Effect of Seawater Curing Agent on the Flavor Profile of Dry-Cured Bacon Determined by Sensory Evaluation, Electronic Nose, and Fatty Composition Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Wet-/Dry-Cured and Dry-Aged Bacon with Various Curing Agents
2.2. Physicochemical and Microbial Composition Analysis
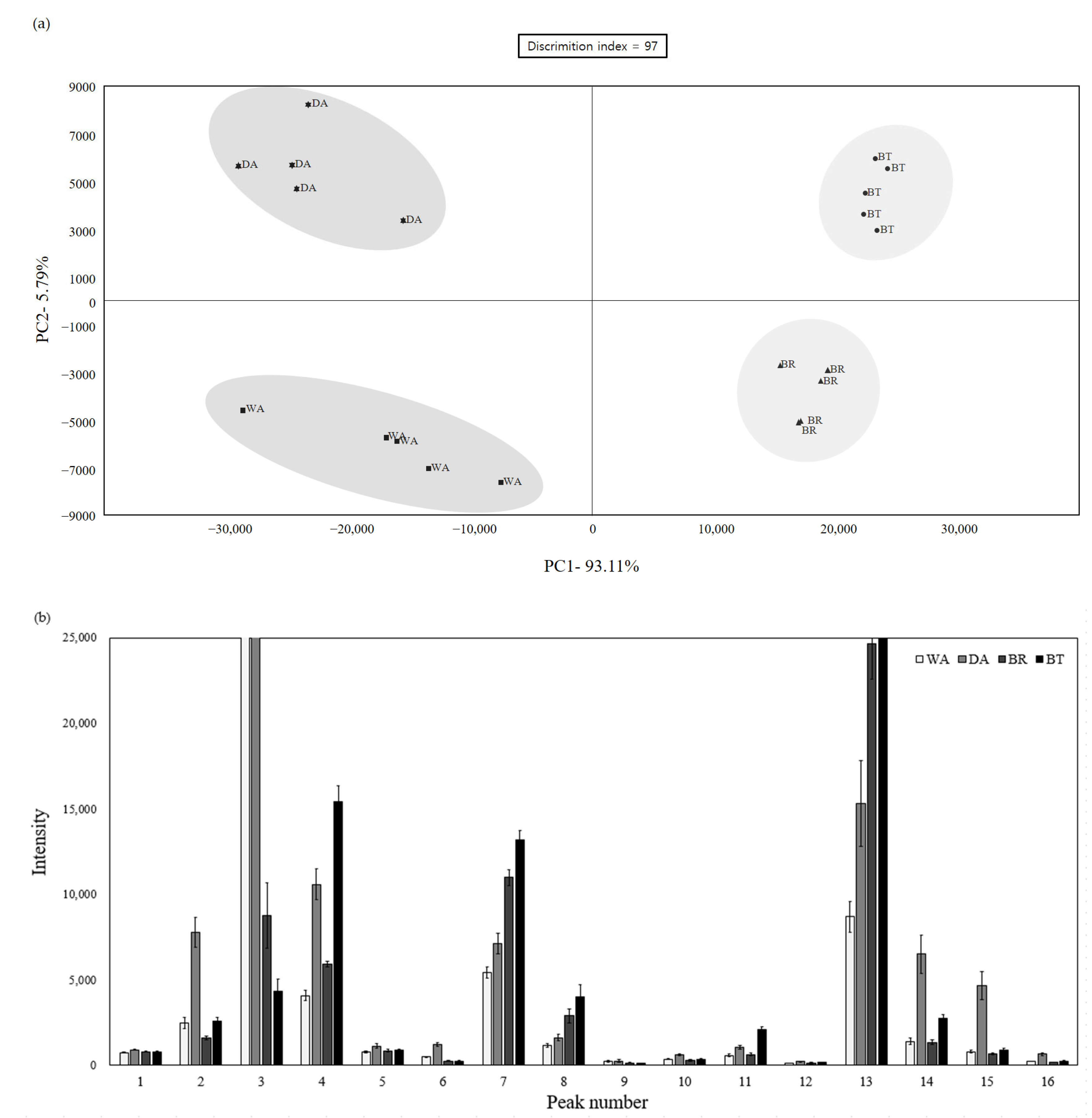
2.3. Electronic Nose
2.4. Fatty Acid Composition
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Properties
3.2. Microbial Composition
3.3. Electronic Nose
3.4. Fatty Acid Composition
3.5. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rysová, J.; Šmídová, Z. Effect of salt content reduction on food processing technology. Foods 2021, 10, 2237. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.E.; Glass, K.A. Sodium reduction and its effect on food safety, food quality, and human health. Compr. Rev. Food Sci. Food Saf. 2010, 9, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Flores, M. Understanding the implications of current health trends on the aroma of wet and dry cured meat products. Meat Sci. 2018, 144, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Ju, M.; Piao, C.; Zhang, J.; Mu, B.; Li, G.; Zhang, W. Hydrolysis of pork sarcoplasmic protein extracts by unique staphylococci isolated from low-salt dry-cured ham. LWT 2022, 164, 113639. [Google Scholar] [CrossRef]
- Sebranek, J.G. Basic curing ingredients. In Ingredients in Meat Products: Properties, Functionality and Applications; Springer: New York, NY, USA; CRC Press: Boca Raton, FL, USA, 2009; pp. 1–23. [Google Scholar]
- Toldrá, F. Curing dry. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, UK, 2014; pp. 425–429. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Carballo, J.; Cofrades, S. Healthier meat and meat products: Their role as functional foods. Meat Sci. 2001, 59, 5–13. [Google Scholar] [CrossRef]
- Tian, X.Y.; Aheto, J.H.; Huang, X.; Zheng, K.; Dai, C.; Wang, C.; Bai, J.W. An evaluation of biochemical, structural and volatile changes of dry-cured pork using a combined ion mobility spectrometry, hyperspectral and confocal imaging approach. J. Sci. Food Agric. 2021, 101, 5972–5983. [Google Scholar] [CrossRef]
- Flores, M. The eating quality of meat: III—Flavor. In Lawrie’s Meat Science; Woodhead Publishing: Sawston, UK, 2023; pp. 421–455. [Google Scholar]
- Kumar, Y. Development of low-fat/reduced-fat processed meat products using fat replacers and analogues. Food Rev. Int. 2021, 37, 296–312. [Google Scholar] [CrossRef]
- Dunkel, A.; Steinhaus, M.; Kotthoff, M.; Nowak, B.; Krautwurst, D.; Schieberle, P.; Hofmann, T. Nature’s chemical signatures in human olfaction: A foodborne perspective for future biotechnology. Angew. Chem. Int. Ed. 2014, 53, 7124–7143. [Google Scholar] [CrossRef]
- del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.; Plotto, A.; Dea, S. Electronic noses and tongues: Applications for the food and pharmaceutical industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef]
- Lehotay, S.J.; Hajšlová, J. Application of gas chromatography in food analysis. TrAC Trends Anal. Chem. 2002, 21, 686–697. [Google Scholar] [CrossRef]
- Jia, W.; Liang, G.; Jiang, Z.; Wang, J. Advances in electronic nose development for application to agricultural products. Food Anal. Methods 2019, 12, 2226–2240. [Google Scholar] [CrossRef]
- Suleman, R.; Hui, T.; Wang, Z.; Alarcon-Rojo, A.D.; Liu, H.; Zhang, D. Semi-quantitative and qualitative distinction of aromatic and flavour compounds in charcoal grilled, electric barbecue grilled, infrared grilled and superheated-steam roasted lamb meat patties using GC/MC, E-nose and E-tongue. Separations 2022, 9, 71. [Google Scholar] [CrossRef]
- Kim, Y.H.B.; Kemp, R.; Samuelsson, L.M. Effects of dry-aging on meat quality attributes and metabolite profiles of beef loins. Meat Sci. 2016, 111, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Choe, J.; Kim, M.; Kim, H.C.; Yoon, J.W.; Oh, S.W.; Jo, C. Role of moisture evaporation in the taste attributes of dry-and wet-aged beef determined by chemical and electronic tongue analyses. Meat Sci. 2019, 151, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Bardi, U. Extracting minerals from seawater: An energy analysis. Sustainability 2010, 2, 980–992. [Google Scholar] [CrossRef]
- Davies, P.A.; Knowles, P.R. Seawater bitterns as a source of liquid desiccant for use in solar-cooled greenhouses. Desalination 2006, 196, 266–279. [Google Scholar] [CrossRef]
- Ko, K.H.; Moon, S.H.; Yoo, Y.J.; Kim, I.C. Characteristics of soybean curds manufactured by various bitterns. Korean J. Food Preserv. 2013, 20, 37–44. [Google Scholar] [CrossRef]
- Ryu, J.P.; Yang, J.H.; Chung, Y.B.; Lee, S.I.; Han, E.S. Quality characteristics of baechu-kimchi salted at high salt concentration for a short time. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1913–1919. [Google Scholar] [CrossRef]
- Uttaro, B.; Badoni, M.; Zawadski, S.; Gill, C.O. Effects of the pressure, flow rate and delivered volume of brine on the distributions of brine and bacteria in brine-injected meat. Food Control 2011, 22, 180–185. [Google Scholar] [CrossRef]
- Sujiwo, J.; Kim, D.; Jang, A. Relation among quality traits of chicken breast meat during cold storage: Correlations between freshness traits and wor values. Poult. Sci. 2018, 97, 2887–2894. [Google Scholar] [CrossRef] [PubMed]
- Tatledgis, B.G.; Pearson, K.M.; Dugan, L.R. A distillation method for the qualitative determination of malonaldehyde in rancid food. J. Am. Oil Chem. Soc. 1960, 37, 44–47. [Google Scholar] [CrossRef]
- Hwang, J.; Lee, S.; Jo, M.; Cho, W.; Moon, J. The effect of sustainability-related information on the sensory evaluation and purchase behavior towards salami products. Food Sci. Anim. Res. 2021, 41, 95. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Cañedo, A.; Martínez-Onandi, N.; Gaya, P.; Nuñez, M.; Picon, A. Effect of high-pressure processing and chemical composition on lipid oxidation, aminopeptidase activity and free amino acids of Serrano dry-cured ham. Meat Sci. 2021, 172, 108349. [Google Scholar] [CrossRef]
- Hereu, A.; Bover-Cid, S.; Garriga, M.; Aymerich, T. High hydrostatic pressure and biopreservation of dry-cured ham to meet the Food Safety Objectives for Listeria monocytogenes. Int. J. Food Microbiol. 2012, 154, 107–112. [Google Scholar] [CrossRef]
- Jittinandana, S.; Kenney, P.B.; Slider, S.D.; Kiser, R.A. Effect of brine concentration and brining time on quality of smoked rainbow trout fillets. J. Food Sci. 2002, 67, 2095–2099. [Google Scholar] [CrossRef]
- Chaijan, M. Physicochemical changes of tilapia (Oreochromis niloticus) muscle during salting. Food Chem. 2011, 129, 1201–1210. [Google Scholar] [CrossRef]
- Yavuz, M.; Kasavi, C.; Öner, E.T. Developments in effective use of volatile organic compound analysis to assess flavour formation during cheese ripening. J. Dairy Res. 2021, 88, 461–467. [Google Scholar] [CrossRef]
- Montel, M.C.; Masson, F.; Talon, R. Bacterial role in flavour development. Meat Sci. 1998, 49, S111–S123. [Google Scholar] [CrossRef]
- Sanz, Y.; Toldrá, F. Purification and characterization of an arginine aminopeptidase from Lactobacillus sakei. Appl. Environ. Microbiol. 2002, 68, 1980–1987. [Google Scholar] [CrossRef]
- Zagorec, M.; Champomier-Vergès, M.C. Lactobacillus sakei: A starter for sausage fermentation, a protective culture for meat products. Microorganisms 2017, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hou, J.; Zhang, X.; Hu, J.; Yu, Z.; Zhu, Y. Improving the flavor of fermented sausage by increasing its bacterial quality via inoculation with lactobacillus plantarum MSZ2 and staphylococcus xylosus YCC3. Foods 2022, 11, 736. [Google Scholar] [CrossRef] [PubMed]
- Flores, M.; Toldra, F. Microbial enzymatic activities for improved fermented meats. Trends Food Sci. Technol. 2011, 22, 81–90. [Google Scholar] [CrossRef]
- Benito, M.J.; Rodríguez, M.; Núnez, F.; Asensio, M.A.; Bermúdez, M.E.; Córdoba, J.J. Purification and characterization of an extracellular protease from Penicillium chrysogenum Pg222 active against meat proteins. Appl. Environ. Microbiol. 2002, 68, 3532–3536. [Google Scholar] [CrossRef] [PubMed]
- Mota, I.; Teixeira-Santos, R.; Rufo, J.C. Detection and identification of fungal species by electronic nose technology: A systematic review. Fungal Biol. Rev. 2021, 37, 59–70. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Li, J. Aroma compounds in wine. In Grape and Wine Biotechnol; InTech: London, UK, 2016; pp. 273–283. [Google Scholar]
- Lee, D.; Lee, H.J.; Yoon, J.W.; Kim, M.; Jo, C. Effect of different aging methods on the formation of aroma volatiles in beef strip loins. Foods 2021, 10, 146. [Google Scholar] [CrossRef]
- Khan, M.I.; Jo, C.; Tariq, M.R. Meat flavor precursors and factors influencing flavor precursors—A systematic review. Meat Sci. 2015, 110, 278–284. [Google Scholar] [CrossRef]
- Xu, Y.; Shui, M.; Chen, D.; Ma, X.; Feng, T. Optimization of Jinhua ham classification method based on volatile flavor substances and determination of key odor biomarkers. Molecules 2022, 27, 7087. [Google Scholar] [CrossRef]
- Aquilani, C.; Pérez-Palacios, T.; Sirtori, F.; Jiménez-Martín, E.; Antequera, T.; Franci, O.; Acciaioli, A.; Bozzi, R.; Pugliese, C. Enrichment of Cinta Senese burgers with omega-3 fatty acids. Effect of type of addition and storage conditions on quality characteristics. Grasas Aceites 2008, 69, e235. [Google Scholar] [CrossRef]
- Sara, L.; Konda, S.; Nikitha, B.; Palupanuri, N. Phospholipid fatty acid profile of Spirulina platensis. In GeNeDis 2020: Genetics and Neurodegenerative Diseases; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 1339, pp. 161–167. [Google Scholar] [CrossRef]
- Canalis, M.B.; Baroni, M.V.; León, A.E.; Ribotta, P.D. Effect of peach puree incorporation on cookie quality and on simulated digestion of polyphenols and antioxidant properties. Food Chem. 2020, 333, 127464. [Google Scholar] [CrossRef]
- Chen, X.; Luo, J.; Lou, A.; Wang, Y.; Yang, D.; Shen, Q.W. Duck breast muscle proteins, free fatty acids and volatile compounds as affected by curing methods. Food Chem. 2021, 338, 128138. [Google Scholar] [CrossRef] [PubMed]
- Nogalski, Z.; Pogorzelska-Przybyłek, P.; Sobczuk-Szul, M.; Nogalska, A.; Modzelewska-Kapituła, M.; Purwin, C. Carcass characteristics and meat quality of bulls and steers slaughtered at two different ages. Ital. J. Anim. Sci. 2018, 17, 279–288. [Google Scholar] [CrossRef]
- Hunt, M.R.; Legako, J.F.; Dinh, T.T.N.; Garmyn, A.J.; O’Quinn, T.G.; Corbin, C.H.; Rathmann, R.J.; Brooks, J.C.; Miller, M.F. Assessment of volatile compounds, neutral and polar lipid fatty acids of four beef muscles from USDA Choice and Select graded carcasses and their relationships with consumer palatability scores and intramuscular fat content. Meat Sci. 2016, 116, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Hossain, A. Role of Lipids in Food Flavor Generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]

| Table | Treatments 1 | p-Value | |||
|---|---|---|---|---|---|
| WA | DA | BR | BT | ||
| pH | 5.65 ± 0.05 bc | 5.68 ± 0.01 b | 5.61 ± 0.05 c | 5.92 ± 0.08 a | *** |
| Water activity (%) | 93.16 ± 0.55 b | 92.41 ± 0.06 c | 92.51 ± 0.04 c | 93.83 ± 0.16 a | ** |
| Salinity (%) | 1.41 ± 0.18 b | 1.61 ± 0.27 a | 1.35 ± 0.11 b | 1.38 ± 0.13 b | * |
| Aging yield (%) | 75.66 ± 1.66 NS | 74.95 ± 1.30 | 75.87 ± 1.56 | 75.36 ± 1.12 | NS |
| VBN (mg/100 g) | 4.01 ± 0.86 b | 5.08 ± 1.38 a | 2.89 ± 1.11 c | 3.45 ± 0.25 bc | *** |
| TBARS (MDA/g) | 0.96 ± 0.07 b | 2.27 ± 0.24 a | 0.84 ± 0.07 b | 0.81 ± 0.04 b | *** |
| Table 1 | Treatments 2 | Aging Period (Week) | p-Value 3 | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| AC | WA | 6.78 ± 0.88 b | 7.41 ±0.36 Aa | 7.46 ± 0.36 a | 7.26 ± 0.42 ab | T ** P *** T × P NS |
| DA | 6.75 ± 0.93 | 6.91 ± 0.48 AB | 7.17 ± 0.48 | 7.06 ± 0.48 | ||
| BR | 6.59 ±0.43 b | 7.16 ± 0.60 ABa | 7.37 ± 0.37 a | 7.26 ± 0.50 a | ||
| BT | 6.53 ± 0.30 | 6.70 ± 0.92 B | 7.02 ± 0.28 | 6.81 ± 0.51 | ||
| MRS | WA | 6.01 ± 0.34 b | 6.29 ± 0.33 b | 6.87 ± 0.58 ABa | 6.19 ± 0.89 b | T NS P *** T × P NS |
| DA | 5.92 ± 0.33 b | 6.25 ± 0.46 a | 6.42 ± 0.12 Ba | 6.20 ± 0.26 ab | ||
| BR | 5.80 ± 0.18 c | 6.42 ± 0.46 b | 7.10 ± 0.74 Aa | 6.30 ± 0.72 bc | ||
| BT | 5.87 ± 0.51 c | 6.15 ± 0.35 bc | 6.62 ± 0.32 ABa | 6.35 ± 0.04 ab | ||
| MSA | WA | 4.59 ± 0.26 b | 5.03 ± 0.89 b | 5.24 ± 0.75 ab | 5.72 ± 0.34 Ba | T NS P *** T × P NS |
| DA | 4.64 ± 1.22 b | 4.93 ± 0.80 b | 5.18 ± 0.81 ab | 5.83 ± 0.49 Ba | ||
| BR | 4.03 ± 0.35 c | 4.64 ± 1.25 bc | 5.04 ± 1.13 b | 6.14 ± 0.58 ABa | ||
| BT | 4.26 ± 0.59 b | 4.72 ± 1.17 b | 5.10 ± 0.55 b | 6.52 ± 0.37 Aa | ||
| PDA | WA | 4.34 ± 0.69 Bc | 4.99 ± 0.65 b | 5.96 ± 0.54 a | 6.15 ± 0.24 Aa | T NS P *** T × P NS |
| DA | 4.29 ± 0.55 Bb | 5.19 ± 0.98 a | 5.50 ± 1.02 a | 5.66 ± 0.26 Ca | ||
| BR | 5.16 ± 0.32 Ac | 5.37 ± 0.60 bc | 5.68 ± 0.66 ab | 5.91 ± 0.16 Ba | ||
| BT | 4.91 ± 0.31 Ab | 5.15 ± 1.22 ab | 5.60 ± 0.30 ab | 5.85 ± 0.31 BCa | ||
| Traits 1 | Treatments 2 | p-Value | |||
|---|---|---|---|---|---|
| WA | DA | BR | BT | ||
| C14:0 | 1.400 ± 0.02 NS | 1.458 ± 0.07 | 1.459 ± 0.02 | 1.379 ± 0.04 | NS |
| C16:0 | 24.937 ± 0.22 NS | 26.541 ± 1.58 | 25.040 ± 1.10 | 24.987 ± 1.70 | NS |
| C16:1n7 | 1.482 ± 0.01 NS | 1.423 ± 0.14 | 0.935 ± 0.12 | 1.466 ± 0.04 | NS |
| C18:0 | 12.434 ± 0.68 NS | 10.691 ± 1.79 | 13.544 ± 0.71 | 10.155 ± 1.36 | NS |
| C18:1n9 | 42.800 ± 0.43 NS | 41.338 ± 1.77 | 43.340 ± 1.02 | 42.598 ± 0.82 | NS |
| C18:1n7 | 0.102 ± 0.04 NS | 0.127 ± 0.02 | 0.104 ± 0.05 | 0.120 ± 0.05 | NS |
| C18:2n6 | 14.696 ± 0.14 NS | 15.952 ± 1.03 | 13.502 ± 0.23 | 16.739 ± 0.97 | NS |
| C18:3n6 | 0.017 ± 0.01 b | 0.018 ± 0.01 b | 0.023 ± 0.00 b | 0.029 ± 0.01 a | *** |
| C18:3n3 | 1.169 ± 0.06 NS | 1.330 ± 0.18 | 0.991 ± 0.04 | 1.397 ± 0.16 | NS |
| C20:1n9 | 0.588 ± 0.08 NS | 0.704 ± 0.09 | 0.649 ± 0.09 | 0.632 ±0.24 | NS |
| C20:4n6 | 0.267 ± 0.01 NS | 0.306 ± 0.01 | 0.300 ± 0.01 | 0.362 ± 0.02 | NS |
| C20:5n3 | 0.013 ± 0.01 b | 0.015 ± 0.01 ab | 0.011 ± 0.01 b | 0.019 ± 0.02 a | * |
| C22:4n6 | 0.066 ± 0.01 NS | 0.068 ± 0.01 | 0.077 ± 0.01 | 0.083 ± 0.01 | NS |
| C22:6n3 | 0.029 ± 0.01 NS | 0.030 ± 0.01 | 0.025 ± 0.01 | 0.036 ± 0.01 | NS |
| SFA | 38.770 ± 0.13 NS | 38.691 ± 1.01 | 40.043 ± 1.06 | 36.521 ± 0.62 | NS |
| UFA | 61.230 ± 0.48 NS | 61.309 ± 1.18 | 59.957 ± 1.83 | 63.479 ± 0.34 | NS |
| MUFA | 44.973 ± 0.56 NS | 43.591 ± 1.97 | 45.027 ± 1.00 | 44.815 ± 0.49 | NS |
| PUFA | 16.257 ± 0.08 bc | 17.718 ± 1.21 ab | 14.929 ± 0.18 c | 18.664 ± 1.11 a | * |
| UFA/SFA | 1.580 ± 0.03 NS | 1.597 ± 0.22 | 1.500 ± 0.11 | 1.739 ± 0.05 | NS |
| MUFA/SFA | 1.160 ± 0.03 NS | 1.135 ± 0.15 | 1.127 ± 0.10 | 1.227 ± 0.01 | NS |
| PUFA/SFA | 0.419 ± 0.01 ab | 0.462 ± 0.07 ab | 0.373 ± 0.01 b | 0.511 ± 0.04 a | NS |
| n6 | 15.046 ± 0.14 bc | 16.344 ± 1.03 ab | 13.902 ± 0.22 b | 17.211 ± 0.95 a | * |
| n3 | 1.211 ± 0.06 ab | 1.374 ± 0.18 ab | 1.028 ±0.04 b | 1.452 ± 0.16 a | NS |
| n6/n3 | 12.439 ± 0.73 NS | 11.959 ± 0.77 | 13.541 ± 0.77 | 11.886 ± 0.63 | NS |
| Traits | Treatments 1 | p-Value | ||||
|---|---|---|---|---|---|---|
| WA | DA | BR | BT | |||
| Taste | Salty | 4.38 ± 0.95 | 4.82 ± 0.88 | 4.05 ± 0.93 | 5.09 ± 1.26 | NS |
| Sour | 4.56 ± 1.07 | 5.20 ± 0.88 | 4.27 ± 1.12 | 5.36 ± 1.03 | NS | |
| Greasy | 4.91 ± 0.71 | 5.14 ± 0.81 | 4.82 ± 0.94 | 5.27 ± 0.99 | NS | |
| Overall | 5.36 ± 1.42 | 5.50 ± 0.61 | 5.45 ± 0.82 | 5.73 ± 1.42 | NS | |
| Flavor | Milky | 4.25 ± 0.89 b | 5.17 ± 1.33 ab | 4.92 ± 1.02 ab | 6.00 ± 0.89 a | * |
| Cheesy | 4.83 ± 0.75 b | 5.30 ± 1.06 b | 4.86 ± 1.07 b | 6.14 ± 0.69 a | * | |
| Rancid | 5.63 ± 1.30 | 5.50 ± 1.07 | 5.38 ± 1.19 | 5.89 ± 0.93 | NS | |
| Overall | 4.83 ± 1.22 b | 5.50 ± 0.79 ab | 5.06 ± 0.81 b | 6.09 ± 0.83 a | * | |
| Texture | Juicy | 4.82 ± 1.17 b | 5.91 ± 0.94 a | 5.82 ± 0.75 a | 5.94 ± 0.81 a | * |
| Gummy | 5.00 ± 1.73 b | 5.78 ± 0.83 ab | 5.55 ± 0.69 ab | 6.10 ± 0.74 a | NS | |
| Overall | 4.86 ± 1.52 b | 5.77 ± 1.09 ab | 5.50 ± 0.53 ab | 6.09 ± 0.83 a | NS | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Kim, H.-Y. Effect of Seawater Curing Agent on the Flavor Profile of Dry-Cured Bacon Determined by Sensory Evaluation, Electronic Nose, and Fatty Composition Analysis. Foods 2023, 12, 1974. https://doi.org/10.3390/foods12101974
Lee S-H, Kim H-Y. Effect of Seawater Curing Agent on the Flavor Profile of Dry-Cured Bacon Determined by Sensory Evaluation, Electronic Nose, and Fatty Composition Analysis. Foods. 2023; 12(10):1974. https://doi.org/10.3390/foods12101974
Chicago/Turabian StyleLee, Sol-Hee, and Hack-Youn Kim. 2023. "Effect of Seawater Curing Agent on the Flavor Profile of Dry-Cured Bacon Determined by Sensory Evaluation, Electronic Nose, and Fatty Composition Analysis" Foods 12, no. 10: 1974. https://doi.org/10.3390/foods12101974
APA StyleLee, S.-H., & Kim, H.-Y. (2023). Effect of Seawater Curing Agent on the Flavor Profile of Dry-Cured Bacon Determined by Sensory Evaluation, Electronic Nose, and Fatty Composition Analysis. Foods, 12(10), 1974. https://doi.org/10.3390/foods12101974








