Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial/Fungal Strains and Chemicals
2.2. Preparation of Partially Purified Enterocin Gr17
2.3. Synergistic Antimicrobial Effect of Enterocin Gr17 and EOs
2.4. Preparation of the Nanoemulsions
2.5. Physicochemical Characteristics of Nanoemulsions
2.5.1. Particle Size and Zeta Potential
2.5.2. Stability Analysis
2.5.3. Encapsulation Efficiency
2.5.4. Shear Viscosity Measurement
2.6. Antibacterial and Antioxidant Activity of Nanoemulsions
2.7. Effects of Nanoemulsions System Application on the Quality Characteristics of Liquid-Smoked Salmon Fillets
2.7.1. Treatment of Liquid-Smoked Salmon Fillets Samples
2.7.2. Physicochemical Parameters
2.7.3. Microbiological Analysis
2.7.4. Volatile Compounds Analysis
2.7.5. Sensory Analysis
2.8. Statistical Analysis
3. Results
3.1. Antimicrobial Effect of Enterocin Gr17 and EOs Combinations on Bacterial and Fungal Strains
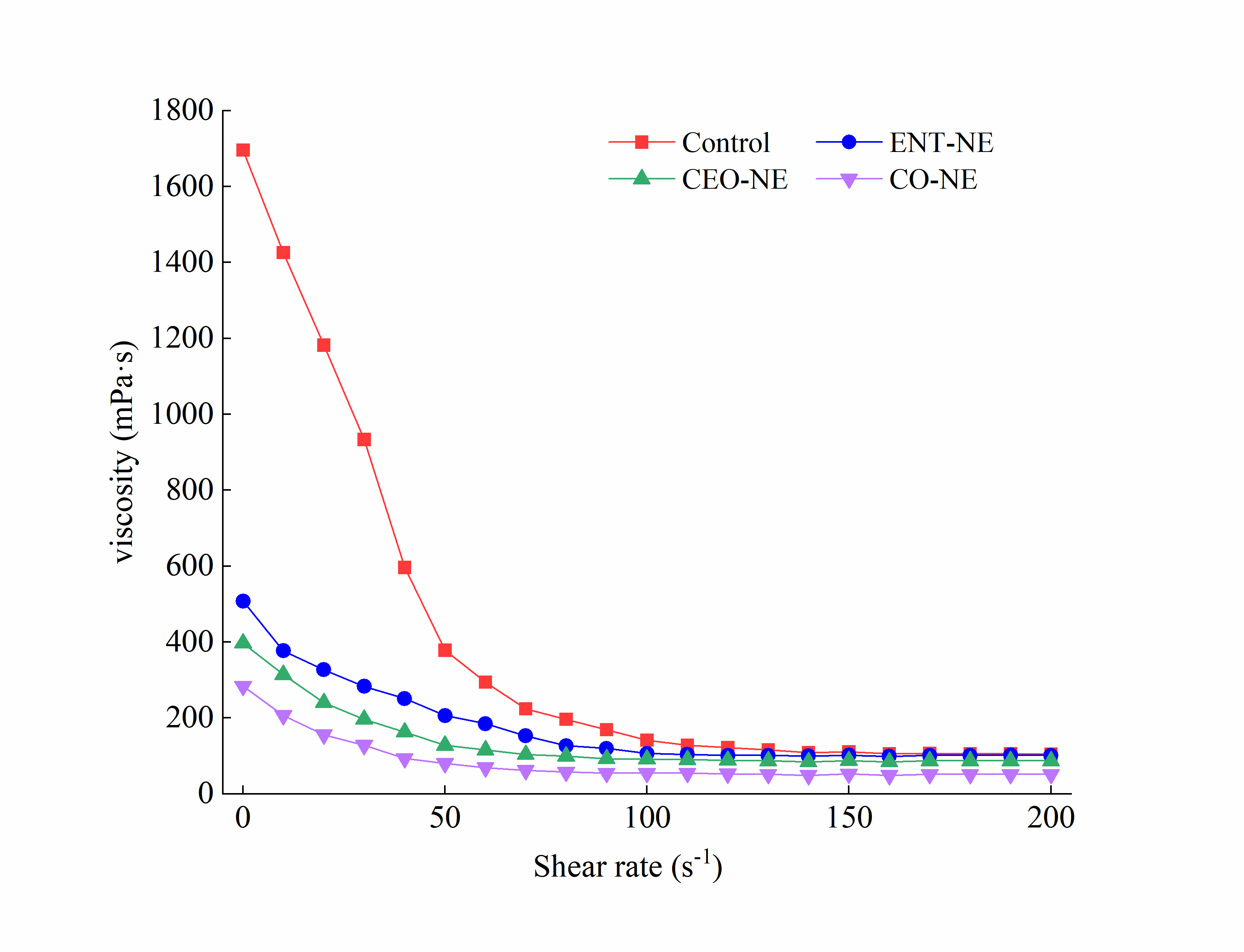
3.2. Effect of Emulsifier Type on Properties of Nanoemulsions
3.3. Effects of Nanoemulsions System Applied on Liquid-Smoked Salmon Fillets
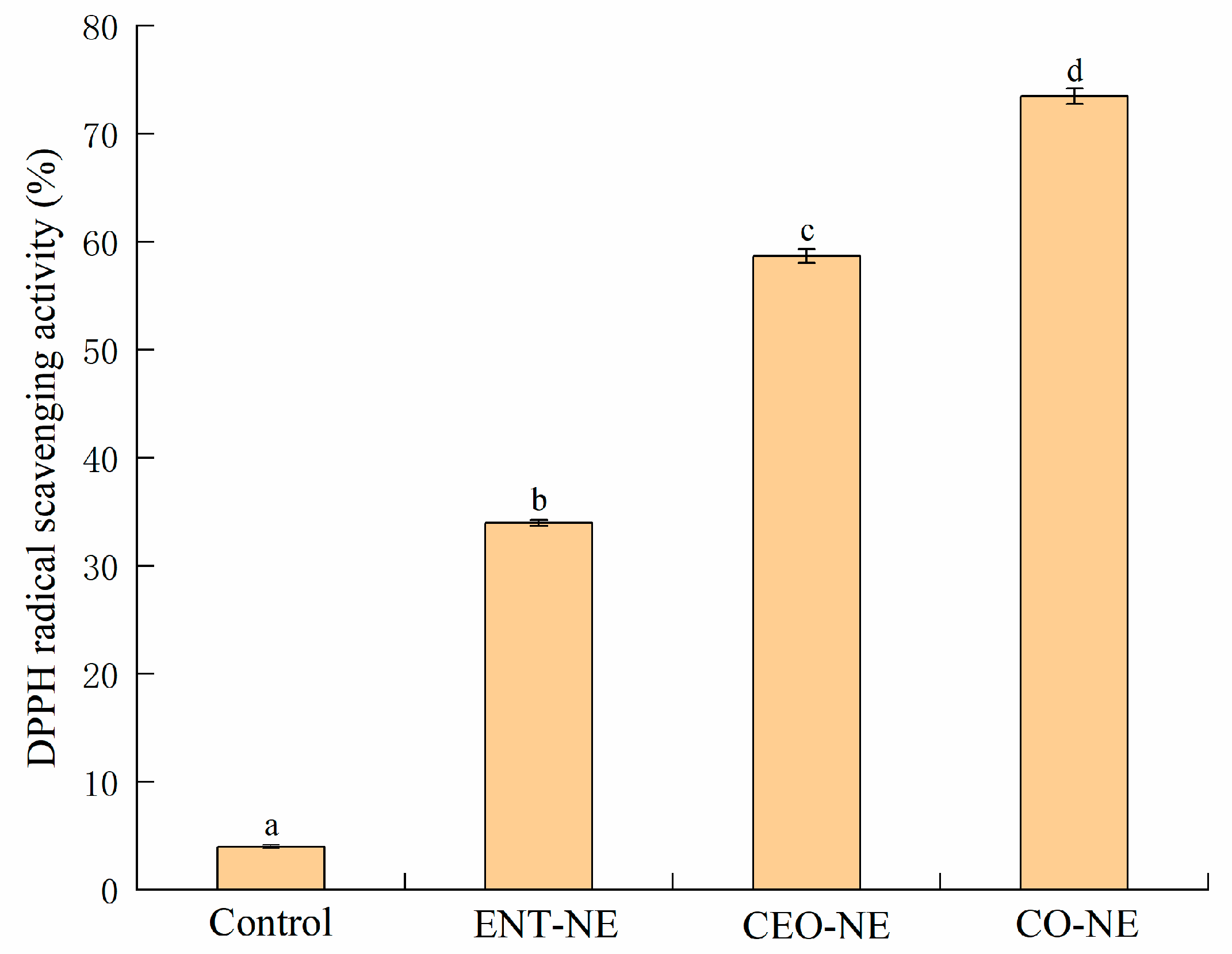
3.4. Antimicrobial and Antioxidant Activity of the SPI-Based Nanoemulsions
3.5. Effect of Nanoemulsions on the Quality Caracteristics of Liquid-Smoked Salmon Fillets
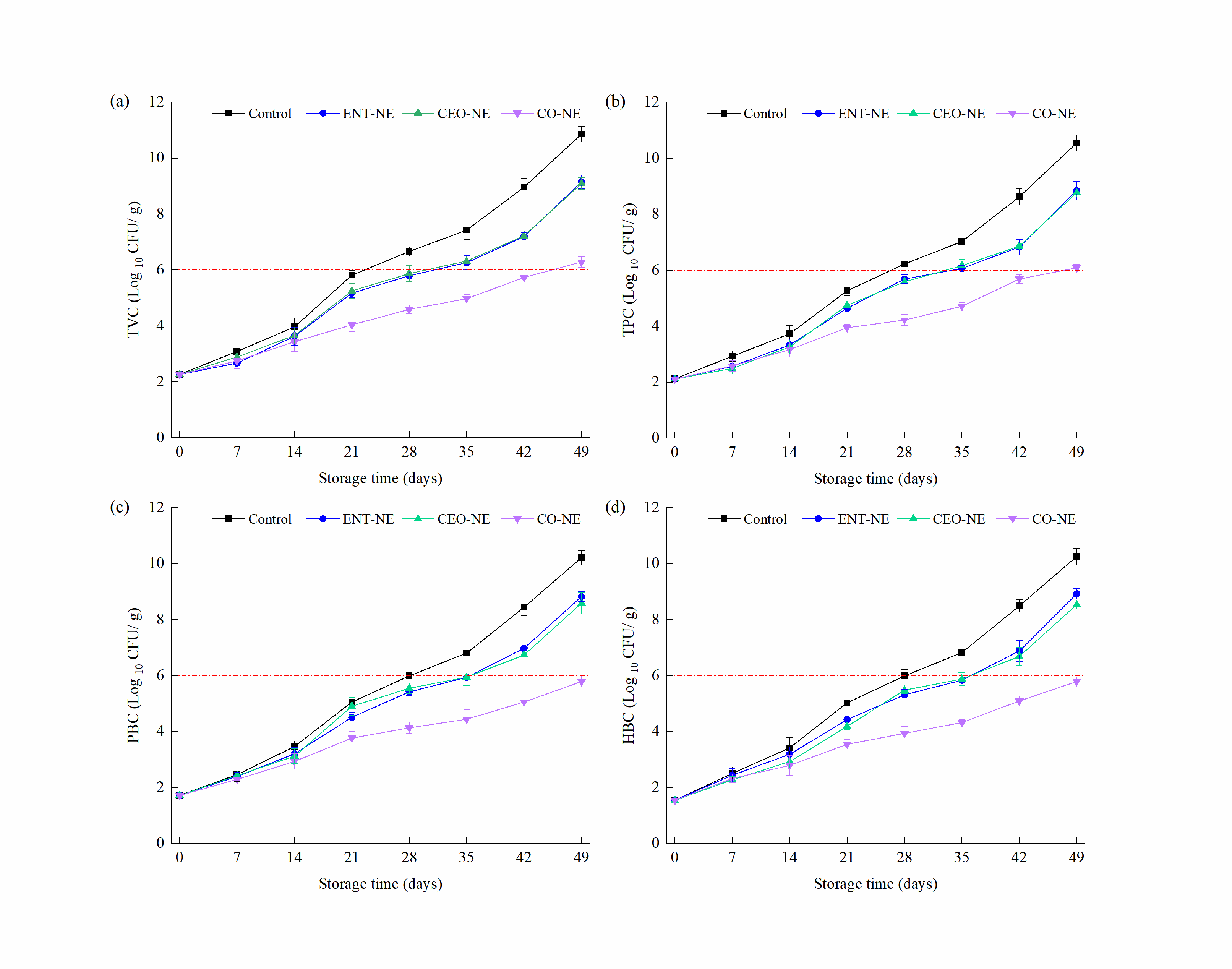
3.5.1. Microbiological Parameters
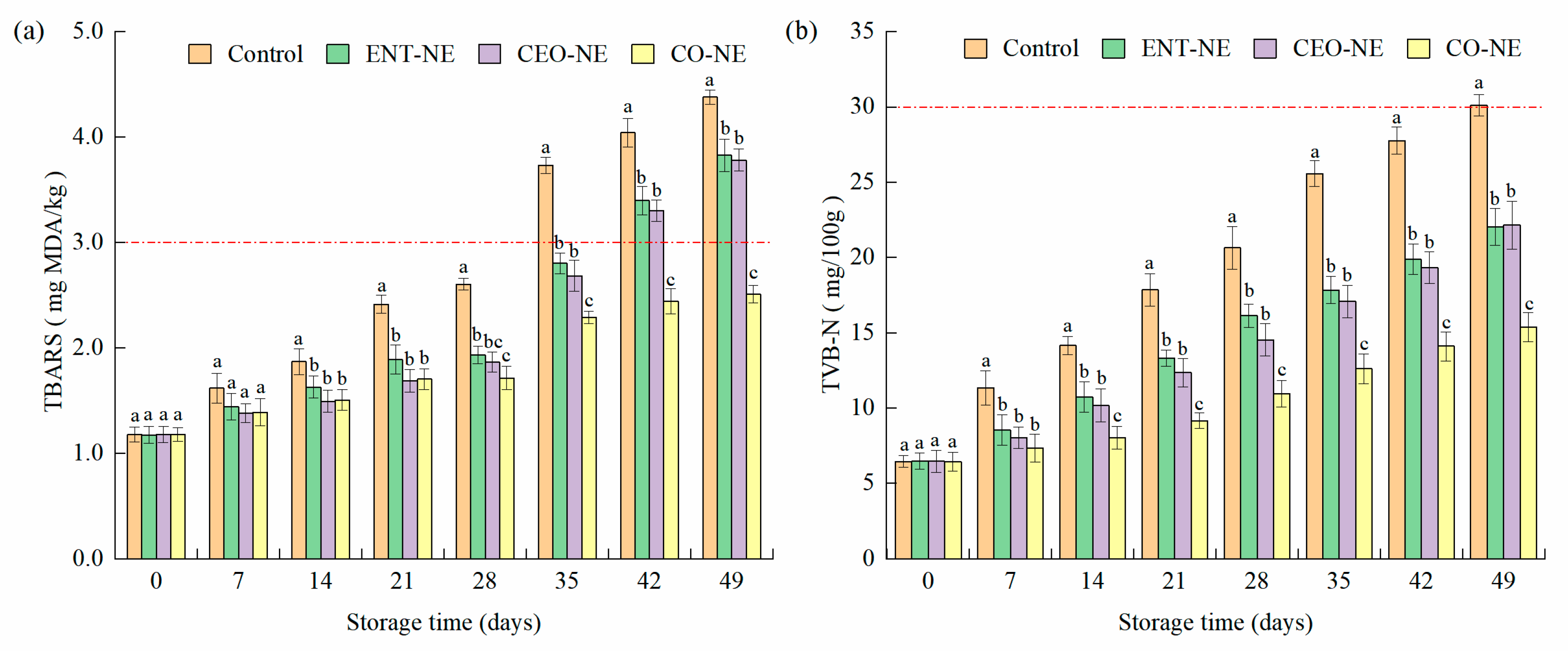
3.5.2. Lipid Oxidation and Protein Degradation
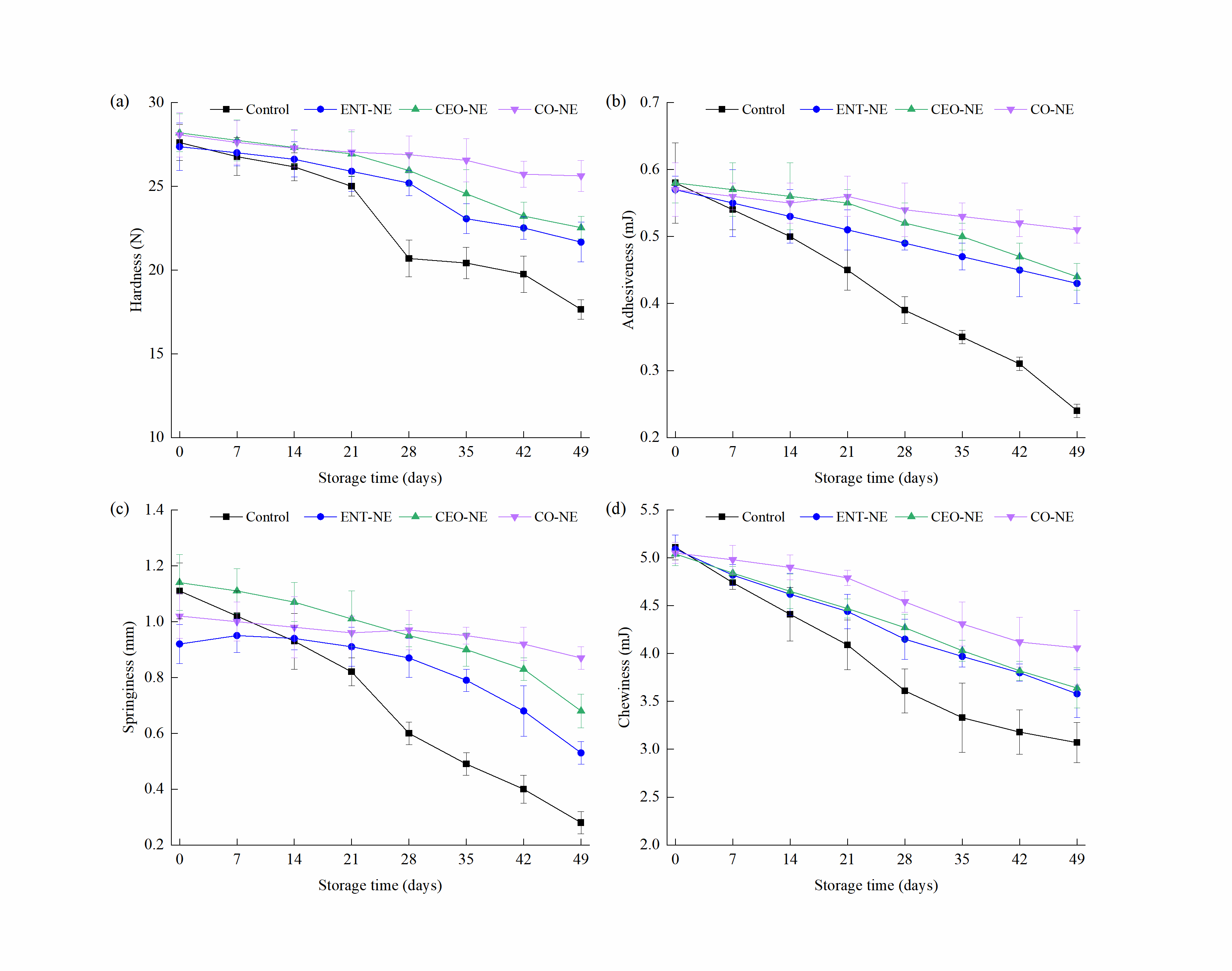
3.5.3. Color and Texture Properties
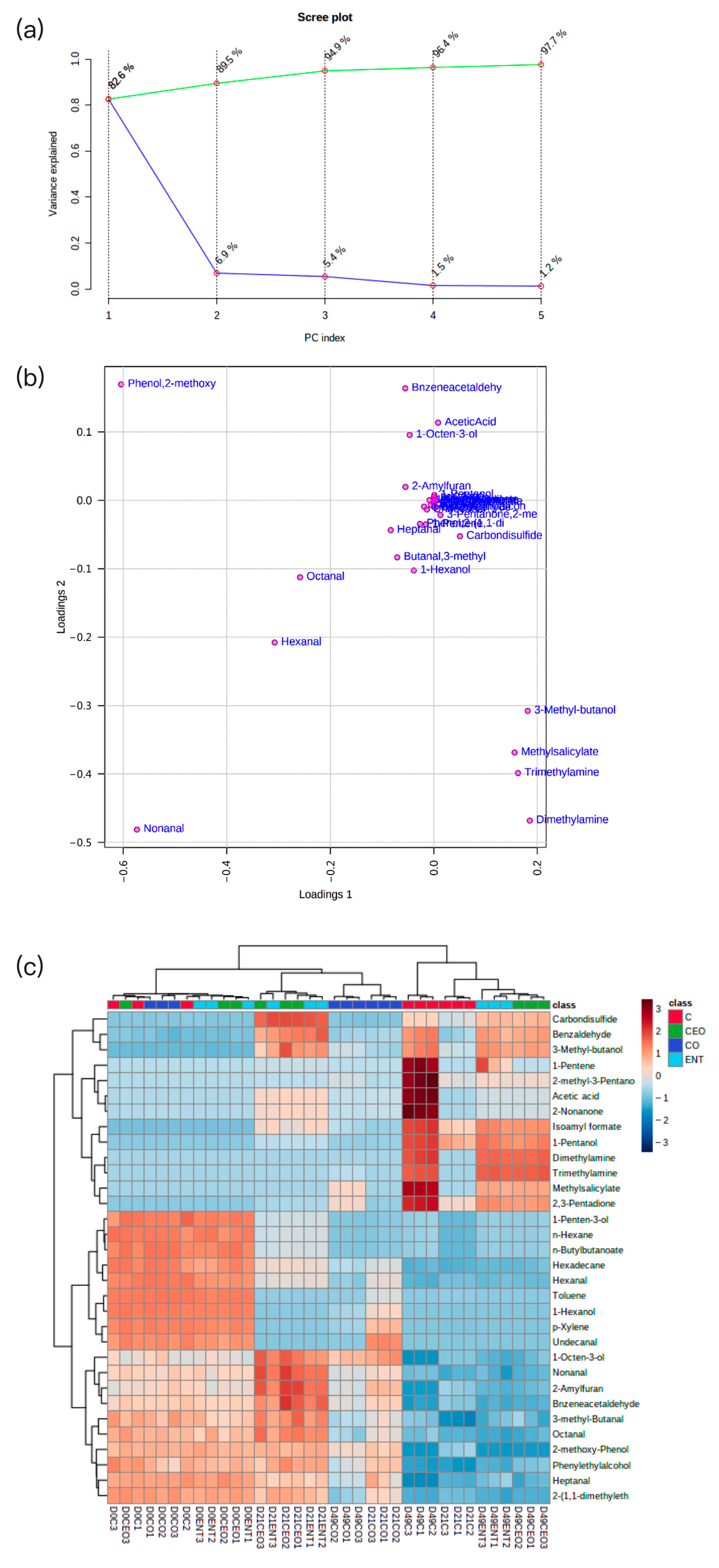
3.5.4. Volatile Compound Profile
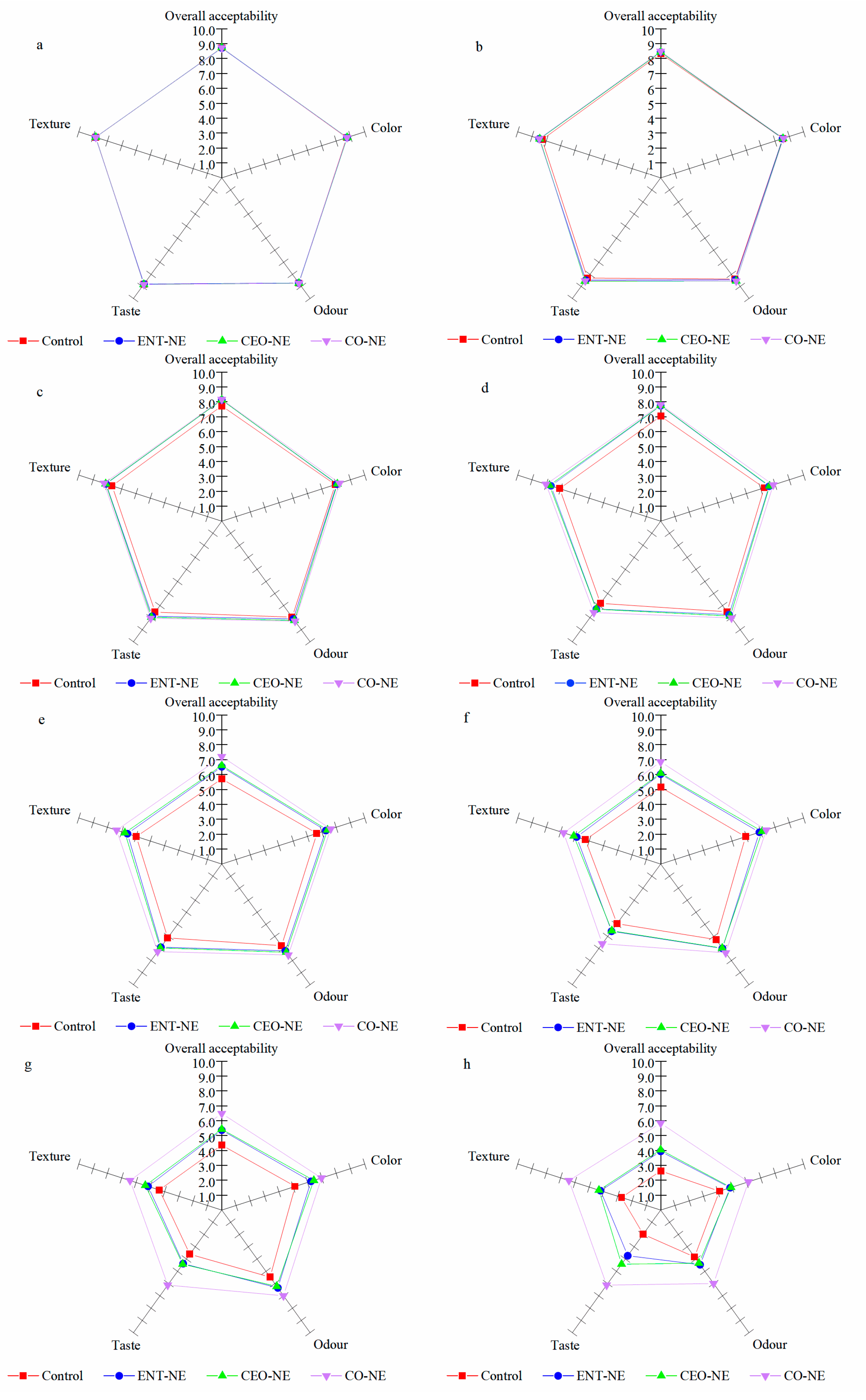
3.5.5. Sensory Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cui, H.Y.; Yang, M.; Shi, C.; Li, C.Z.; Lin, L. Application of Xanthan-Gum-Based Edible Coating Incorporated with Litsea Cubeba Essential oil Nanoliposomes in Salmon Preservation. Foods 2022, 11, 1535. [Google Scholar] [CrossRef]
- Lerfall, J.; Skuland, A.V.; Skare, E.H.; Hasli, P.R.; Rotabakk, B.T. Quality Characteristics and Consumer Acceptance of Diploid and Triploid Cold Smoked Atlantic Salmon Reared at 5, 10 and 15 degrees C. LWT-Food Sci. Technol. 2017, 85, 45–51. [Google Scholar] [CrossRef]
- Aymerich, T.; Rodriguez, M.; Garriga, M.; Bover-Cid, S. Assessment of the Bioprotective Potential of Lactic Acid Bacteria against Listeria monocytogenes on Vacuum-Packed Cold-Smoked Salmon Stored at 8 Degrees C. Food Microbiol. 2019, 83, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Moghanjougi, Z.M.; Bari, M.R.; Khaneghah, A.M. Natural Protective Agents and Their Applications as Bio-Preservatives in the Food Industry: An Overview of Current and Future Applications. Ital. Food Sci. 2021, 33, 55–68. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural Products with Preservative Properties for Enhancing the Microbiological Safety and Extending the Shelf-life of Seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Liu, G.R.; Wang, Y.; Li, X.; Hao, X.; Xu, D.X.; Zhou, Y.N.; Mehmood, A.; Wang, C.T. Genetic and Biochemical Evidence that Enterococcus faecalis Gr17 Produces a Novel and Sec-Dependent Bacteriocin, Enterocin Gr17. Front. Microbiol. 2019, 10, 102504. [Google Scholar] [CrossRef]
- Liu, G.; Nie, R.; Liu, Y.; Mehmood, A. Combined Antimicrobial Effect of Bacteriocins with Other Hurdles of Physicochemic and Microbiome to Prolong Shelf Life of Food: A Review. Sci. Total Environ. 2022, 825, 154058. [Google Scholar] [CrossRef]
- Zgheib, H.; Drider, D.; Belguesmia, Y. Broadening and Enhancing Bacteriocins Activities by Association with Bioactive Substances. Int. J. Environ. Res. Public Health 2020, 17, 7835. [Google Scholar] [CrossRef]
- Turgis, M.; Vu, K.D.; Dupont, C.; Lacroix, M. Combined Antimicrobial Effect of Essential oils and Bacteriocins against Foodborne Pathogens and Food Spoilage Bacteria. Food Res. Int. 2012, 48, 696–702. [Google Scholar] [CrossRef]
- Ghrairi, T.; Hani, K. Enhanced Bactericidal Effect of Enterocin A in Combination with Thyme Essential oils against L. Monocytogenes and E. coli O157:H7. J. Food Sci. Technol. 2015, 52, 2148–2156. [Google Scholar] [CrossRef]
- Alves, F.; Barbosa, L.; Andrade, B.; Albano, M.; Furtado, F.; Pereira, A.; Rall, V.; Fernandes, A. Short Communication: Inhibitory Activities of the Lantibiotic Nisin Combined with Phenolic Compounds against Staphylococcus aureus and Listeria monocytogenes in Cow Milk. J. Dairy Sci. 2016, 99, 1831–1836. [Google Scholar] [CrossRef] [PubMed]
- Stergiou, V.A.; Thomas, L.V.; Adams, M.R. Interactions of Nisin with Glutathione in a Model Protein System and Meat. J. Food Prot. 2006, 69, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Sant’Anna, V.; Quadros, D.; Motta, A.; Brandelli, A. Antibacterial Activity of Bacteriocin-like Substance P34 on Listeria monocytogenes in Chicken Sausage. Braz. J. Microbiol. 2013, 44, 1163–1167. [Google Scholar] [CrossRef][Green Version]
- Fernandez-Lopez, J.; Viuda-Martos, M. Introduction to the Special Issue: Application of Essential oils in Food Systems. Foods 2018, 7, 56. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Das, A.K.; Dhar, P.; Nanda, P.K.; Chatterjee, N. Nanoemulsion-Based Technologies for Delivering Natural Plant-Based Antimicrobials in Foods. Front. Sustain. Food Syst. 2021, 5, 643208. [Google Scholar] [CrossRef]
- Ling, J.K.U.; Chan, Y.S.; Nandong, J. Insights into the Release Mechanisms of Antioxidants from Nanoemulsion Droplets. J. Food Sci. Technol. -Mysore 2022, 59, 1677–1691. [Google Scholar] [CrossRef]
- Mavalizadeh, A.; Fazlara, A.; PourMahdi, M.; Bavarsad, N. The Effect of Separate and Combined Treatments of Nisin, Rosmarinus Officinalis Essential oil (Nanoemulsion and Free Form) and Chitosan Coating on the Shelf Life of Refrigerated Chicken Fillets. J. Food Meas. Charact. 2022, 16, 4497–4513. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of Nanoemulsion-Based Active Coatings with Composite Mixture of Star Anise Essential oil, Polylysine, and Nisin on the Quality and Shelf Life of Ready-to-Eat Yao Meat Products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Buldain, D.; Buchamer, A.V.; Marchetti, M.L.; Aliverti, F.; Bandoni, A.; Mestorino, N. Combination of Cloxacillin and Essential oil of Melaleuca Armillaris as an Alternative against Staphylococcus aureus. Front. Vet. Sci. 2018, 5, 177. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Niakousari, M.; Parastouei, K.; Mokhtarian, M.; Es, I.; Khaneghah, A.M. Co-Encapsulation of Vitamin D3 and Saffron Petals’ Bioactive Compounds in Nanoemulsions: Effects of Emulsifier and Homogenizer Types. J. Food Process. Preserv. 2020, 44, 14629. [Google Scholar] [CrossRef]
- Sui, X.; Bi, S.; Qi, B.; Wang, Z.; Zhang, M.; Li, Y.; Jiang, L. Impact of ultrasonic treatment on an emulsion system stabilized with soybean protein isolate and lecithin: Its emulsifying property and emulsion stability. Food Hydrocoll. 2017, 63, 727–734. [Google Scholar] [CrossRef]
- Nam, S.H.; Choi, Y.J.; Kim, Y.-W.; Jun, K.; Jeong, N.-H.; Oh, S.-G.; Kang, H.-C. Syntheses and characterization of new photoresponsive surfactants, N-(azobenzene-4-oxy-2-hydroxypropyl)-N-(alkyloxy-2-hydroxypropyl) aminopropyl sulfonic acid sodium salt. J. Ind. Eng. Chem. 2020, 90, 203–213. [Google Scholar] [CrossRef]
- Singh, B.; Chaudhari, A.; Das, S.; Tiwari, S.; Dubey, N. Preparation and Characterization of a Novel Nanoemulsion Consisting of Chitosan and Cinnamomum Tamala Essential oil and Its Effect on Shelf-Life Lengthening of Stored Millets. Pestic. Biochem. Physiol. 2022, 187, 105214. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Rodriguez, M.; Prieto, P.; Garcia, M.; Martin-Pinero, M.; Munoz, J. Influence of Nanoemulsion/Gum Ratio on Droplet Size Distribution, Rheology and Physical Stability of Nanoemulgels Containing Inulin and Omega-3 Fatty Acids. J. Sci. Food Agric. 2022, 102, 6397–6403. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. A Novel Active Bionanocomposite Film Incorporating Rosemary Essential oil and Nanoclay into Chitosan. J. Food Eng. 2012, 111, 343–350. [Google Scholar] [CrossRef]
- Birkeland, S.; Akse, L. Study of the Quality Characteristics in Cold-Smoked Salmon (Salmo salar) Originating from Pre- or Post-Rigor Raw Material. J. Food Sci. 2010, 75, E580–E587. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.L.d.; Montenegro Stamford, T.L.; Oliveira Lima, E.d. Sensitivity of Spoiling and Pathogen Food-related Bacteria to Origanum vulgare L. (Lamiaceae) Essential oil. Braz. J. Microbiol. 2006, 37, 527–532. [Google Scholar] [CrossRef][Green Version]
- Setyowati, W.; Susanti, V. Antibacterial Activity of Natural Paper from Banana Peel (Musa paradisiaca Linn.) with Additive Essential oils. Int. Conf. Adv. Mater. Better Future 2019, 578, 012048. [Google Scholar] [CrossRef]
- Almasi, H.; Azizi, S.; Amjadi, S. Development and Characterization of Pectin Films Activated by Nanoemulsion and Pickering Emulsion Stabilized Marjoram (Origanum majorana L.) Essential oil. Food Hydrocoll. 2020, 99, 105338. [Google Scholar] [CrossRef]
- Guo, Y.C.; Huang, J.C.; Sun, X.B.; Lu, Q.; Huang, M.; Zhou, G.H. Effect of Normal and Modified Atmosphere Packaging on Shelf Life of Roast Chicken Meat. J. Food Saf. 2018, 38, 2493. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.; Villalba-Villalba, A.; Pacheco-Aguilar, R.; Ramirez-Suarez, J.; Lugo-Sanchez, M.; Garcia-Sanchez, G.; Carvallo-Ruiz, M. Changes in Quality Parameters of Monterey Sardine (Sardinops Sagax Caerulea) Muscle during the Canning Process. Food Chem. 2010, 122, 482–487. [Google Scholar] [CrossRef]
- Cardinal, M.; Gunnlaugsdottir, H.; Bjoernevik, M.; Ouisse, A.; Vallet, J.; Leroi, F. Sensory Characteristics of Cold-Smoked Atlantic Salmon (Salmo salar) from European Market and Relationships with Chemical, Physical and Microbiological Measurements. Food Res. Int. 2004, 37, 181–193. [Google Scholar] [CrossRef]
- Filho, P.R.C.d.O.; Araujo, I.B.; Raul, L.J.; Maciel, M.I.S.; Shinohara, N.K.S.; Gloria, M.B.A. Stability of Refrigerated Traditional and Liquid Smoked Catfish (Sciades herzbergii) Sausages. J. Food Sci. 2021, 86, 2939–2948. [Google Scholar] [CrossRef] [PubMed]
- Espe, M.; Kiessling, A.; Lunestad, B.; Torrissen, O.; Rora, A. Quality of Cold Smoked Salmon Collected in One French Hypermarket during a Period of 1 Year. LWT-Food Sci. Technol. 2004, 37, 627–638. [Google Scholar] [CrossRef]
- Chan, S.; Roth, B.; Jessen, F.; Lovdal, T.; Jakobsen, A.; Lerfall, J. A Comparative Study of Atlantic Salmon Chilled in Refrigerated Seawater versus on Ice: From Whole Fish to Cold-Smoked Fillets. Sci. Rep. 2020, 10, 17160. [Google Scholar] [CrossRef]
- Guo, H.X.; Feng, T.; Qi, W.Y.; Kong, Q.L.; Yue, L.; Wang, H.H. Effects of Electron-Beam Irradiation on Volatile Flavor Compounds of Salmon Fillets by the Molecular Sensory Science Technique. J. Food Sci. 2021, 86, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottir, K.; Martinsdottir, E.; Green-Petersen, D.; Hyldig, G.; Schelvis, R.; Delahunty, C. Sensory Characteristics of Different Cod Products Related to Consumer Preferences and Attitudes. Food Qual. Prefer. 2009, 20, 120–132. [Google Scholar] [CrossRef]
- Mei, J.; Ma, X.; Xie, J. Review on Natural Preservatives for Extending Fish Shelf Life. Foods 2019, 8, 490. [Google Scholar] [CrossRef]
- Pathania, R.; Najda, A.; Chawla, P.; Kaushik, R.; Khan, M.A. Low-Energy Assisted Sodium Alginate Stabilized Phyllanthus Niruri Extract Nanoemulsion: Characterization, in Vitro Antioxidant and Antimicrobial Application. Biotechnol. Rep. 2022, 33, e00711. [Google Scholar] [CrossRef]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A.; et al. Improving the Oxidative Stability of Fish Oil Nanoemulsions by Co-Encapsulation with Curcumin and Resveratrol. Colloids Surf. B-Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef]
- Fogarty, C.; Whyte, P.; Brunton, N.; Lyng, J.; Smyth, C.; Fagan, J.; Bolton, D. Spoilage Indicator Bacteria in Farmed Atlantic Salmon (Salmo salar) Stored on Ice for 10 Days. Food Microbiol. 2019, 77, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Li, D.; Luo, Y. Characterization of the Microbiota in Lightly Salted Bighead Carp (Aristichthys Nobilis) Fillets Stored at 4 Degrees C. Food Microbiol. 2017, 62, 106–111. [Google Scholar] [CrossRef]
- Kenari, R.E.; Razavi, R. Encapsulation of Bougainvillea (Bougainvillea spectabilis) Flower Extract in Urtica Dioica L. Seed Gum: Characterization, Antioxidant/Antimicrobial Properties, and in Vitro Digestion. Food Sci. Nutr. 2022, 10, 3436–3443. [Google Scholar] [CrossRef] [PubMed]
- Ouahioune, L.A.; Wrona, M.; Nerin, C.; Djenane, D. Novel Active Biopackaging Incorporated with Macerate of Carob (Ceratonia siliqua L.) to Extend Shelf-Life of Stored Atlantic Salmon Fillets (Salmo salar L.). LWT-Food Sci. Technol. 2022, 156, 113015. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.L.; Zheng, P.A.; Tian, F.; Zhang, Z.Z.; Sun, Z.D.; Cai, L.Y. Development of Edible Composite Film Based on Chitosan Nanoparticles and Their Application in Packaging of Fresh Red Sea Bream Fillets. Food Control 2022, 132, 108545. [Google Scholar] [CrossRef]
- Schubring, R. Thermal Stability, Texture, Liquid Holding Capacity and Colour of Smoked Salmon on Retail Level. Thermochim. Acta 2006, 445, 168–178. [Google Scholar] [CrossRef]
- Sveinsdottir, K.; Martinsdottir, E.; Hyldig, G.; Jorgensen, B.; Kristbergsson, K. Application of Quality Index Method (QIM) Scheme in Shelf-Life Study of Farmed Atlantic Salmon (Salmo salar). J. Food Sci. 2002, 67, 1570–1579. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Ornaghi, M.G.; Kempinski, E.M.B.C.; Sary, C.; Monteschio, J.D.; Matumoto-Pintro, P.T.; Ribeiro, R.P.; Do Prado, I.N. Quality and Sensory Acceptability of Fish Fillet (Oreochromis niloticus) with Alginate-Based Coating Containing Essential oils. J. Food Sci. Technol. -Mysore 2018, 55, 4945–4955. [Google Scholar] [CrossRef]
- Meral, R.; Alav, A.; Karakas, C.; Dertli, E.; Yilmaz, M.T.; Ceylan, Z. Effect of Electrospun Nisin and Curcumin Loaded Nanomats on the Microbial Quality, Hardness and Sensory Characteristics of Rainbow Trout Fillet. LWT-Food Sci. Technol. 2019, 113, 108292. [Google Scholar] [CrossRef]
- Li, P.; Zhou, Q.; Chu, Y.; Lan, W.; Mei, J.; Xie, J. Effects of Chitosan and Sodium Alginate Active Coatings containing ε-polysine on Qualities of Cultured Pufferfish (Takifugu obscurus) during Cold Storage. Int. J. Biol. Macromol. 2020, 160, 418–428. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, I.; Ucar, Y.; Durmus, M.; Kosker, A.; Oz, M.; Ozogul, F. Evaluation of Effects of Nanoemulsion Based on Herb Essential oils (Rosemary, Laurel, Thyme and Sage) on Sensory, Chemical and Microbiological Quality of Rainbow Trout (Oncorhynchus mykiss) Fillets during Ice Storage. LWT-Food Sci. Technol. 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Hansen, L.T.; Gill, T.; Hussa, H.H. Effects of Salt and Storage Temperature on Chemical, Microbiological and Sensory Changes in Cold-Smoked Salmon. Food Res. Int. 1995, 28, 123–130. [Google Scholar] [CrossRef]
- Erkan, N.; Üretener, G.; Alpas, H.; Selçuk, A.; Özden, Ö.; Buzrul, S. The Effect of Different High Pressure Conditions on the Quality and Shelf Life of Cold Smoked Fish. Innov. Food Sci. Emerg. Technol. 2011, 12, 104–110. [Google Scholar] [CrossRef]

| Indicator Strains | MIC (g/L) | FICI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ENT | EEO | TEO | CAEO | CEO | MEO | LEO | ENT and EEO | ENT and TEO | ENT and CAEO | ENT and CEO | ENT and MEO | ENT and LEO | ||
| S. aureus | 5 | 2.5 | 5 | 2.5 | 0.3125 | 1.25 | 1.25 | 1.031 | 0.531 | 0.563 | 0.125 | 1.25 | 1.031 | |
| B. cereus | 1.25 | 2.5 | 5 | 1.25 | 0.3125 | 1.25 | 0.625 | 0.125 | 0.156 | 1.5 | 0.063 | 0. 5 | 1.25 | |
| L. monocytogenes | 1.25 | 1.25 | 0.3125 | 0.3125 | 0.1563 | 0.625 | 0.3125 | 0.625 | 1.125 | 0.156 | 0.094 | 0.563 | 0.625 | |
| S. enterica | 1.25 | 0.3125 | 5 | 0.1563 | 0.3125 | 2.5 | 0.3125 | 1.125 | 1.25 | 0.625 | 0.125 | 0.75 | 1.063 | |
| E. coli | 5 | 2.5 | 5 | 0.3125 | 0.3125 | 2.5 | 0.625 | 0.125 | 0.563 | 0.75 | 0.063 | 0.625 | 1.5 | |
| P. aeruginosa | 5 | 1.25 | 0.625 | 0.3125 | 0.3125 | 5 | 0.625 | 1.5 | 0.625 | 1.031 | 0.125 | 1.125 | 0.563 | |
| P. fluorescens | 5 | 1.25 | 0.625 | 0.3125 | 0.3125 | 5 | 5 | 0.375 | 1.25 | 0.188 | 0.125 | 0.75 | 0.75 | |
| S. putrefaciens | 5 | 1.25 | 0.625 | 0.3125 | 0.3125 | 5 | 2.5 | 2 | 0.75 | 0.625 | 0.125 | 1.25 | 1.25 | |
| C. albicans | 10 | 0.3125 | 0.1563 | 0.0781 | 0.0195 | 0.1563 | 0.0781 | 1.5 | 0.563 | 1.031 | 0.188 | 2 | 2 | |
| A. flavus | ND | 0.3125 | 0.3125 | 0.1563 | 0.0391 | 0.1563 | 0.1563 | 1.25 | 1.031 | 1.5 | 0.125 | 1.063 | 0.75 | |
| P. expansum | ND | 0.625 | 0.625 | 0.3125 | 0.3125 | 0.3125 | 0.1563 | 1.063 | 1.5 | 2 | 0.125 | 1.031 | 1.5 | |
| A. alternata | 20 | 0.3125 | 0.625 | 0.1563 | 0.0781 | 0.625 | 0.625 | 1.031 | 1.5 | 0.75 | 0.188 | 1.25 | 1.5 | |
| Emulsifier | Average Droplet Size (nm) | Zeta Potential (mV) | PDI | TSI (12 h) |
|---|---|---|---|---|
| SPI | 161.26 ± 6.40 f | −32.51 ± 0.83 d | 0.235 | 2.50 ± 0.06 f |
| WPI | 219.23 ± 6.93 e | −21.41 ± 0.49 b | 0.283 | 5.56 ± 0.07 e |
| SCN | 344.62 ± 8.08 d | −27.60 ± 0.48 c | 0.241 | 6.79 ± 0.09 d |
| HPMC | 609.13 ± 7.11 b | −12.41 ± 0.38 a | 0.689 | 12.45 ± 0.52 a |
| KGM | 372.94 ± 8.53 c | −13.23 ± 0.21 a | 0.501 | 11.03 ± 0.41 b |
| XGM | 1215.00 ± 8.89 a | −43.64 ± 0.98 e | 0.747 | 8.43 ± 0.40 c |
| Samples | Inhibitory Zone (mm) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | B. cereus | L. monocytogenes | S. enterica | E. coli | P. aeruginosa | P. fluorescens | S. putrefaciens | C. albicans | A. flavus | P. expansum | A. alternata | |
| Control | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| CEO-NE | 20.7 ± 0.65 b | 17.0 ± 0.59 b | 18.77 ± 0.55 b | 15.87 ± 0.64 b | 14.90 ± 0.46 b | 18.97 ± 0.93 b | 12.30 ± 0.44 b | 11.80 ± 0.40 b | 26.13 ± 0.25 b | 21.7 ± 0.65 b | 15.13 ± 0.83 a | 16.97 ± 0.45 b |
| ENT-NE | 12.8 ± 0.26 c | 13.7 ± 0.45 c | 12.43 ± 0.15 c | 13.13 ± 0.25 c | 11.80 ± 0.26 c | 10.87 ± 0.42 c | 12.07 ± 0.29 b | 11.67 ± 0.50 b | 11.73 ± 0.49 c | ND | ND | 9.90 ± 0.44 c |
| CO-NE | 42.4 ± 0.31 a | 34.6 ± 0.66 a | 30.63 ± 0.80 a | 33.67 ± 0.35 a | 25.17 ± 0.76 a | 30.83 ± 0.45 a | 25.40 ± 0.40 a | 21.83 ± 0.96 a | 38.93 ± 0.78 a | 22.2 ± 0.72 a | 16.33 ± 0.90 a | 30.30 ± 0.45 a |
| Storage Time (days) | Control | ENT-NE | CEO-NE | CO-NE | |
|---|---|---|---|---|---|
| L*(Lightness) | 0 | 53.15 ± 2.07 Aa | 53.83 ± 1.15 Aa | 54.62 ± 0.63 Aa | 53.93 ± 0.99 Aa |
| 7 | 53.84 ± 1.25 Aa | 54.40 ± 0.49 Aa | 54.19 ± 1.04 Aa | 53.52 ± 1.25 Aa | |
| 14 | 53.67 ± 0.72 Aa | 54.15 ± 1.10 Aa | 54.14 ± 0.40 Aa | 53.39 ± 0.34 Aa | |
| 21 | 52.85 ± 0.81 Aba | 52.47 ± 0.47 Aba | 53.74 ± 0.81 Aba | 53.45 ± 1.69 Aa | |
| 28 | 50.51 ± 0.46 Bb | 51.83 ± 0.57 Bab | 52.61 ± 0.97 Bca | 53.51 ± 1.31 Aa | |
| 35 | 44.07 ± 1.82 Cb | 51.38 ± 0.61 Ba | 51.88 ± 0.25 Ca | 53.17 ± 1.85 Aa | |
| 42 | 40.57 ± 1.38 Dd | 47.59 ± 0.76 Cc | 50.00 ± 0.84 Db | 53.03 ± 0.75 Aa | |
| 49 | 37.64 ± 1.70 Ec | 45.40 ± 0.88 Db | 46.34 ± 0.80 Eb | 52.86 ± 1.64 Aa | |
| a*(redness) | 0 | 22.06 ± 1.16 Aa | 22.09 ± 0.90 Aa | 22.14 ± 1.46 Aa | 21.78 ± 0.90 Aa |
| 7 | 20.45 ± 0.99 Aba | 21.99 ± 0.98 Aa | 22.24 ± 0.87 Aa | 21.89 ± 1.67 Aa | |
| 14 | 19.22 ± 1.40 Ba | 21.65 ± 0.72 Aa | 21.29 ± 1.02 Aa | 20.76 ± 1.01 Aa | |
| 21 | 19.79 ± 0.94 Bb | 20.91 ± 0.65 Aab | 21.44 ± 0.71 Aa | 20.88 ± 0.59 Aa | |
| 28 | 16.64 ± 0.49 Cb | 21.13 ± 1.08 Aa | 22.26 ± 0.85 Aa | 22.11 ± 1.05 Aa | |
| 35 | 15.64 ± 0.72 Cc | 19.26 ± 1.12 BCb | 19.80 ± 1.02 Aab | 22.04 ± 2.27 Aa | |
| 42 | 13.76 ± 1.30 Db | 17.70 ± 1.63 Ca | 17.70 ± 1.33 Ba | 20.19 ± 0.64 Aa | |
| 49 | 11.65 ± 0.95 Ec | 15.54 ± 1.96 Db | 17.04 ± 1.72 Bb | 21.14 ± 1.28 Aa | |
| b*(yellowness) | 0 | 28.28 ± 1.55 Ba | 27.35 ± 0.97 Aa | 28.30 ± 1.20 Aa | 27.92 ± 0.66 Aa |
| 7 | 28.18 ± 0.87 Ba | 27.75 ± 1.09 Aa | 28.11 ± 0.96 Aa | 28.09 ± 0.91 Aa | |
| 14 | 28.35 ± 0.60 Ba | 28.14 ± 0.51 Aa | 28.24 ± 1.66 Aa | 28.13 ± 1.54 Aa | |
| 21 | 28.57 ± 0.51 Ba | 28.45 ± 2.06 Aa | 28.54 ± 1.50 Aa | 28.09 ± 1.06 Aa | |
| 28 | 29.08 ± 1.88 Aba | 28.60 ± 0.69 Aa | 28.45 ± 1.67 Aa | 28.23 ± 1.72 Aa | |
| 35 | 30.08 ± 1.49 Aba | 29.03 ± 1.60 Aa | 29.04 ± 1.95 Aa | 28.27 ± 1.18 Aa | |
| 42 | 31.41 ± 1.70 Aa | 29.27 ± 1.86 Aa | 29.28 ± 1.53 Aa | 28.32 ± 1.91 Aa | |
| 49 | 30.60 ± 1.82 Aa | 29.63 ± 1.93 Aa | 29.51 ± 1.39 Aa | 28.55 ± 1.26 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, J.; Nie, R.; Du, J.; Sun, H.; Liu, G. Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets. Foods 2023, 12, 78. https://doi.org/10.3390/foods12010078
Duan J, Nie R, Du J, Sun H, Liu G. Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets. Foods. 2023; 12(1):78. https://doi.org/10.3390/foods12010078
Chicago/Turabian StyleDuan, Jiaojiao, Rong Nie, Jing Du, Haoxuan Sun, and Guorong Liu. 2023. "Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets" Foods 12, no. 1: 78. https://doi.org/10.3390/foods12010078
APA StyleDuan, J., Nie, R., Du, J., Sun, H., & Liu, G. (2023). Effect of Nanoemulsion Containing Enterocin GR17 and Cinnamaldehyde on Microbiological, Physicochemical and Sensory Properties and Shelf Life of Liquid-Smoked Salmon Fillets. Foods, 12(1), 78. https://doi.org/10.3390/foods12010078






