Effects of pH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Characterization of the Emulsion
2.3.1. pH Treatment of Emulsion
2.3.2. Ionic Strength
2.3.3. Zeta Potential
2.3.4. Particle Size Distribution
2.3.5. Creaming Stability
2.3.6. Protein Mass on Fat Globule Surface
2.3.7. Viscosity
2.4. Characterization of Aerated Emulsions
2.4.1. Overrun
2.4.2. Firmness
2.5. Data Analysis
3. Results and Discussion
3.1. Effects of pH on the Stability and Aeration Characteristics of Dairy Emulsion
3.1.1. Emulsion Stability
3.1.2. Aeration Characteristics
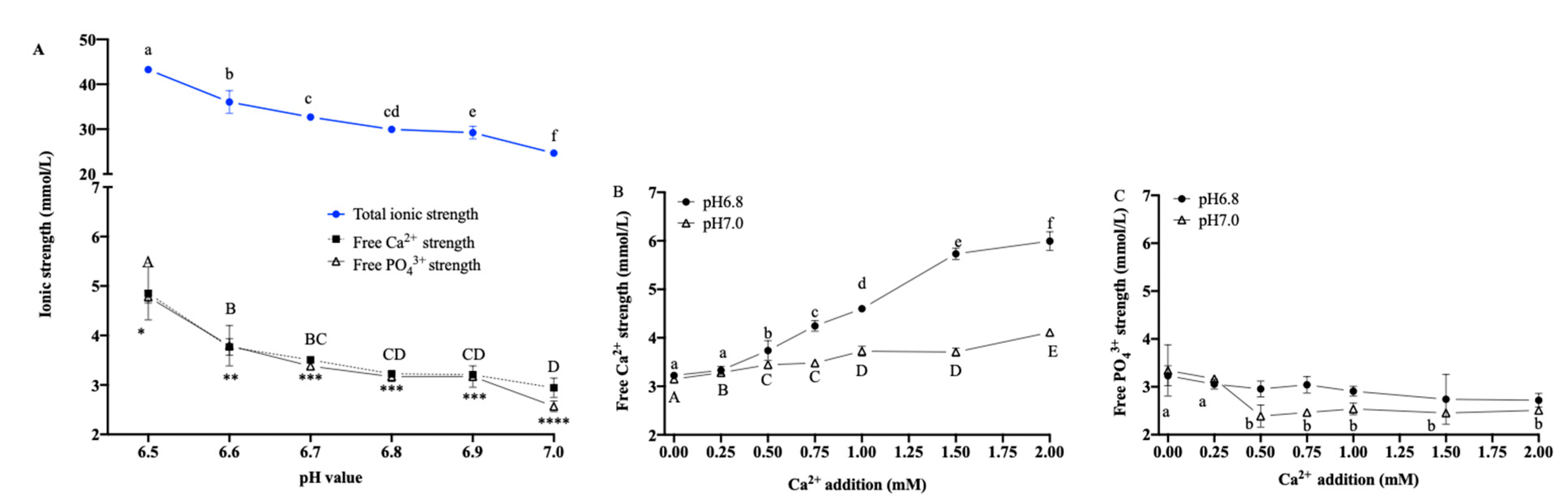
3.2. Ionic Strength with Multiple pH Values
3.3. Effects of Ionic Strength on the Stability and Aeration Characteristics of Dairy Emulsion
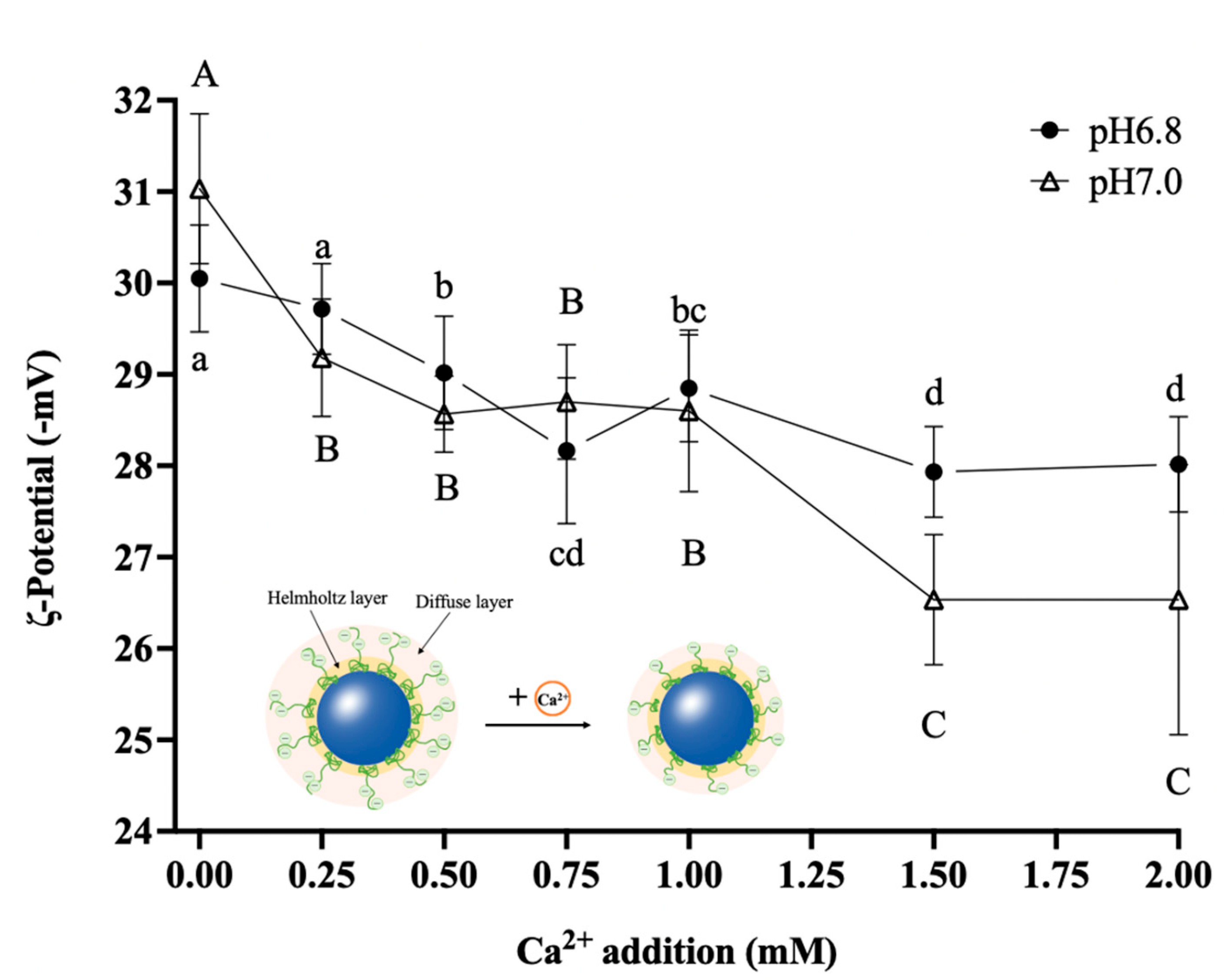
3.3.1. Zeta Potential
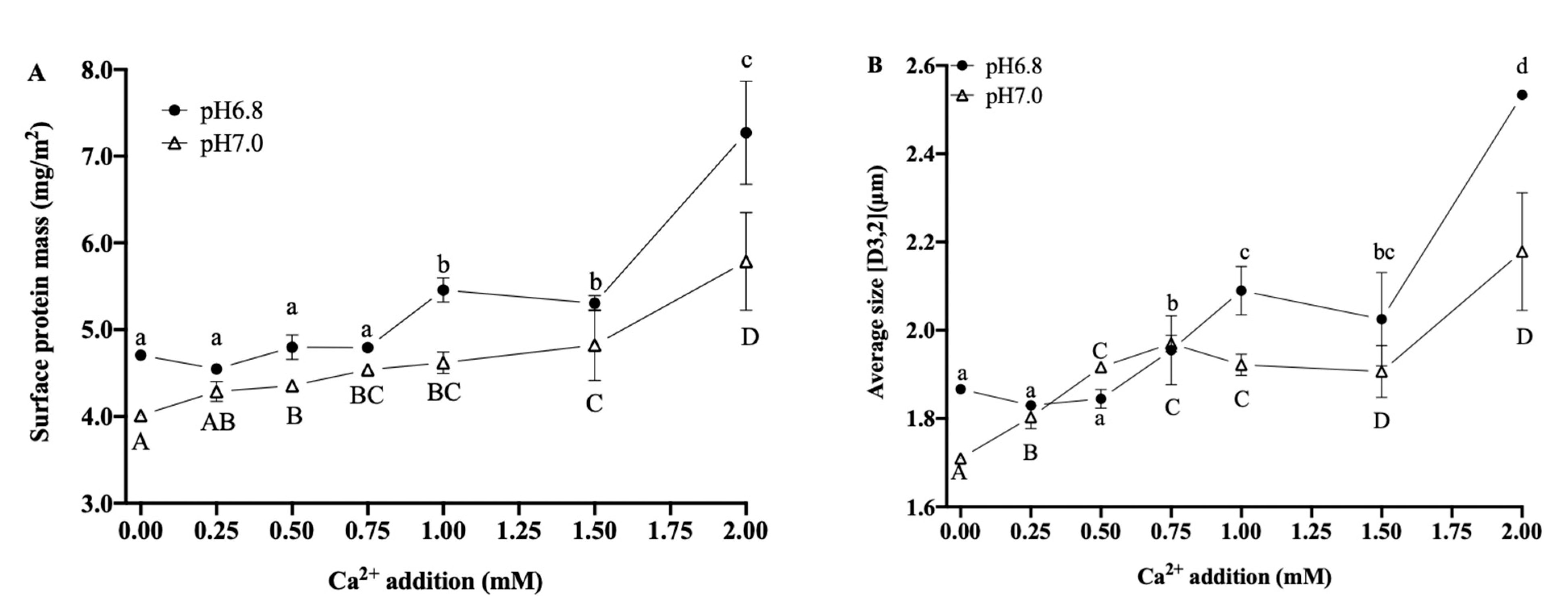
3.3.2. Interfacial Proteins and Particle Size
3.3.3. O/W Emulsion Stability
3.3.4. Aeration Characteristics
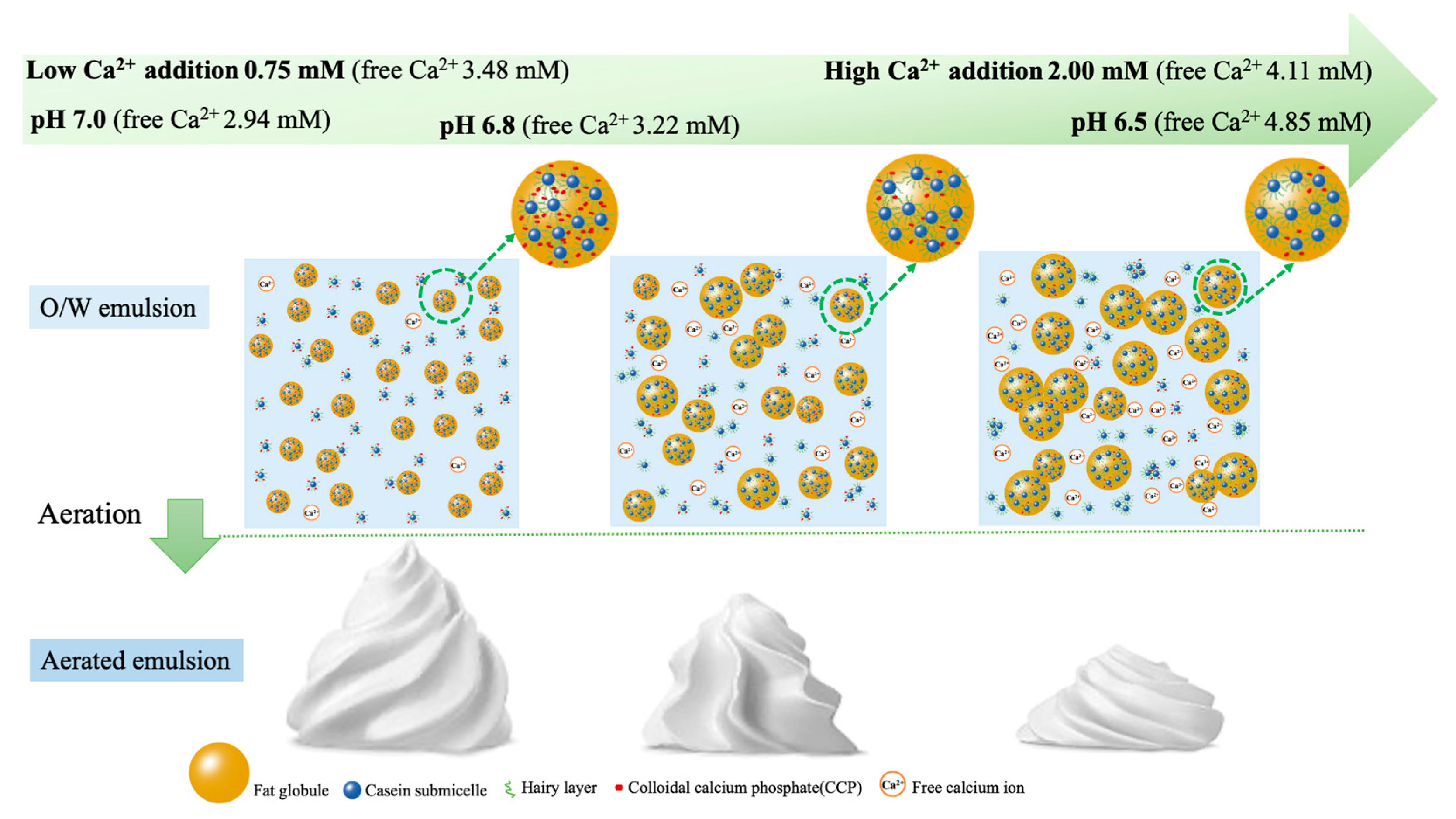
3.4. Effects of Free Ca2+ Strength on Emulsion Stability and Aeration Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sajedi, M.; Nasirpour, A.; Keramat, J.; Desobry, S. Effect of modified whey protein concentrate on physical properties and stability of whipped cream. Food Hydrocoll. 2014, 36, 93–101. [Google Scholar] [CrossRef]
- Wang, Y.; Hartel, R.W.; Zhang, L. The stability of aerated emulsions: Effects of emulsifier synergy on partial coalescence and crystallization of milk fat. J. Food Eng. 2021, 291, 110257. [Google Scholar] [CrossRef]
- Hunt, J.A.; Dalgleish, D.G. Heat Stability of Oil-in-Water Emulsions Containing Milk Proteins: Effect of Ionic Strength and pH. J. Food Sci. 1995, 60, 1120–1123. [Google Scholar] [CrossRef]
- Zhang, Z.; Dalgleish, D.G.; Goff, H.D. Effect of pH and ionic strength on competitive protein adsorption to air/water interfaces in aqueous foams made with mixed milk proteins. Colloids Surf. B Biointerfaces 2004, 34, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Petursson, S.; Decker, E.A.; Mcclements, D.J. Stabilization of Oil-in-Water Emulsions by Cod Protein Extracts. J. Agric. Food Chem. 2004, 52, 3996–4001. [Google Scholar] [CrossRef]
- Bengoechea, C.; Romero, A.; Aguilar, J.M.; Cordobés, F.; Guerrero, A. Temperature and pH as factors influencing droplet size distribution and linear viscoelasticity of O/W emulsions stabilised by soy and gluten proteins. Food Hydrocoll. 2010, 24, 783–791. [Google Scholar] [CrossRef]
- Anema, S.G.; Lowe, E.K.; Li, Y. Effect of pH on the viscosity of heated reconstituted skim milk. Int. Dairy J. 2004, 14, 541–548. [Google Scholar] [CrossRef]
- Allen, K.E.; Dickinson, E.; Murray, B. Acidified sodium caseinate emulsion foams containing liquid fat: A comparison with whipped cream. LWT-Food Sci. Technol. 2006, 39, 225–234. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, D.; Wang, Y.; Li, M.; Zhang, L. The effect of caseins on the stability and whipping properties of recombined dairy creams. Int. Dairy J. 2020, 105, 104658. [Google Scholar] [CrossRef]
- Holt, C.; Carver, J.A.; Ecroyd, H.; Thorn, D.C. Invited review: Caseins and the casein micelle: Their biological functions, structures, and behavior in foods. J. Dairy Sci. 2013, 96, 6127–6146. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, D.; Yang, H.; Zhang, J.; Liu, X.; Regenstein, J.M.; Hemar, Y.; Zhou, P. Effect of calcium sequestration by ion-exchange treatment on the dissociation of casein micelles in model milk protein concentrates. Food Hydrocoll. 2016, 60, 59–66. [Google Scholar] [CrossRef]
- Lin, L.; Oh, H.; Deeth, H.; Wong, M. The effects of casein and whey proteins on the rheological properties of calcium-induced skim milk gels. Int. Dairy J. 2021, 113, 104893. [Google Scholar] [CrossRef]
- Shao, P.; Ma, H.; Zhu, J.; Qiu, Q. Impact of ionic strength on physicochemical stability of o/w emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocoll. 2017, 69, 202–209. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, A.L.; O’Mahony, J.A.; Fenelon, M.A. Sensitivity of emulsions stabilised by bovine β-casein and lactoferrin to heat and CaCl2. Food Hydrocoll. 2014, 35, 420–428. [Google Scholar] [CrossRef]
- Schiffer, S.; Scheidler, E.; Kiefer, T.; Kulozik, U. Effect of temperature, added calcium and pH on the equilibrium of caseins between micellar state and milk serum. Foods 2021, 10, 822. [Google Scholar] [CrossRef]
- Keowmaneechai, E.; Mcclements, D.J. Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2. J. Agric. Food Chem. 2002, 50, 7145–7153. [Google Scholar] [CrossRef]
- Phan-Xuan, T.; Durand, D.; Nicolai, T.; Donato, L.; Schmitt, C.; Bovetto, L. Heat induced formation of beta-lactoglobulin microgels driven by addition of calcium ions. Food Hydrocoll. 2014, 34, 227–235. [Google Scholar] [CrossRef]
- Considine, T.; Patel, H.; Anema, S.; Singh, H.; Creamer, L. Interactions of milk proteins during heat and high hydrostatic pressure treatments—A review. Innov. Food Sci. Emerg. Technol. 2007, 8, 1–23. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; D’arcy, B.R.; Deeth, H.C.; Oh, H.E. The rheological properties of calcium-induced milk gels. J. Food Eng. 2014, 130, 45–51. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, D.; Li, Y.; Li, M.; Wang, Y.; Li, Y.; Zhang, L. Thermodynamic and whipping properties of milk fat in whipped cream: A study based on DSC and TD-NMR. Int. Dairy J. 2019, 97, 149–157. [Google Scholar] [CrossRef]
- Fredrick, E.; Heyman, B.; Moens, K.; Fischer, S.; Verwijlen, T.; Moldenaers, P.; Van der Meeren, P.; Dewettinck, K. Monoacylglycerols in dairy recombined cream: II. The effect on partial coalescence and whipping properties. Food Res. Int. 2013, 51, 936–945. [Google Scholar] [CrossRef]
- Ezeh, V.N.; Lewis, M.J. Milk reversibility following reduction and restoration of pH. Int. J. Dairy Technol. 2011, 64, 179–187. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, J.; Zhou, Y.; Cheng, Y.; Nirasawa, S. Improving solubility and emulsifying property of wheat gluten by deamidation with four different acids: Effect of replacement of folded conformation by extended structure. Food Hydrocoll. 2017, 72, 105–114. [Google Scholar] [CrossRef]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and citrate on thermal stability of whey protein stabilized oil-in-water emulsions containing calcium chloride. Food Res. Int. 2006, 39, 230–239. [Google Scholar] [CrossRef]
- Ye, A.; Srinivasan, M.; Singh, H. Influence of NaCl Addition on the Properties of Emulsions Formed with Commercial Calcium Caseinate. Food Chem. 2000, 69, 237–244. [Google Scholar] [CrossRef]
- International IDF Standard. 20B:1993: Milk: Determination of nitrogen content: Inspection by Attributes; International Dairy Federation: Schaerbeek, Belgium, 1993. [Google Scholar]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Effects of pH on protein–protein interactions and implications for protein phase behavior. Biochim. Biophys. Acta 2008, 1784, 600–610. [Google Scholar] [CrossRef]
- Fredrick, E.; Walstra, P.; Dewettinck, K. Factors governing partial coalescence in oil-in-water emulsions. Adv. Colloid Interface Sci. 2010, 153, 30–42. [Google Scholar] [CrossRef]
- Bigikocin, E.; Mert, B.; Alpas, H. Effect of high hydrostatic pressure and high dynamic pressure on stability and rheological properties of model oil-in-water emulsions. High Press. Res. 2011, 31, 462–474. [Google Scholar] [CrossRef]
- Gonzalez-Jordan, A.; Thomar, P.; Nicolai, T.; Dittmer, J. The effect of pH on the structure and phosphate mobility of casein micelles in aqueous solution. Food Hydrocoll. 2015, 51, 88–94. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Cao, C.; Kong, B.; Wang, H.; Zhang, H.; Liu, Q. Effects of different pH conditions on interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Hydrocoll. 2022, 131, 107752. [Google Scholar] [CrossRef]
- Sriprablom, J.; Luangpituksa, P.; Wongkongkatep, J.; Pongtharangkul, T.; Suphantharika, M. Influence of pH and ionic strength on the physical and rheological properties and stability of whey protein stabilized o/w emulsions containing xanthan gum. J. Food Eng. 2019, 242, 141–152. [Google Scholar] [CrossRef]
- Ladjal-Ettoumi, Y.; Boudries, H.; Chibane, M.; Romero, A. Pea, chickpea and lentil protein isolates: Physicochemical characterization and emulsifying properties. Food Biophys. 2016, 11, 43–51. [Google Scholar] [CrossRef]
- Hotrum, N.E.; Stuart, M.A.C.; van Vliet, T.; Avino, S.F.; van Aken, G.A. Elucidating the relationship between the spreading coefficient, surface-mediated partial coalescence and the whipping time of artificial cream. Colloids Surf. A Physicochem. Eng. Asp. 2005, 260, 71–78. [Google Scholar] [CrossRef]
- Zhao, Q.; Kuang, W.; Long, Z.; Fang, M.; Liu, D.; Yang, B.; Zhao, M. Effect of sorbitan monostearate on the physical characteristics and whipping properties of whipped cream. Food Chem. 2013, 141, 1834–1840. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, M.; Li, J.; Yang, B.; Su, G.; Cui, C.; Jiang, Y. Effect of hydroxypropyl methylcellulose on the textural and whipping properties of whipped cream. Food Hydrocoll. 2009, 23, 2168–2173. [Google Scholar] [CrossRef]
- Comas, D.; Wagner, J.; Tomás, M. Creaming stability of oil in water (O/W) emulsions: Influence of pH on soybean protein–lecithin interaction. Food Hydrocoll. 2006, 20, 990–996. [Google Scholar] [CrossRef]
- Mancuso, J.R.; McClements, D.J.; Decker, E.A. The effects of surfactant type, pH, and chelators on the oxidation of salmon oil-in-water emulsions. J. Agric. Food Chem. 1999, 47, 4112–4116. [Google Scholar] [CrossRef]
- Marchin, S.; Putaux, J.-L.; Pignon, F.; Léonil, J. Effects of the environmental factors on the casein micelle structure studied by cryo transmission electron microscopy and small-angle x-ray scattering/ultrasmall-angle X-ray scattering. J. Chem. Phys. 2007, 126, 01B617. [Google Scholar] [CrossRef]



| pH | Aeration Characteristics | Ca2+ addition (mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 0.25 | 0.50 | 0.75 | 1.00 | 1.50 | 2.00 | ||
| 6.8 | Aeration time/s | 116.00 ± 11.37 a | 114.25 ± 2.22 ab | 102.5 ± 5.32 c | 106.25 ± 4.72 bc | 105.25 ± 7.14 bc | 98.75 ± 1.50 cd | 92.33 ± 4.16 d |
| Overrun/% | 158.44 ± 2.26 ab | 159.99 ± 3.24 a | 159.43 ± 0.64 a | 155.17 ± 0.94 ab | 154.41 ± 5.12 ab | 153.07 ± 2.88 b | 153.31 ± 2.48 b | |
| Foam firmness/N | 10.02 ± 1.50 a | 11.00 ± 1.30 a | 9.77 ± 1.39 a | 5.89 ± 2.02 b | 6.23 ± 1.38 b | 6.10 ± 1.25 b | 5.65 ± 2.45 b | |
| 7 | Aeration time/s | 146.25 ± 4.99 A | 147.67 ± 11.24 A | 121.25 ± 2.87 B | 121.75 ± 9.22 B | 108.5 ± 1.29 C | 106.25 ± 6.39 C | 101.00 ± 5.944 C |
| Overrun/% | 177.08 ± 2.32 A | 175.89 ± 2.42 A | 175.116 ± 0.79 A | 173.73 ± 3.56 A | 166.63 ± 2.49 B | 159.15 ± 5.85 B | 149.98 ± 2.78 C | |
| Foam firmness /N | 10.21 ± 0.88 A | 8.72 ± 2.68 A | 8.99 ± 2.77 A | 9.58 ± 1.36 A | 8.51 ± 1.30 A | 8.56 ± 2.85 A | 6.34 ± 0.29 A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cui, X.; Li, Y.; Wang, S.; Yan, G.; Zhang, L.; Li, Y. Effects of pH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion. Foods 2023, 12, 1976. https://doi.org/10.3390/foods12101976
Wang Y, Cui X, Li Y, Wang S, Yan G, Zhang L, Li Y. Effects of pH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion. Foods. 2023; 12(10):1976. https://doi.org/10.3390/foods12101976
Chicago/Turabian StyleWang, Yunna, Xin Cui, Yang Li, Shiran Wang, Guosen Yan, Liebing Zhang, and Yan Li. 2023. "Effects of pH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion" Foods 12, no. 10: 1976. https://doi.org/10.3390/foods12101976
APA StyleWang, Y., Cui, X., Li, Y., Wang, S., Yan, G., Zhang, L., & Li, Y. (2023). Effects of pH and Ionic Strength in Calcium on the Stability and Aeration Characteristics of Dairy Emulsion. Foods, 12(10), 1976. https://doi.org/10.3390/foods12101976






