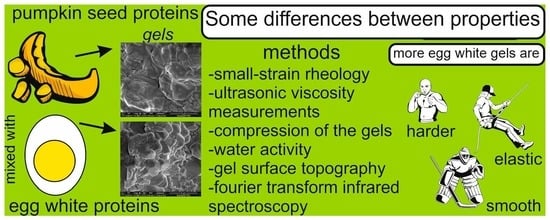
Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Small-Strain Rheology
2.4. Ultrasonic Viscosity Measurements
2.5. Compression of the Gels
2.6. Water Activity
2.7. Gel Surface Topography
2.8. Polarizing Optical Microscopy
2.9. Scanning Electron Microscopy (SEM)
2.10. Fourier Transform Infrared Spectroscopy
2.11. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties
3.2. Microstructure
3.3. Fourier Transform Infrared Spectroscopy
3.4. Water Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Huertas, S.; Terpiłowski, K.; Tomczyńska-Mleko, M.; Nishinari, K.; Mleko, S. Surface and rheological properties of egg white albumin/gelatin dispersions gelled on cold plasma-activated glass. Food Hydrocoll. 2015, 96, 224–230. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Sobral, P.J.D.A. Plant protein-based delivery systems: An emerging approach for increasing the efficacy of lipophilic bioactive compounds. Molecules 2021, 27, 60. [Google Scholar] [CrossRef] [PubMed]
- Weng, Y.; Sun, Z. Major cucurbit crops. In Genetics, Genomics and Breeding of Cucurbits; Wang, Y.-H., Behera, T.K., Kole, C., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 1–16. [Google Scholar]
- Miedzianka, J.; Zambrowicz, A.; Zielińska-Dawidziak, M.; Drożdż, W.; Nemś, A. Effect of acetylation on physicochemical and functional properties of commercial pumpkin protein concentrate. Molecules 2021, 26, 1575. [Google Scholar] [CrossRef]
- Tu, G.L.; Bui, T.H.N.; Tran, T.T.T.; Ton, N.M.N.; Le, V.V.M. Comparison of enzymatic and ultrasonic extraction of albumin from defatted pumpkin (Cucurbita pepo) seed powder. Food Technol. Biotechnol. 2015, 53, 479–487. [Google Scholar] [CrossRef]
- Fu, C.L.; Huan, S.; Li, Q.H. A review on pharmacological activities and utilization technologies of pumpkin. Plant Foods Hum. Nutr. 2006, 61, 70–77. [Google Scholar] [CrossRef]
- Olaofe, O.; Adeyemi, F.O.; Adediran, G.O. Amino acid and mineral compositions and functional properties of some oilseeds. J. Agric. Food Chem. 1994, 42, 878–881. [Google Scholar] [CrossRef]
- Hida, A.; Hasegawa, Y.; Mekata, Y.; Usuda, M.; Masuda, Y.; Kawano, H.; Kawano, Y. Effects of egg white protein supplementation on muscle strength and serum free amino acid concentrations. Nutrients 2012, 4, 1504–1517. [Google Scholar] [CrossRef]
- Glynn, E.L.; Fry, C.S.; Drummond, M.J.; Timmerman, K.L.; Dhanani, S.; Volpi, E.; Rasmussen, B.B. Excess leucine intake enhances muscle anabolic signaling but not net protein anabolism in young men and women. J. Nutr. 2010, 140, 1970–1976. [Google Scholar] [CrossRef]
- Zeng, L.; Wang, Z.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Physicochemical and gel properties of pumpkin seed protein: A comparative study. Int. J. Food Sci. Technol. 2023, 57, 1639–1651. [Google Scholar] [CrossRef]
- Alavi, F.; Emam-Djomeh, Z.; Chen, L. Acid-induced gelation of thermal co-aggregates from egg white and hempseed protein: Impact of microbial transglutaminase on mechanical and microstructural properties of gels. Food Hydrocoll. 2020, 107, 105960. [Google Scholar] [CrossRef]
- Schmitt, C.; Silva, J.V.; Amagliani, L.; Chassenieux, C.; Nicolai, T. Heat-induced and acid-induced gelation of dairy/plant protein dispersions and emulsions. Curr. Opin. Food Sci. 2019, 27, 43–48. [Google Scholar] [CrossRef]
- Rafe, A.; Vahedi, E.; Hasan-Sarei, A.G. Rheology and microstructure of binary mixed gel of rice bran protein-whey: Effect of heating rate and whey addition. J. Sci. Food Agric. 2016, 96, 3890. [Google Scholar] [CrossRef] [PubMed]
- Ashakayeva, R.; Assenova, B.; Tumenova, G.; Nurgazezova, A.; Zhumanova, G.; Atambayeva, Z.; Baikadamova, A.; II, D.; Dautova, A. A Pumpkin-Based Emulsion Gel as a Texture Improvement of Mixed Horsemeat Semi-Smoked Sausages. Foods 2022, 11, 3886. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Method. In AOAC Official Methods of Analysis, 15th ed.; AOAC International: Arlington, VA, USA; Washington, DC, USA, 1995. [Google Scholar]
- Mousavi, S.M.R.; Rafe, A.; Yeganehzad, S. Structure-rheology relationships of composite gels: Alginate and Basil seed gum/guar gum. Carbohydr. Polym. 2020, 232, 115809. [Google Scholar] [CrossRef] [PubMed]
- Wesołowska-Trojanowska, M.; Tomczyńska-Mleko, M.; Terpiłowski, K.; Muszyński, S.; Nishinari, K.; Nastaj, M.; Mleko, S. Co-gelation of gluten and gelatin as a novel functional material formation method. J. Food Sci. Technol. 2020, 57, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, S.; Rook, R.; De Haas, D.; Verschuur, E.D.J.; Rohde, M.; Kloosterman, J.L. An ultrasonic shear wave viscometer for low viscosity Newtonian liquids. Meas. Sci. Technol. 2021, 32, 125305. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Ozimek, L. Ultrasound viscosity measurements allow determination of gas volume fraction in foamed gels. J. Food Process. Eng. 2013, 36, 572–578. [Google Scholar] [CrossRef]
- Jose, J.; Pouvreau, L.; Martin, A.H. Mixing whey and soy proteins: Consequences for the gel mechanical response and water holding. Food Hydrocoll. 2016, 60, 216–224. [Google Scholar] [CrossRef]
- Rezig, L.; Chibani, F.; Chouaibi, M.; Dalgalarrondo, M.; Hessini, K.; Guéguen, J.; Hamdi, S. Pumpkin (Cucurbita maxima) Seed Proteins: Sequential Extraction Processing and Fraction Characterization. J. Agric. Food Chem. 2013, 61, 7715–7721. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Su, Y.; Chang, C.; Gu, L.; Yang, Y.; Li, J. Effect of soybean protein isolate and egg white mixture on gelation of chicken myofibrillar proteins under salt/-free conditions. LWT 2021, 149, 111871. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Nishinari, K.; Handa, A. Ca-induced egg white protein gels with various microstructure. Food Sci. Technol. Res. 2014, 20, 1207–1212. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Handa, A.; Wesołowska-Trojanowska, M.; Terpiłowski, K.; Kwiatkowski, C.; Mleko, S. New controlled release material: Aerated egg white gels induced by calcium ions. Eur. Food Res. Technol. 2016, 242, 1235–1243. [Google Scholar] [CrossRef]
- Lin, J.; Huang, Y.; Wang, S. The Hofmeister effect on protein hydrogels with stranded and particulate microstructures. Colloids Surf. B Biointerfaces 2020, 196, 111332. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J. Study of the Interactions and Physicochemical Properties of Pea and Egg White Protein Mixtures: From the Colloidal to the Gelled State. Ph.D. Thesis, Environmental and Society, Université Bourgogne Franche-Comté, Besançon, France, 2022. [Google Scholar]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Dugo, G. The role of water in protein’s behavior: The two dynamical crossovers studied by NMR and FTIR techniques. Comput. Struct. Biotechnol. J. 2015, 13, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Phuhongsung, P.; Zhang, M.; Devahastin, S. Influence of surface pH on color, texture and flavor of 3D printed composite mixture of soy protein isolate, pumpkin, and beetroot. Food Bioprocess Technol. 2020, 13, 1600–1610. [Google Scholar] [CrossRef]
- Kopjar, M.; Buljeta, I.; Jelić, I.; Kelemen, V.; Šimunović, J.; Pichler, A. Encapsulation of cinnamic acid on plant-based proteins: Evaluation by HPLC, DSC and FTIR-ATR. Plants 2021, 10, 2158. [Google Scholar] [CrossRef]
- Byler, D.M.; Susi, H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers 1986, 25, 469–487. [Google Scholar] [CrossRef]
- Uysal, R.S.; Boyaci, I.H. Authentication of liquid egg composition using ATR-FTIR and NIR spectroscopy in combination with PCA. J. Sci. Food Agric. 2020, 100, 855–862. [Google Scholar] [CrossRef]
- Kusio, K.; Szafrańska, J.O.; Radzki, W.; Sołowiej, B.G. Effect of Whey Protein Concentrate on Physicochemical, Sensory and Antioxidative Properties of High-Protein Fat-Free Dairy Desserts. Appl. Sci. 2020, 10, 7064. [Google Scholar] [CrossRef]
- Theys, T.E.; Geeraerd, A.H.; Verhulst, A.; Poot, K.; Van Bree, I.; Devlieghere, F.; Moldenaers, P.; Wilson, D.; Brocklehurst, T.; Van Impe, J.F. Effect of pH, water activity and gel micro-structure, including oxygen profiles and rheological characterization, on the growth kinetics of Salmonella Typhimurium. Int. J. Food Microbiol. 2008, 128, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Agoda-Tandjawa, G.; Dieudé-Fauvel, E.; Girault, R.; Baudez, J.C. Using water activity measurements to evaluate rheological consistency and structure strength of sludge. J. Chem. Eng. 2013, 228, 799–805. [Google Scholar] [CrossRef]
- Bielska, P.; Cais-Sokolińska, D.; Teichert, J.; Biegalski, J.; Kaczyński, Ł.K.; Chudy, S. Effect of honeydew honey addition on the water activity and water holding capacity of kefir in the context of its sensory acceptability. Sci. Rep. 2021, 11, 22956. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomczyńska-Mleko, M.; Terpiłowski, K.; Pérez-Huertas, S.; Sapiga, V.; Polischuk, G.; Sołowiej, B.; Nastaj, M.; Wesołowska-Trojanowska, M.; Mleko, S. Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein. Foods 2023, 12, 2030. https://doi.org/10.3390/foods12102030
Tomczyńska-Mleko M, Terpiłowski K, Pérez-Huertas S, Sapiga V, Polischuk G, Sołowiej B, Nastaj M, Wesołowska-Trojanowska M, Mleko S. Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein. Foods. 2023; 12(10):2030. https://doi.org/10.3390/foods12102030
Chicago/Turabian StyleTomczyńska-Mleko, Marta, Konrad Terpiłowski, Salvador Pérez-Huertas, Viktoria Sapiga, Galina Polischuk, Bartosz Sołowiej, Maciej Nastaj, Marta Wesołowska-Trojanowska, and Stanisław Mleko. 2023. "Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein" Foods 12, no. 10: 2030. https://doi.org/10.3390/foods12102030
APA StyleTomczyńska-Mleko, M., Terpiłowski, K., Pérez-Huertas, S., Sapiga, V., Polischuk, G., Sołowiej, B., Nastaj, M., Wesołowska-Trojanowska, M., & Mleko, S. (2023). Co-Gelation of Pumpkin-Seed Protein with Egg-White Protein. Foods, 12(10), 2030. https://doi.org/10.3390/foods12102030