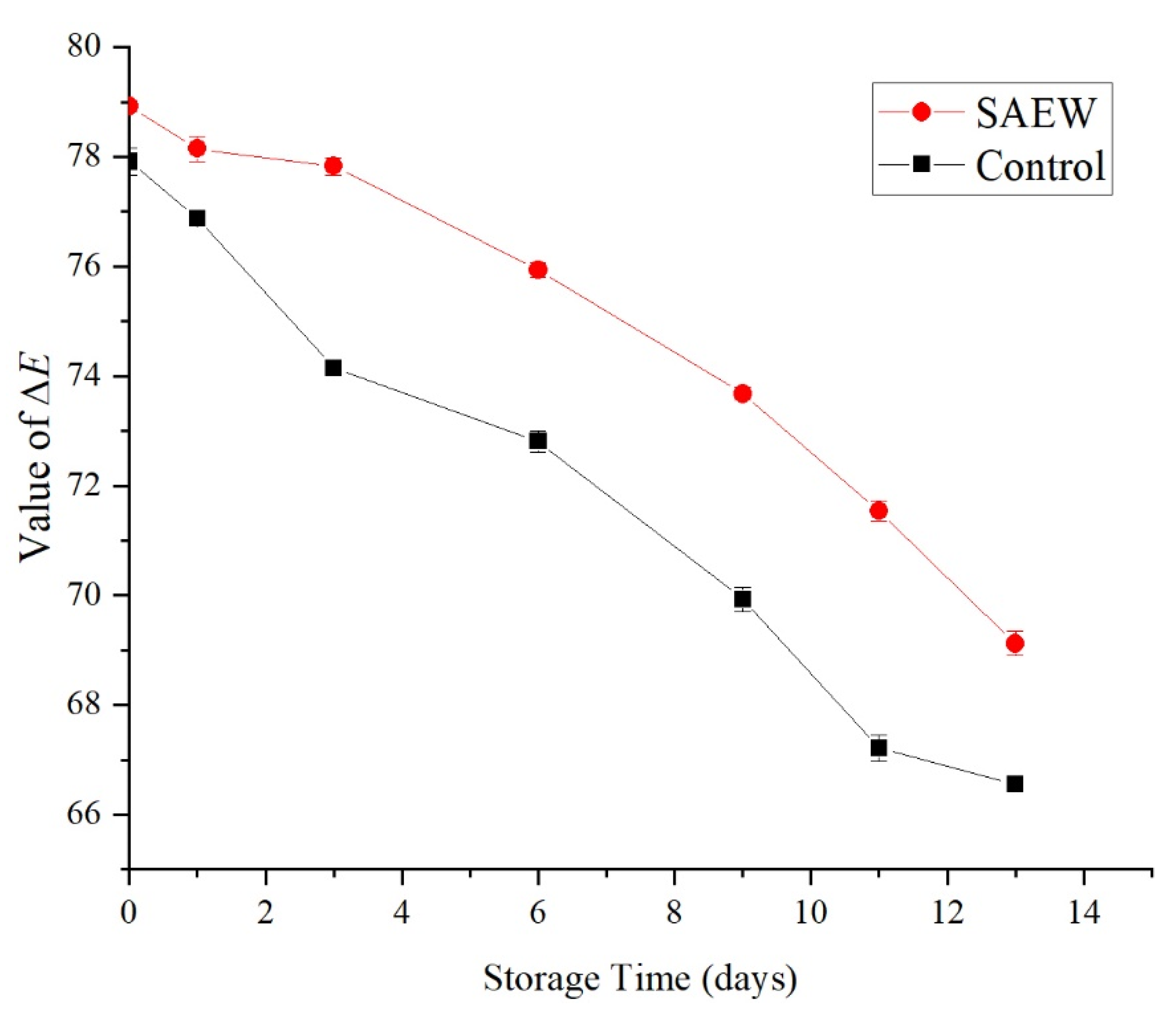
3.2.3. Effect of an SAEW Treatment on the Color of Fresh-Cut Samples
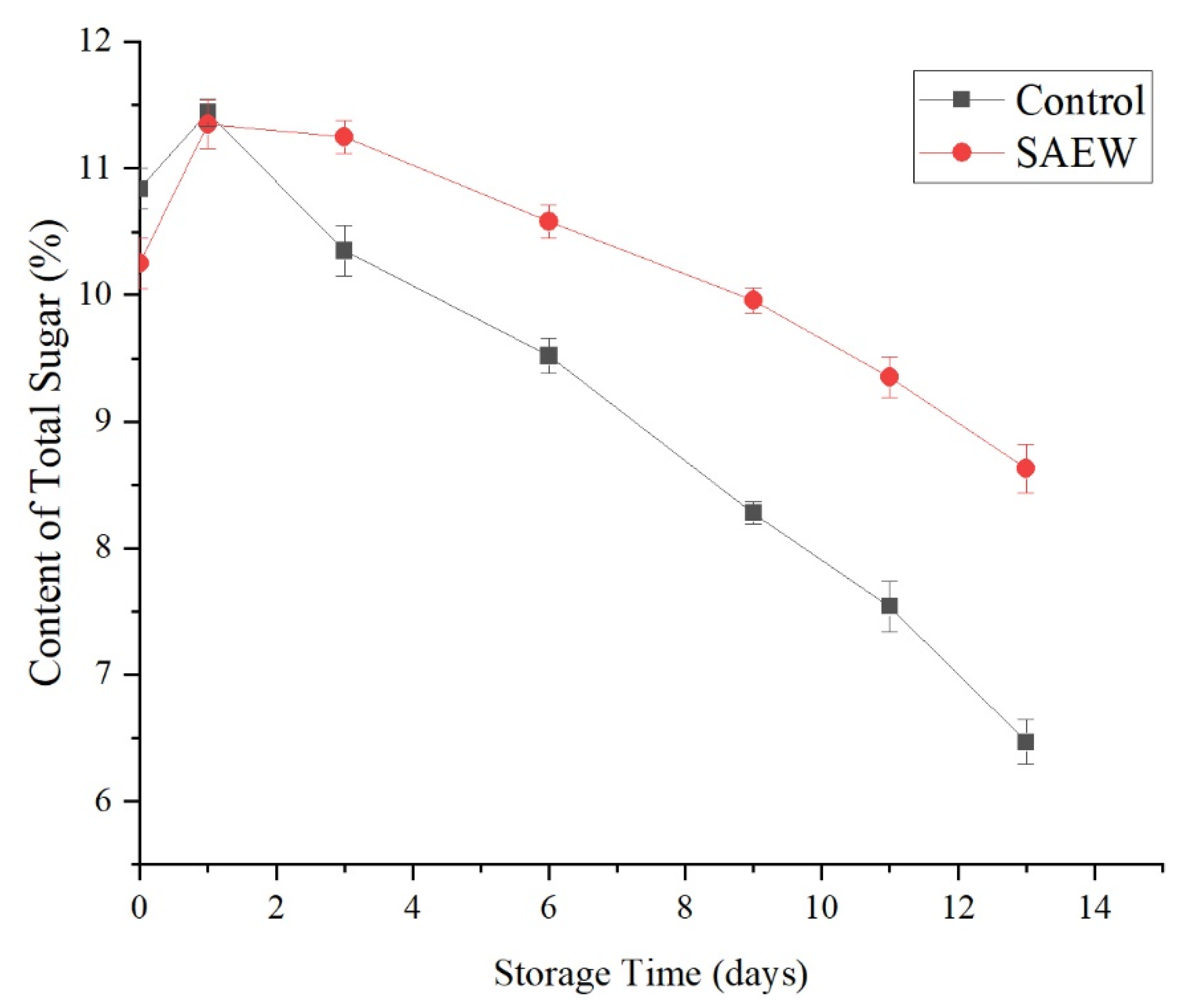
3.2.4. Effects of the SAEW Treatment on the Total Sugar Content of Fresh-Cut Apple
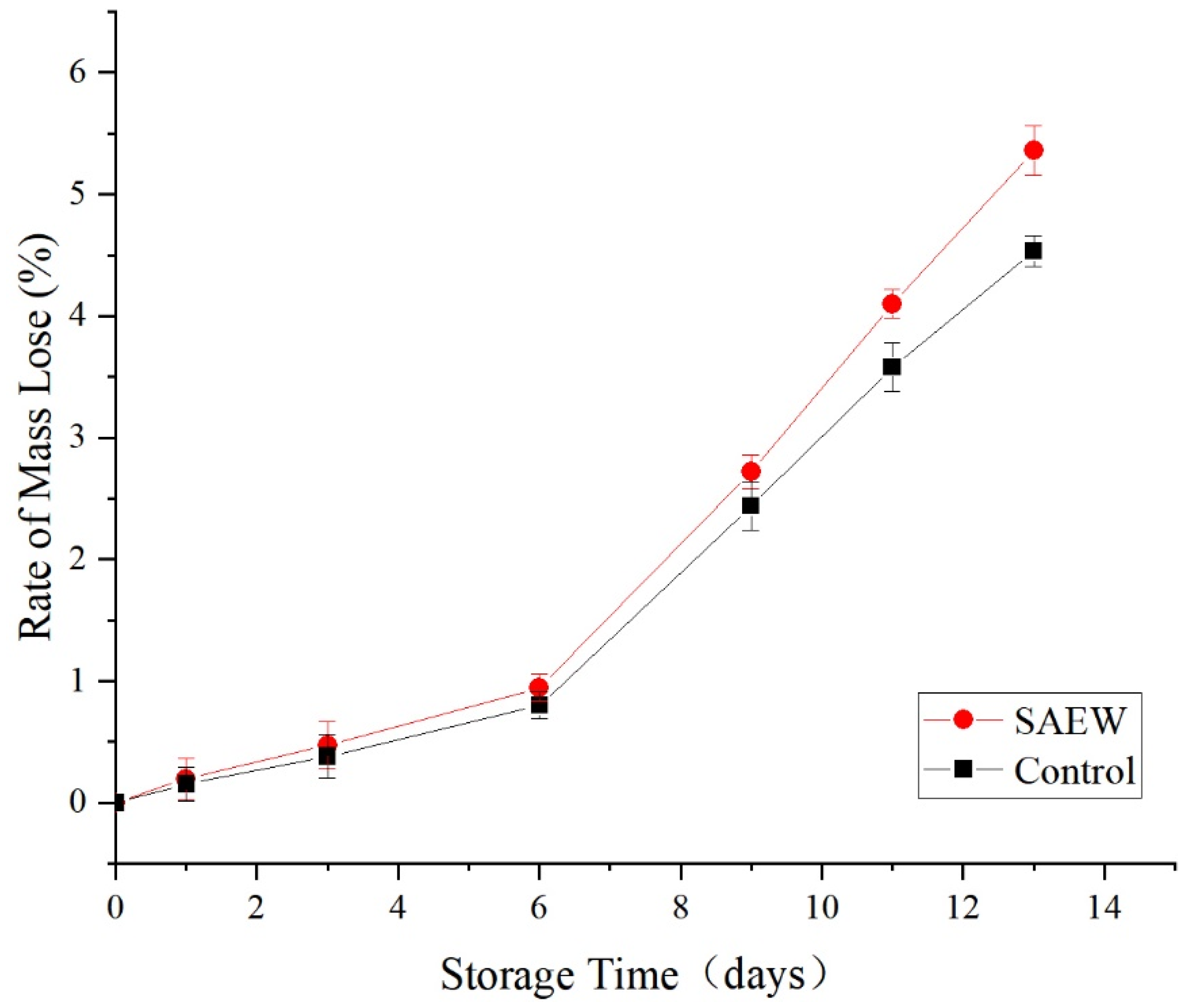
3.2.5. Effects of the SAEW Treatment on the Weight Loss Rate of Fresh-Cut Apple
3.2.6. Effects of the SAEW Treatment on the pH Value of Fresh-Cut Apple
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasan, S.M.K.; Ferrentino, G.; Scampicchio, M. Nanoemulsion as advanced edible coatings to preserve the quality of fresh-cut fruits and vegetables: A review. Int. J. Food Sci. Technol. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Ranjha, M.; Rezek Jambrak, A.; Qureshi, M.I.; Khan, N.; Manuel Lorenzo, J.; Aadil, R.M. Nonthermal food processing: A step towards a circular economy to meet the sustainable development goals. Food Chem. X 2022, 16, 100516. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A. Food preservation techniques and nanotechnology for increased shelf life of fruits, vegetables, beverages and spices: A review. Environ. Chem. Lett. 2021, 19, 1715–1735. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Sun, Y.; Li, C.; Li, Y.; Zhang, Q.; Wu, Z. Reduction of Escherichia coli O157:H7 and naturally present microbes on fresh-cut lettuce using lactic acid and aqueous ozone. RSC Adv. 2019, 9, 22636–22643. [Google Scholar] [CrossRef] [Green Version]
- Collazo, C.; Noguera, V.; Aguilo-Aguayo, I.; Abadias, M.; Colas-Meda, P.; Nicolau, I.; Vinas, I. Assessing water-assisted UV-C light and its combination with peroxyacetic acid and Pseudomonas graminis CPA-7 for the inactivation and inhibition of Listeria monocytogenes and Salmonella enterica in fresh-cut ‘Iceberg’ lettuce and baby spinach leaves. Int. J. Food Microbiol. 2019, 297, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Yildiz, G.; Izli, G.; Aadil, R.M. Comparison of chemical, physical, and ultrasound treatments on the shelf life of fresh-cut quince fruit (Cydonia oblonga Mill.). J. Food Process. Preserv. 2019, 44, e14366. [Google Scholar] [CrossRef]
- Santo, D.; Graca, A.; Nunes, C.; Quintas, C. Survival and growth of Cronobacter sakazakii on fresh-cut fruit and the effect of UV-C illumination and electrolyzed water in the reduction of its population. Int. J. Food Microbiol. 2016, 231, 10–15. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, S.; Zhou, X.; He, J.; Yu, Y.; Ye, Z. Preservative effects of the combined treatment of slightly acidic electrolyzed water and ice on pomfret. Int. J. Agric. Biol. Eng. 2021, 14, 230–236. [Google Scholar] [CrossRef]
- Yan, P.; Jo, H.Y.; Chelliah, R.; Jo, K.H.; Woo, N.C.; Wook, M.S.; Oh, D.H. Optimization and Effect of Water Hardness for the Production of Slightly Acidic Electrolyzed Water on Sanitization Efficacy. Front. Microbiol. 2022, 13, 816671. [Google Scholar] [CrossRef]
- Li, F.; Zhong, Q.; Kong, B.; Pan, N.; Xia, X.; Bao, Y. Synergistic effect and disinfection mechanism of combined treatment with ultrasound and slightly acidic electrolyzed water and associated preservation of mirror carp (Cyprinus carpio L.) during refrigeration storage. Food Chem. 2022, 386, 132858. [Google Scholar] [CrossRef]
- Chen, X.; Tyagi, A.; Vijayalakshmi, S.; Chelliah, R.; Shabbir, U.; Oh, D.H. Anti-adhesion and anti-biofilm activity of slightly acidic electrolyzed water combined with sodium benzoate against Streptococcus mutans: A novel ecofriendly oral sanitizer to prevent cariogenesis. Microb. Pathog. 2022, 166, 105535. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.; Xue, F.; Ma, S.; Zhao, M.; Zhao, D.; Hao, J. Contribution of slightly acidic electrolytic water (SAEW) to food safety, nutrients enrichment, and allergenicity reduction of peanut sprouts. J. Food Process. Preserv. 2022, 46, e16396. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, B.; Yoon, S.R.; Yang, J.S.; Ha, J.H. Physicochemical stability and virucidal effect of diluted, slightly acidic electrolyzed water against human norovirus. Food Sci. Biotechnol. 2022, 31, 131–138. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Zhao, D.; Hao, J. Efficacy of the two-step disinfection with slightly acidic electrolyzed water for reduction of Listeria monocytogenes contamination on food raw materials. LWT 2021, 140, 110699. [Google Scholar] [CrossRef]
- Brasil, C.C.B.; de Menezes, C.R.; Jacob-Lopes, E.; Barin, J.S.; Zepka, L.Q.; Campagnol, P.C.B.; Wagner, R.; Cichoski, A.J. Combined application of electrolysed water and ultrasound to improve the sanitation of knives in the meat industry. Int. J. Food Sci. Technol. 2019, 55, 1136–1144. [Google Scholar] [CrossRef]
- Wang, H.; Duan, D.; Wu, Z.; Xue, S.; Xu, X.; Zhou, G. Primary concerns regarding the application of electrolyzed water in the meat industry. Food Control. 2019, 95, 50–56. [Google Scholar] [CrossRef]
- Han, R.; Liao, X.; Ai, C.; Ding, T.; Wang, J. Sequential treatment with slightly acidic electrolyzed water (SAEW) and UVC light-emitting diodes (UVC-LEDs) for decontamination of Salmonella Typhimurium on lettuce. Food Control. 2021, 123, 107738. [Google Scholar] [CrossRef]
- Yan, P.; Chelliah, R.; Jo, K.H.; Selvakumar, V.; Chen, X.; Jo, H.Y.; Oh, D.H. Stability and Antibiofilm Efficiency of Slightly Acidic Electrolyzed Water Against Mixed-Species of Listeria monocytogenes and Staphylococcus aureus. Front. Microbiol. 2022, 13, 865918. [Google Scholar] [CrossRef]
- Kang, M.; Park, B.; Ha, J.H. Kinetic Modeling of Slightly Acidic Electrolyzed Water Decay Characteristics in Fresh Cabbage Disinfection Against Human Norovirus. Front Microbiol. 2021, 12, 616297. [Google Scholar] [CrossRef]
- Tamime, A. Cleaning-in-Place: Dairy, Food and Beverage Operations, 3rd ed.; Blackwell Publishing Ltd: Hoboken, NJ, USA, 13 May 2008; pp. 223–242. [Google Scholar]
- Cruz, G.A.G.A.S.R.; Valenzuela, R.C.; Zavala, J.F.A.; Rosa, L.A.D.L.; Alvarez-Parrilla, E. New Technologies to Preserve Quality of Fresh-Cut Produce; Food Engineering; Integrated Approaches: New York, NY, USA, 2008; pp. 105–115. [Google Scholar]
- Ejoh, S.I.; Wireko-Manu, F.D.; Page, D.; Renard, C.M.G.C. Traditional green leafy vegetables as underutilised sources of micronutrients in a rural farming community in south-west Nigeria I: Estimation of vitamin C, carotenoids and mineral contents. S. Afr. J. Clin. Nutr. 2019, 34, 40–45. [Google Scholar] [CrossRef]
- Goisser, S.; Wittmann, S.; Fernandes, M.; Mempel, H.; Ulrichs, C. Comparison of colorimeter and different portable food-scanners for non-destructive prediction of lycopene content in tomato fruit. Postharvest Biol. Technol. 2020, 167, 111232. [Google Scholar] [CrossRef]
- Coultate, T. Food. The Chemistry of Its Components, 6th ed.; Poyal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Hao, J.; Zhang, J.; Zheng, X.; Zhao, D. Bactericidal efficacy of slightly acidic electrolyzed water (SAEW) against Listeria monocytogenes planktonic cells and biofilm on food-contact surfaces. Food Qual. Saf. 2022, 6, fyab038. [Google Scholar] [CrossRef]
- Chen, X.; Tango, C.N.; Daliri, E.B.; Oh, S.Y.; Oh, D.H. Disinfection Efficacy of Slightly Acidic Electrolyzed Water Combined with Chemical Treatments on Fresh Fruits at the Industrial Scale. Foods 2019, 8, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaro, A.L.; Spadafora, N.D.; Pereira, M.J.; Dhorajiwala, R.; Herbert, R.J.; Muller, C.T.; Rogers, H.J.; Pintado, M. Multitrait analysis of fresh-cut cantaloupe melon enables discrimination between storage times and temperatures and identifies potential markers for quality assessments. Food Chem. 2018, 241, 222–231. [Google Scholar] [CrossRef]
- Pasini, L.; Ragni, L.; Rombolà, A.D.; Berardinelli, A.; Guarnieri, A.; Marangoni, B. Influence of the Fertilisation System on the Mechanical Damage of Apples. Biosyst. Eng. 2004, 88, 441–452. [Google Scholar] [CrossRef]
- Ling, J.; Li, J.; Kang, M. Application of slightly acidic electrolyzed water (SAEW) in preservation of zizania latifolia stems. Food Science 2015, 36, 250–254. [Google Scholar] [CrossRef]
- Spricigo, P.C.; Freitas, T.P.; Purgatto, E.; Ferreira, M.D.; Correa, D.S.; Bai, J.; Brecht, J.K. Visually imperceptible mechanical damage of harvested tomatoes changes ethylene production, color, enzyme activity, and volatile compounds profile. Postharvest Biol. Technol. 2021, 176, 111503. [Google Scholar] [CrossRef]
- Liu, X.; Liu, D.H.; Chen, T.; Zhang, J.; Wang, C.L. Watercore Pear Fruit Respiration Changed and Accumulated gamma-Aminobutyric Acid (GABA) in Response to Inner Hypoxia Stress. Genes 2022, 13, 977. [Google Scholar] [CrossRef]



| The Variables | Coded | Coded Levels | Levels of Independent | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| ACC (mg/L) | A | X1 | 10 | 20 | 30 |
| Treatment Time (min) | B | X2 | 3 | 5 | 7 |
| Liquid-to-material Ratio (mL/g) | C | X3 | 4:1 | 8:1 | 12:1 |
| No. | ACC A/(mg/L) | Treatment Time B/min | Liquid-to-Material Ratio C/(mL/g) | Microbial Lethality Rate Y1/lg (CFU/g) | Sensory Score Y2 |
|---|---|---|---|---|---|
| 1 | 30 | 7 | 8 | 3.81 | 7.46 |
| 2 | 20 | 7 | 12 | 3.07 | 8.44 |
| 3 | 20 | 5 | 8 | 2.93 | 8.99 |
| 4 | 20 | 5 | 8 | 3.82 | 8.98 |
| 5 | 20 | 5 | 8 | 3.12 | 8.99 |
| 6 | 10 | 7 | 8 | 1.61 | 8.14 |
| 7 | 30 | 5 | 4 | 3.36 | 7.57 |
| 8 | 30 | 5 | 12 | 3.64 | 7.67 |
| 9 | 20 | 5 | 8 | 3.08 | 8.95 |
| 10 | 20 | 3 | 4 | 1.98 | 8.55 |
| 11 | 10 | 3 | 8 | 1.01 | 8.42 |
| 12 | 20 | 5 | 8 | 3.06 | 8.99 |
| 13 | 10 | 5 | 12 | 1.38 | 8.50 |
| 14 | 30 | 3 | 8 | 3.04 | 7.59 |
| 15 | 10 | 5 | 4 | 1.47 | 8.24 |
| 16 | 20 | 7 | 4 | 2.87 | 8.31 |
| 17 | 20 | 3 | 12 | 2.51 | 8.70 |
| Response Value | Source | Sum of Squares | Degree of Freedom | Means Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|---|
| Microbial Lethality Rate (Y1) | Model | 11.91 | 9 | 1.32 | 17.29 | 0.0005 | Highly significant |
| Residual | 0.54 | 7 | 0.077 | ||||
| Lack of fit | 0.038 | 3 | 0.013 | 0.10 | 0.9547 | Not significant | |
| Pure error | 0.50 | 4 | 0.12 | ||||
| Cor. Total | 12.44 | 16 | |||||
| R2 = 0.9569, Radj2 = 0.9016 | |||||||
| Sensory Score (Y2) | Model | 4.66 | 9 | 0.52 | 1198.63 | <0.0001 | Highly significant |
| Residual | 0.0030 | 7 | 0.0004 | ||||
| Lack of fit | 0.00018 | 3 | 0.0006 | 2.03 | 0.2526 | Not significant | |
| Pure error | 0.0012 | 4 | 0.0003 | ||||
| Cor. Total | 4.66 | 16 | |||||
| R2 = 0.9994, Radj2 = 0.9985 | |||||||
| Response Value | Coefficients | Regression Coefficient | Degree of Freedom | Standard Error | F-Value | p-Value |
|---|---|---|---|---|---|---|
| Microbial Lethality Rate (Y1) | Constant Term | 3.20 | 1 | 0.12 | -- | -- |
| A | 8.78 | 1 | 8.78 | 114.70 | <0.0001 | |
| B | 0.99 | 1 | 0.99 | 12.99 | 0.0087 | |
| C | 0.11 | 1 | 0.11 | 1.38 | 0.2781 | |
| AB | 0.0072 | 1 | 0.0073 | 0.094 | 0.7676 | |
| AC | 0.034 | 1 | 0.034 | 0.45 | 0.5251 | |
| BC | 0.027 | 1 | 0.027 | 0.36 | 0.5697 | |
| A2 | 1.01 | 1 | 1.01 | 13.20 | 0.0084 | |
| B2 | 0.50 | 1 | 0.50 | 6.54 | 0.0377 | |
| C2 | 0.26 | 1 | 0.26 | 3.43 | 0.1064 | |
| Sensory Score (Y2) | Constant Term | 8.98 | 1 | 0.0093 | -- | -- |
| A | −0.38 | 1 | 0.0074 | 2620.69 | <0.0001 | |
| B | −0.11 | 1 | 0.0074 | 239.53 | <0.0001 | |
| C | 0.080 | 1 | 0.0074 | 118.48 | <0.0001 | |
| AB | 0.037 | 1 | 0.010 | 13.02 | 0.0086 | |
| AC | −0.040 | 1 | 0.010 | 14.81 | 0.0063 | |
| BC | −0.0050 | 1 | 0.010 | 0.23 | 0.6452 | |
| A2 | −0.79 | 1 | 0.010 | 6100.09 | <0.0001 | |
| B2 | −0.29 | 1 | 0.010 | 798.36 | <0.0001 | |
| C2 | −0.19 | 1 | 0.010 | 365.76 | <0.0001 |
| ACC (mg/L) | Treatment Time (min) | Liquid-to-Material Ratio (mL/g) | Microbial Lethality Rate Y1 | Sensory Evaluation Y2 | ||
|---|---|---|---|---|---|---|
| Predictive Value | Measured Value | Predictive Value | Measured Value | |||
| 2.99 | 8.75 | |||||
| 21 | 5 | 9:1 | 3.37 | 3.14 | 8.77 | 8.80 |
| 3.61 | 8.64 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Yang, Z.; Bi, B.; He, J. Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple. Foods 2023, 12, 39. https://doi.org/10.3390/foods12010039
Gao Q, Yang Z, Bi B, He J. Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple. Foods. 2023; 12(1):39. https://doi.org/10.3390/foods12010039
Chicago/Turabian StyleGao, Qing, Ziyi Yang, Baoliang Bi, and Jinsong He. 2023. "Effects of Slightly Acidic Electrolyzed Water on the Quality of Fresh-Cut Apple" Foods 12, no. 1: 39. https://doi.org/10.3390/foods12010039




