The Effect of Acetylation on the Physicochemical Properties of Chickpea Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Chickpea Starch
2.3. Acetylation of Chickpea Starch
2.4. Measurement of Degree of Substitution
2.5. Determination of Basic Properties
2.5.1. Determination of Solubility and Swelling Power
2.5.2. Measurement of Transparency
2.5.3. Determination of Freeze–Thaw Stability of Starch Paste
2.6. Structure Determination
2.6.1. Observation of Starch Granule Morphology
2.6.2. X-ray Diffraction (XRD)
2.6.3. Fourier Transform Infrared (FT-IR) Spectroscopy
2.6.4. Determination of Starch Gelatinization Curve (RVA)
2.6.5. Determination of Gel Texture of Starch
2.7. Data Analysis
3. Results and Analysis
3.1. Acetyl Content and Degree of Substitution of Chickpea Starch
3.2. Basic Property Analysis
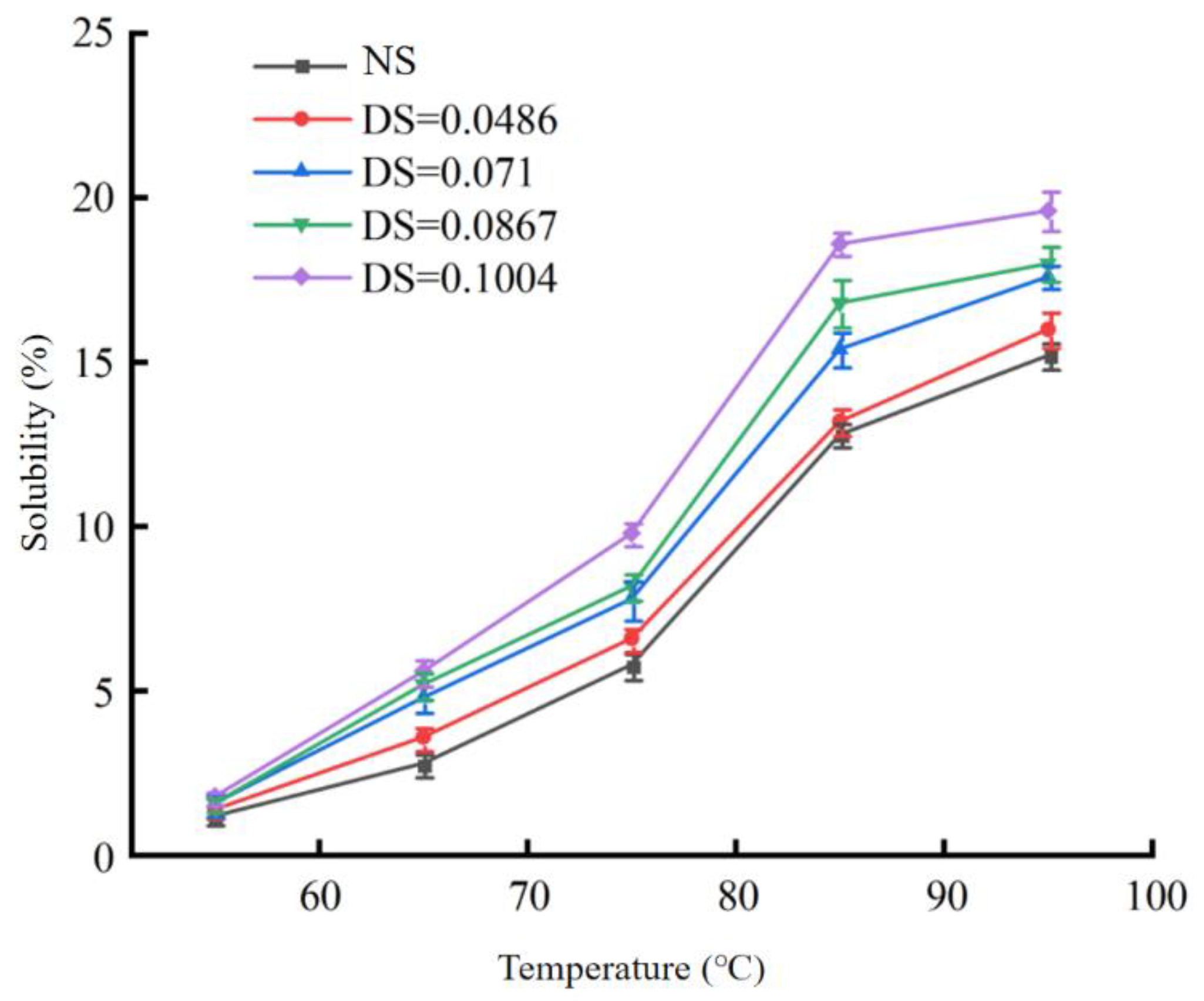
3.2.1. Changes in the Solubility and Swelling Power
3.2.2. Changes in Transparency
3.2.3. Changes in the Syneresis Rate
3.3. Structural Analysis
3.3.1. The Change in Starch Granule Morphology
3.3.2. Analysis of X-ray Diffraction
3.3.3. Analysis of the Fourier Transform Infrared Spectrum
3.3.4. Changes in the Viscosity Properties of Starch Paste
3.3.5. Changes in the Gel Properties of Starch
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaur, R.; Prasad, K. Technological, processing and nutritional aspects of chickpea (Cicer arietinum)—A review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Rehman, A.; Hussain, M.; Siddique, K.H.M. Zinc nutrition in chickpea (Cicer arietinum): A review. Crop Pasture Sci. 2020, 71, 199. [Google Scholar] [CrossRef]
- Garcia-Santos, M.D.S.L.; Conceição, F.S.; Villas Boas, F.; Salotti De Souza, B.M.; Barretto, A.C.D.S. Effect of the addition of resistant starch in sausage with fat reduction on the physicochemical and sensory properties. Food Sci. Technol. 2019, 39, 491–497. [Google Scholar] [CrossRef]
- Mesa, E.; Manjarres-Pinzon, K.; Rodriguez-Sandoval, E. Gluten-free cheese bread from frozen dough: Effect of modified cassava starch. Food Sci. Technol. 2019, 39, 654–661. [Google Scholar] [CrossRef]
- Haqa, F.; Haojie, Y.L.W.; Lisong, T.; Muhammad, H.; Rizwan, U.K.; Mehmooda, S.; Bilal-Ul-Amina; Raja, S.U.; Amin, K.; Ahsan, N. Advances in chemical modifications of starches and their applications. Carbohyd. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef] [PubMed]
- Ariyantoro, A.R.; Affandi, D.R.; Yulviatun, A.; Ishartani, D.; Septiarani, A. Pasting properties of jack bean (Canavalia ensiformis) modified starch with heat moisture treatment. IOP Conf. Ser. Earth Environ. Sci. 2021, 905, 12092. [Google Scholar] [CrossRef]
- Acevedo, B.A.; Villanueva, M.; Chaves, M.G.; Avanza, M.V.; Ronda, F. Modification of structural and physicochemical properties of cowpea (Vigna unguiculata) starch by hydrothermal and ultrasound treatments. Food Hydrocolloid. 2022, 124, 107266. [Google Scholar] [CrossRef]
- Ji, S.; Qiao, D.; Zhao, S.; Xu, Y.; Jia, C.; Niu, M.; Zhang, B. Incorporating acetylated starch regulates the structure and sol-gel performance of wheat starch-based binary system. Food Hydrocolloid. 2023, 140, 108635. [Google Scholar] [CrossRef]
- Tang, H.; He, Q.; Li, Y.; Liu, X. Sulfonated carboxymethyl debranched starch: Preparation, performance and application. J. Polym. Res. 2022, 29, 396. [Google Scholar] [CrossRef]
- Fang, K.; Zhang, W.; Wang, H.; Wang, X.; Xia, F.; Su, X.; Li, J. A synthesis of double modified: Irradiated and oxidized corn starch and its microstructural and physicochemical properties. Mater. Today Commun. 2023, 35, 106343. [Google Scholar] [CrossRef]
- Tappiban, P.; Zhao, J.; Zhang, Y.; Gao, Y.; Zhang, L.; Bao, J. Effects of single and dual modifications through electron beam irradiation and hydroxypropylation on physicochemical properties of potato and corn starches. Int. J. Biol. Macromol. 2022, 220, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Melnyk, O.; Luo, Y. Optimization of heat-moisture treatment on potato starch and study on its physicochemical properties. Technol. Audit. Prod. Reserves 2022, 3, 43–49. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, W.; Yang, Z.; Zhong, Y.; Xing, B.; Wu, Z.; Chen, H.; Wu, D.; Zhang, Q.; Qin, W.; et al. Study on physicochemical properties, digestive properties and application of acetylated starch in noodles. Int. J. Biol. Macromol. 2019, 128, 948–956. [Google Scholar] [CrossRef]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; Da Rosa Zavareze, E.; Dias, A.R.G. Acetylation of rice starch in an aqueous medium for use in food. LWT Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef]
- de Souza Silva, G.M.; de Carvalho Batista Muniz, I.; Veloso, C.M.; Santos, L.S.; de Melo Neto, B.A.; Bonomo, R.C.F. Chemical, morphological, thermal and technological properties of acetylated white inhambu starch. J. Polym. Environ. 2022, 30, 246–257. [Google Scholar] [CrossRef]
- Zhao, X.; Zeng, L.; Huang, Q.; Zhang, B.; Zhang, J.; Wen, X. Structure and physicochemical properties of cross-linked and acetylated tapioca starches affected by oil modification. Food Chem. 2022, 386, 132848. [Google Scholar] [CrossRef]
- Shaari, S.; Samsudin, H.; Uthumporn, U. Effect of acetylation treatment on surface modified tapioca starches. Food Res. 2021, 5, 340–347. [Google Scholar] [CrossRef]
- Olagunju, A.; Omoba, O.; Enujiugha, V.; Alashi, A.; Aluko, R. Technological properties of acetylated pigeon pea starch and its stabilized Set-Type yoghurt. Foods 2020, 9, 957. [Google Scholar] [CrossRef]
- Otemuyiwa, I.O.; Aina, A.F. Physicochemical properties and in-vitro digestibility studies of microwave assisted chemically modified breadfruit (Artocarpus altilis) starch. Int. J. Food Prop. 2021, 24, 140–151. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Liu, S.; Chang, X. Influence of phosphorylation and acetylation on structural, physicochemical and functional properties of chestnut starch. Polymers 2022, 14, 172. [Google Scholar] [CrossRef]
- Kunyanee, K.; Luangsakul, N. The impact of heat moisture treatment on the physicochemical properties and in vitro glycemic index of rice flour with different amylose contents and associated effects on rice dumpling quality. LWT 2022, 154, 112694. [Google Scholar] [CrossRef]
- Rahim, A.; Mahfudz; Muhardi; Kadir, S.; Rostiati; Alam, N.; Hutomo, G.S.; Priyantono, E.; Salingkat, C.A.; Abdullah; et al. Effect of pH and acetic anhydride concentration on physicochemical characteristics of acetylated sago starch. IOP Conf. Ser. Earth Environ. Sci. 2022, 1107, 12124. [Google Scholar] [CrossRef]
- Marboh, V.; Mahanta, C.L. Physicochemical and rheological properties and in vitro digestibility of heat moisture treated and annealed starch of sohphlang (Flemingia vestita) tuber. Int. J. Biol. Macromol. 2021, 168, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Mcclements, D.J.; Dai, L.; He, X.; Li, Y.; Ji, N.; Qin, Y.; Xiong, L.; Sun, Q. Improvement of pasting and gelling properties of potato starch using a direct vapor-heat moisture treatment. Int. J. Biol. Macromol. 2022, 219, 1197–1207. [Google Scholar] [CrossRef]
- Rodboontheng, W.; Uttapap, D.; Wandee, Y.; Udchumpisai, W.; Kotatha, D.; Puttanlek, C.; Rungsardthong, V. Simple thermal and freezing treatments to improve absorption capacity and alter digestibility of canna starch granules. Int. J. Biol. Macromol. 2022, 194, 861–869. [Google Scholar] [CrossRef]
- Zhang, B.; Saleh, A.S.M.; Su, C.; Gong, B.; Zhao, K.; Zhang, G.; Li, W.; Yan, W. The molecular structure, morphology, and physicochemical property and digestibility of potato starch after repeated and continuous heat–moisture treatment. J. Food Sci. 2020, 85, 4215–4224. [Google Scholar] [CrossRef] [PubMed]
- Yassaroh, Y.; Woortman, A.J.J.; Loos, K. Physicochemical properties of heat-moisture treated, stearic acid complexed starch: The effect of complexation time and temperature. Int. J. Biol. Macromol. 2021, 175, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Uzizerimana, F.; Dang, K.; Yang, Q.; Hossain, M.S.; Gao, S.; Bahati, P.; Mugiraneza, N.G.; Yang, P.; Feng, B. Physicochemical properties and in vitro digestibility of tartary buckwheat starch modified by heat moisture treatment: A comparative study. NFS J. 2021, 25, 12–20. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. Physicochemical, rheological and structural characterization of acetylated oat starches. LWT 2017, 80, 19–26. [Google Scholar] [CrossRef]
- Ray, A.; Samajdar, S.; Kumar, K.J. Improving delayed-release potential of winged bean starch by acetylation. Carbohydr. Polym. Technol. Appl. 2021, 2, 100118. [Google Scholar] [CrossRef]
- Sindhu, R.; Devi, A.; Khatkar, B.S. Morphology, structure and functionality of acetylated, oxidized and heat moisture treated amaranth starches. Food Hydrocolloid. 2021, 118, 106800. [Google Scholar] [CrossRef]
- Cuenca, P.; Ferrero, S.; Albani, O. Preparation and characterization of cassava starch acetate with high substitution degree. Food Hydrocolloid. 2020, 100, 105430. [Google Scholar] [CrossRef]
- Zięba, T.; Wilczak, A.; Kobryń, J.; Musiał, W.; Kapelko-żeberska, M.; Gryszkin, A.; Meisel, M. The annealing of acetylated potato starch with various substitution degrees. Molecules 2021, 26, 2096. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Dhital, S.; Zhang, M.; Wang, J.; Chen, Z. Structural, gelatinization, and rheological properties of heat-moisture treated potato starch with added salt and its application in potato starch noodles. Food Hydrocolloid. 2022, 131, 107802. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Liu, M.; Strappe, P.; Li, M.; Wang, A.; Zhuang, M.; Liu, J.; Blanchard, C.; Zhou, Z. Association of starch crystalline pattern with acetylation property and its influence on gut microbota fermentation characteristics. Food Hydrocolloid. 2022, 128, 107556. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wang, J.; Dong, Y. Microwave-assisted synthesis and characterization of acetylated corn starch. Starch Stärke 2014, 66, 515–523. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, H. Hydroxypropylation and acetylation of rice starch: Effects of starch protein content. Food Sci. Biotechnol. 2022, 31, 1169–1177. [Google Scholar] [CrossRef]
- Obioma, O.G.; Doshima, I.B.; Kwagh-Hal, I.J.; Ann, K.N.; Amak, D.A.M. Proximate composition and pasting properties of modified starches of white yam, trifoliate yam and sweet potato. World J. Food Sci. Technol. 2022, 6, 58–68. [Google Scholar]
- Zdybel, E.; Wilczak, A.; Kapelko-żeberska, M.; Tomaszewska-Ciosk, E.; Gryszkin, A.; Gawrońska, A.; Zięba, T. Physicochemical properties and digestion resistance of acetylated starch obtained from annealed starch. Polymers 2021, 13, 4141. [Google Scholar] [CrossRef]








| Acetic Anhydride Dosage/% | Acetyl Group Content/% | Degree of Substitution |
|---|---|---|
| 4 | 1.29 ± 0.02 a | 0.0486 ± 0.002 a |
| 6 | 1.89 ± 0.03 b | 0.071 ± 0.003 b |
| 8 | 2.30 ± 0.03 c | 0.0867 ± 0.001 c |
| 10 | 2.60 ± 0.02 d | 0.1004 ± 0.001 d |
| Samples | NS | DS = 0.0486 | DS = 0.071 | DS = 0.0867 | DS = 0.1004 |
|---|---|---|---|---|---|
| Relative crystallinity (%) | 31.04 | 30.89 | 30.47 | 30.28 | 30.07 |
| Scheme | Peak Viscosity (cP) | Trough Viscosity (cP) | Breakdown Viscosity (cP) | Final Viscosity (cP) | Setback Viscosity (cP) | Gelatinization Temperature (°C) |
|---|---|---|---|---|---|---|
| NS | 4284 ± 31 a | 3110 ± 13.6 a | 1174 ± 36.7 a | 6424 ± 62 a | 3314 ± 57.4 a | 75.05 ± 0.4 a |
| DS = 0.0486 | 3909 ± 173 b | 2841 ± 55 b | 1068 ± 171 b | 4515 ± 50.7 b | 1674 ± 54.2 b | 68.65 ± 1.0 b |
| DS = 0.071 | 3704 ± 92 bc | 2761 ± 52.1 bc | 943 ± 38.7 c | 4119 ± 13.9 bc | 1358 ± 40.8 c | 62.53 ± 11 b |
| DS = 0.0867 | 3636 ± 48.8 b | 2672 ± 45.1 bc | 964 ± 40.8 b | 3873 ± 70.9 cd | 1201 ± 79.9 cd | 58.50 ± 0.9 bc |
| DS = 0.1004 | 3295 ± 117 c | 2450 ± 145 c | 845 ± 54 cd | 3775 ± 13.3 d | 1325 ± 150.5 e | 50.25 ± 0.4 d |
| Species | Hardness/g | Cohesion/g | Elasticity/mm | Adhesion/g·s | Chewiness/mj |
|---|---|---|---|---|---|
| NS | 29.79 ± 0.58 a | 0.76 ± 0.01 a | 5.62 ± 0.15 a | 19.88 ± 0.4 a | 102 ± 5.03 a |
| DS = 0.0486 | 15.08 ± 0.63 b | 0.75 ± 0.01 a | 5.38 ± 0.19 a | 10.72 ± 0.23 b | 42.4 ± 2.44 b |
| DS = 0.071 | 13.15 ± 0.73 c | 0.74 ± 0.04 a | 5.01 ± 0.23 bc | 7.85± 0.27 c | 28.2 ± 3.22 c |
| DS = 0.0867 | 11.41 ± 0.55 d | 0.59 ± 0.03 b | 4.54 ± 0.47 cd | 6.05 ± 0.49 d | 25.6 ± 2.5 c |
| DS = 0.1004 | 10.14 ± 0.4 e | 0.54 ± 0.03 b | 4.12 ± 0.34 d | 3.55 ± 0.44 e | 22.8 ± 3.27 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Du, M.; Cao, T.; Xu, W. The Effect of Acetylation on the Physicochemical Properties of Chickpea Starch. Foods 2023, 12, 2462. https://doi.org/10.3390/foods12132462
Zhang C, Du M, Cao T, Xu W. The Effect of Acetylation on the Physicochemical Properties of Chickpea Starch. Foods. 2023; 12(13):2462. https://doi.org/10.3390/foods12132462
Chicago/Turabian StyleZhang, Chunlan, Mengyao Du, Tiantian Cao, and Wei Xu. 2023. "The Effect of Acetylation on the Physicochemical Properties of Chickpea Starch" Foods 12, no. 13: 2462. https://doi.org/10.3390/foods12132462
APA StyleZhang, C., Du, M., Cao, T., & Xu, W. (2023). The Effect of Acetylation on the Physicochemical Properties of Chickpea Starch. Foods, 12(13), 2462. https://doi.org/10.3390/foods12132462




