Influence of Non-Thermal Plasma Treatment on Structural Network Attributes of Wheat Flour and Respective Dough
Abstract
:1. Introduction
2. Materials and Methods
2.1. NTP Treatment Experimental Setup
2.2. Dough Preparation
2.3. Baking Parameters
2.4. Analysis of Flour, Dough, and Baked Products
2.4.1. Flour Hydration Analysis
2.4.2. Flour Protein Analysis
2.4.3. Other Flour Quality Analysis
2.4.4. Color Analysis of Flour and Products
2.4.5. Baked Product Analysis
2.4.6. Microbiological Analysis
2.5. Experimental Procedure
3. Results and Discussion
3.1. Influence on the Flour Hydration Properties
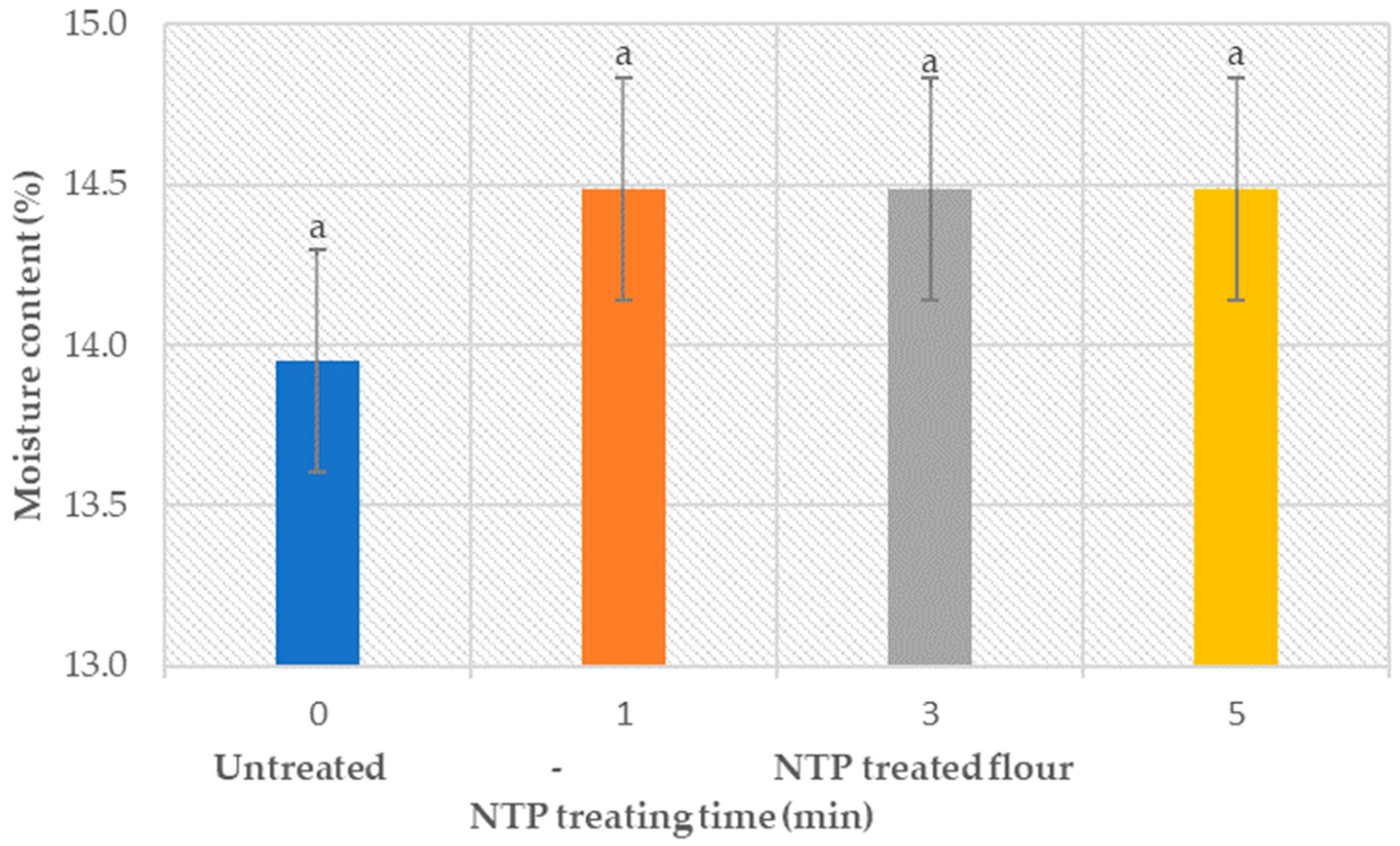
3.1.1. Moisture Content Analysis
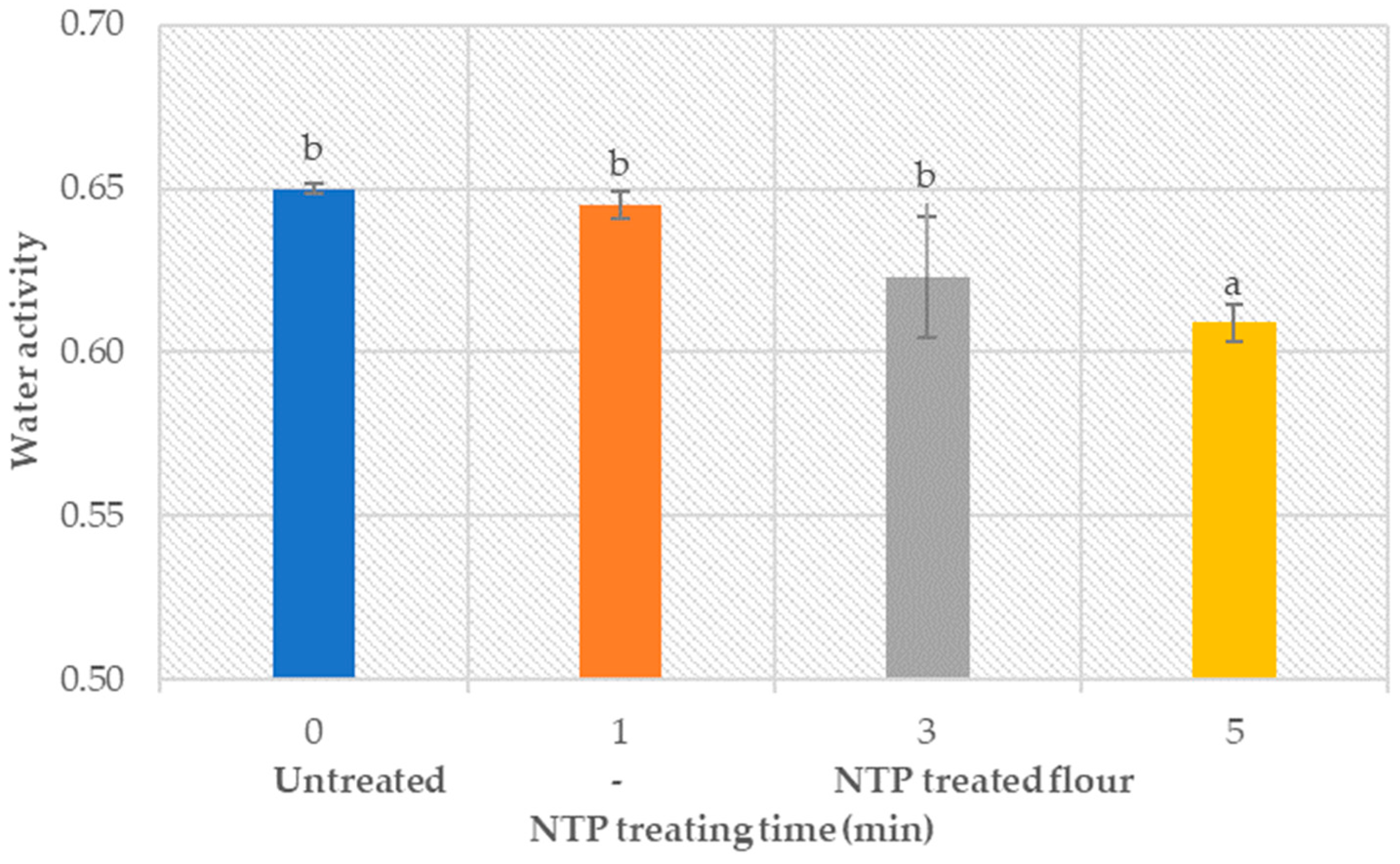
3.1.2. Water Activity Analysis (aw–Value)
3.1.3. Near-Infrared Spectroscopy for Moisture Content Analysis
3.2. Flour Protein Properties
3.2.1. Gluten Content (Wet and Dry) and Gluten Index
3.2.2. Zeleny’s Sedimentation Analysis and Adhesive Properties Analysis
3.2.3. Water Absorption: Farinograph Tests
3.2.4. Dough Stability and Softening: Farinograph
3.2.5. Dough Stretch Resistance, Dough Extensibility, and Their Ratio: Kieffer Rig
3.3. Other Flour Quality Analysis
3.3.1. Falling Number
3.3.2. Starch Gelatinization Temperature, Viscosity, and Starch Damage
3.4. Color Properties of Flour and Baked Products
3.4.1. Color Analysis of Flour
3.4.2. Color Analysis of Baked Products
3.5. Baking Product Quality
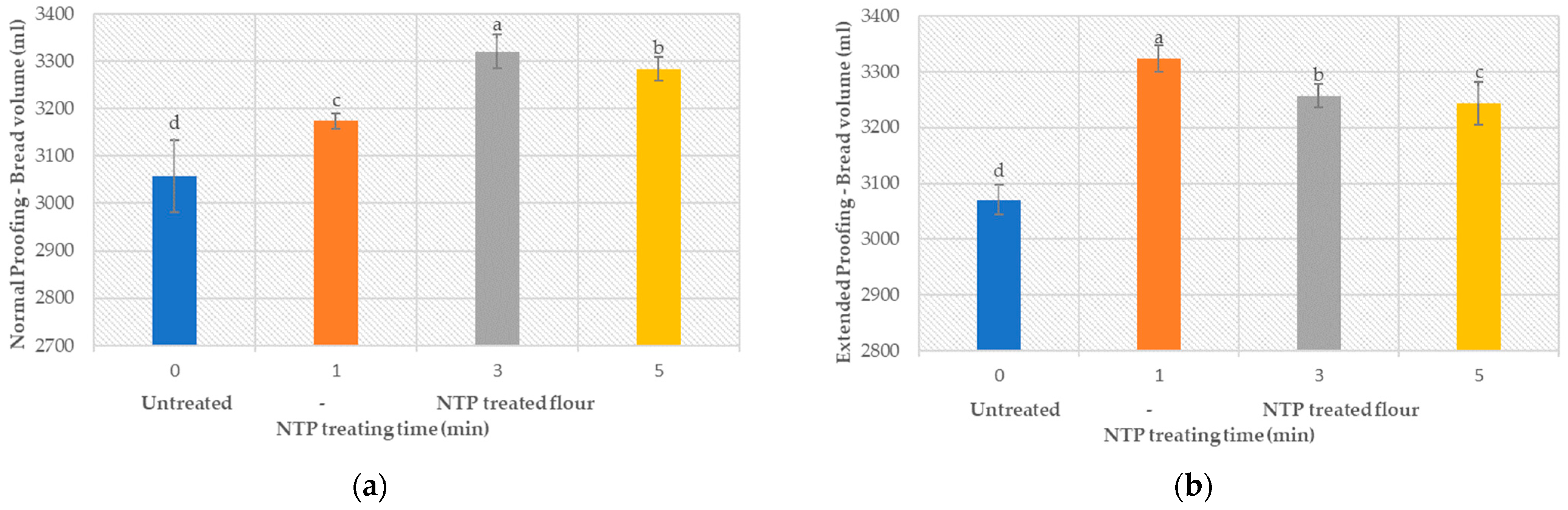
3.5.1. Product Volume
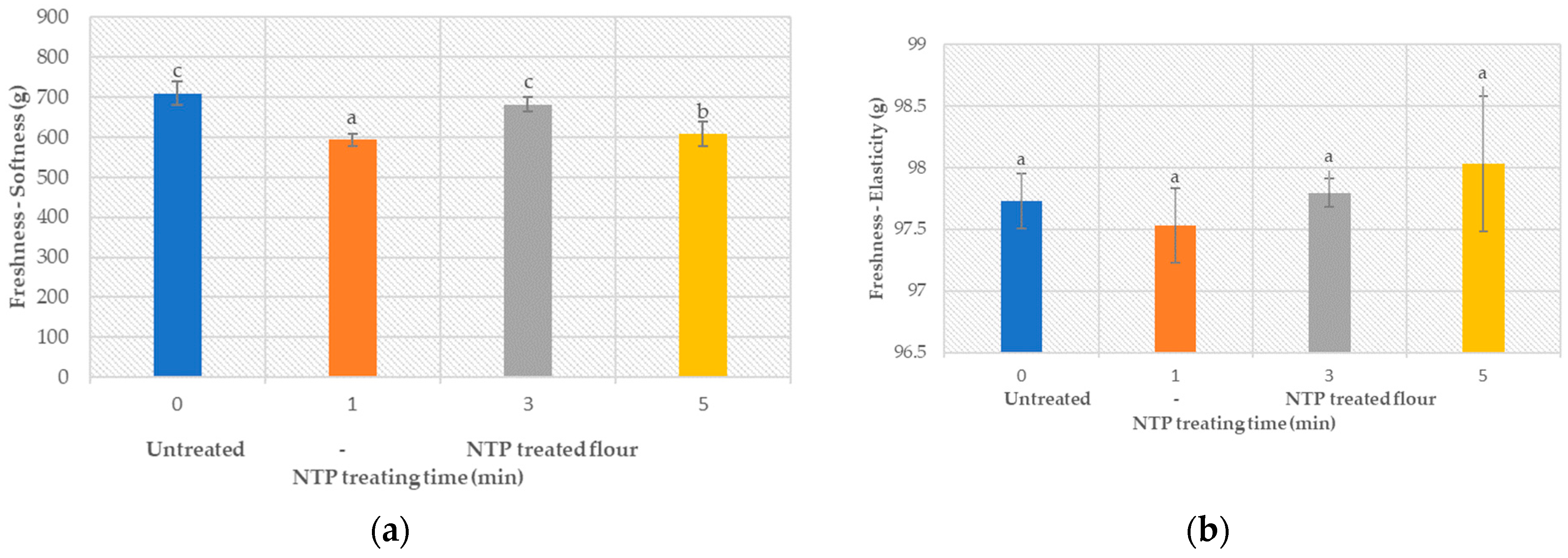
3.5.2. Product Freshness
3.5.3. Sensory Analysis
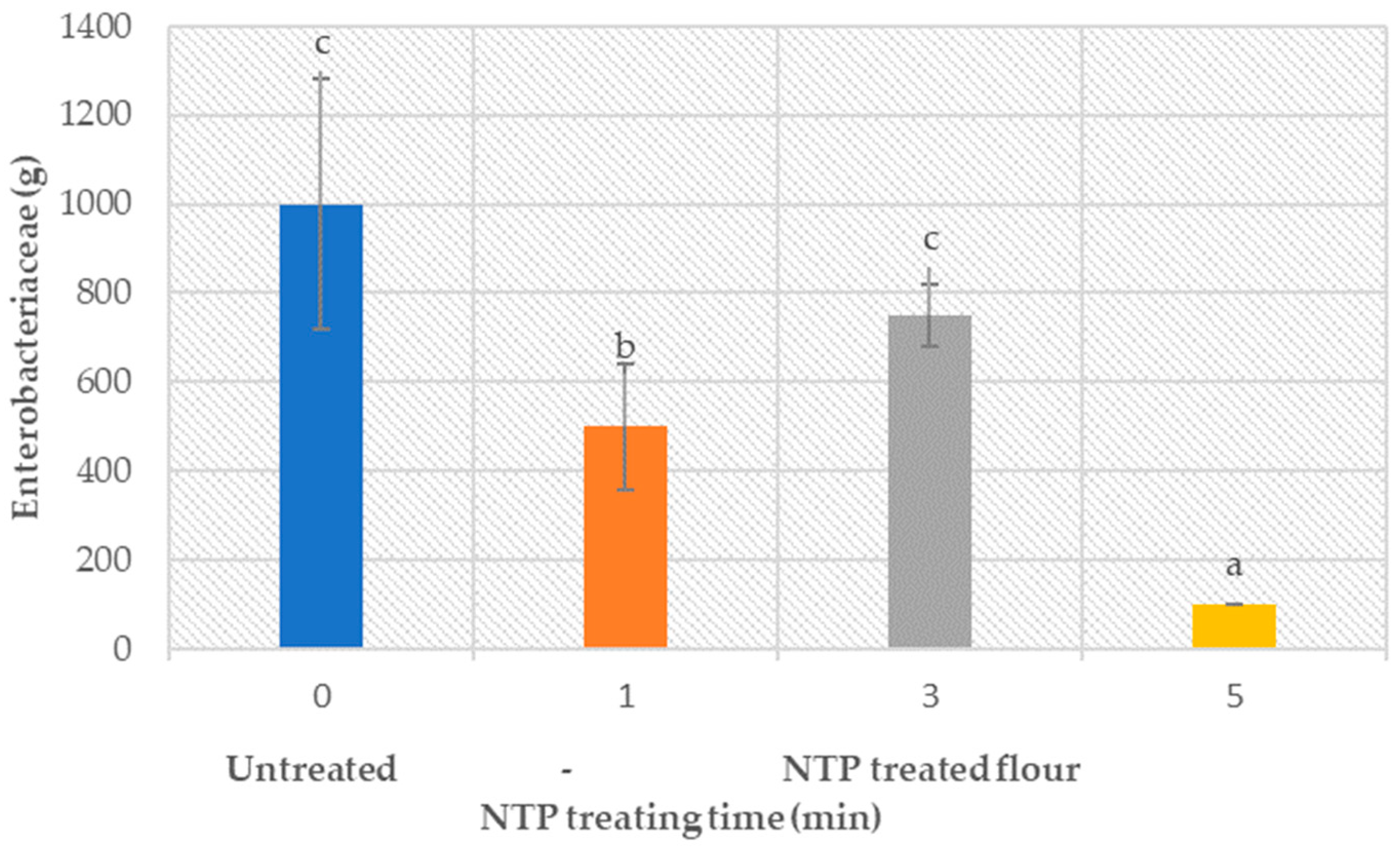
3.6. Flour Microbiology (Bacteria and Enzymes)
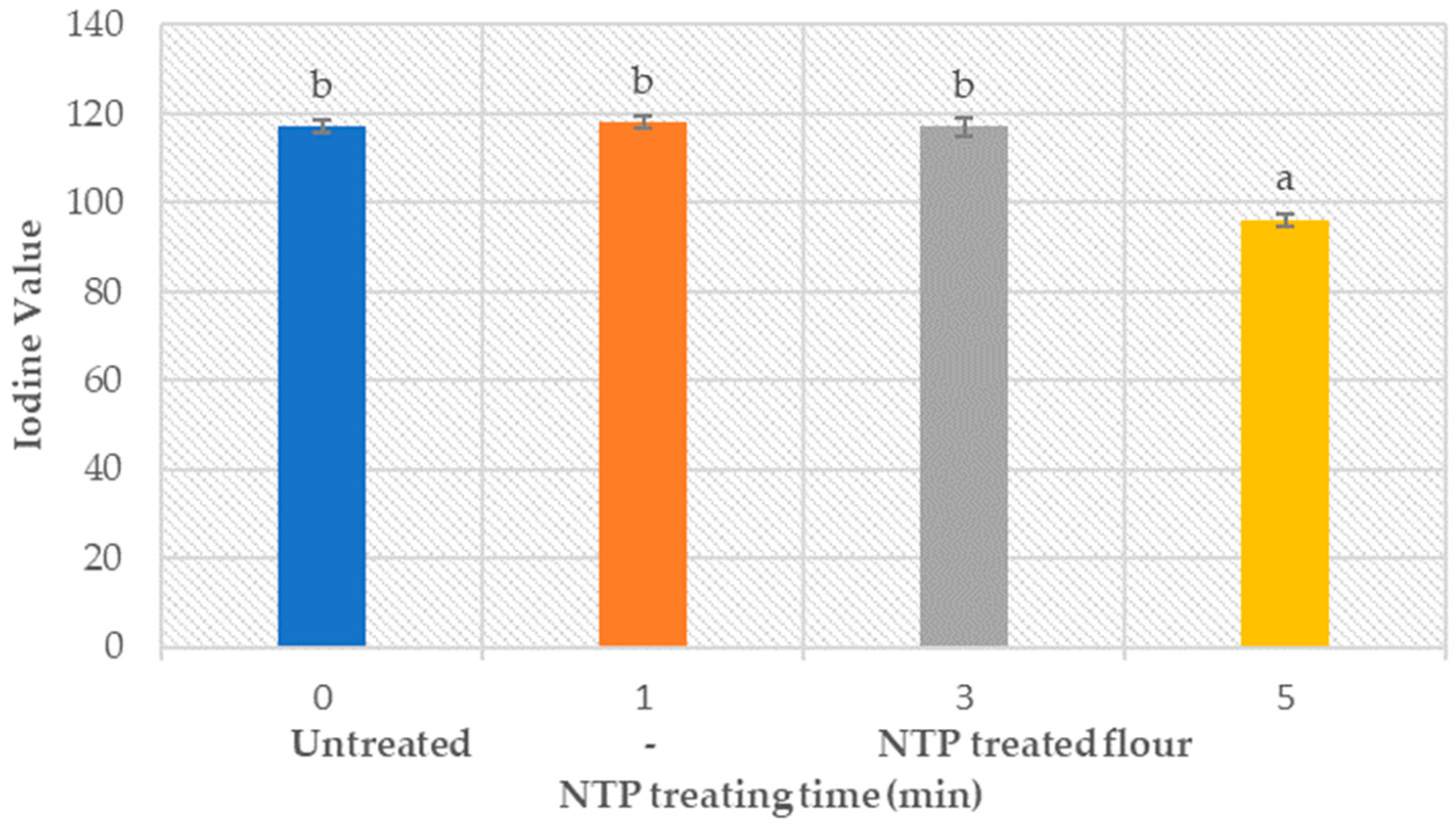
3.7. Flour Fat Amount
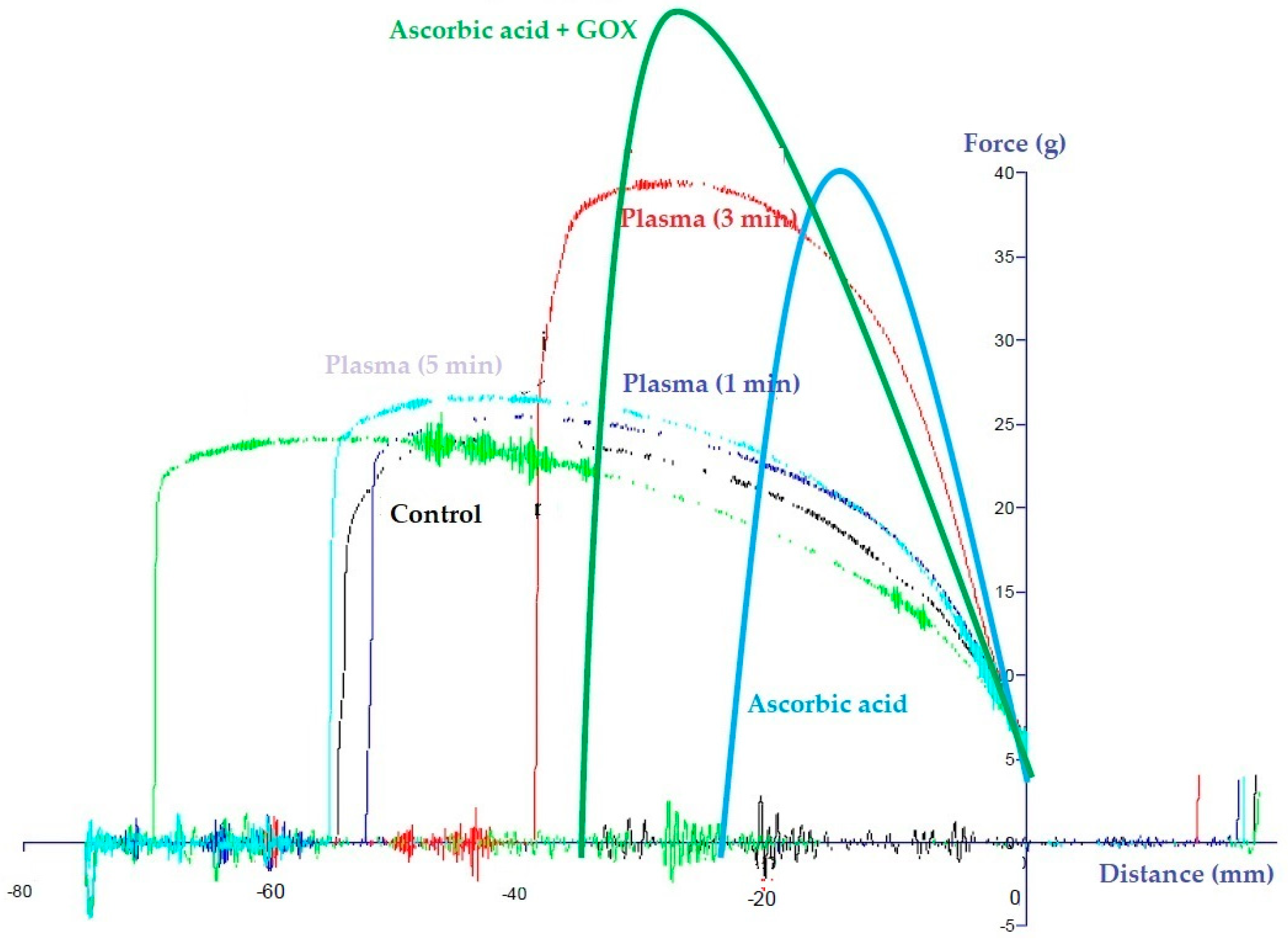
3.8. NTP Treatment vs. Ascorbic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laskowski, W.; Górska-Warsewicz, H.; Rejman, K.; Czeczotko, M.; Zwolińska, J. How Important are Cereals and Cereal Products in the Average Polish Diet? Nutrients 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.R.; Xu, Y. Rheology of Food Materials: Impact on and Relevance in Food Processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Ramli, A.N.M.; Hong, P.K.; Manas, N.H.A.; Azelee, N.I.W. An overview of enzyme technology used in food industry. In Value-Addition in Food Products and Processing Through Enzyme Technology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Vitale, M.; Schillaci, D. Food Processing and Foodborne Illness. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Park, S.H.; Lamsal, B.P.; Balasubramaniam, V.M. Principles of Food Processing. In Food Processing Principles and Applications, 2nd ed.; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Lee, S.H.; Choi, W.; Jun, S. Conventional and Emerging Combination Technologies for Food Processing. Food Eng. Rev. 2016, 8, 414–434. [Google Scholar] [CrossRef]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. Food Preservation with Chemicals BT-Modern Food Microbiology; Jay, J.M., Ed.; Springer: Boston, MA, USA, 1995; pp. 273–303. [Google Scholar]
- D’souza, A.A.; Kumari, D.; Banerjee, R. Nanocomposite biosensors for point-of-care—Evaluation of food quality and safety. Nanobiosensors 2017, 629–676. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.W. High pressure processing of foods. An Overview. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef]
- McHugh, T. Putting ultrasound to use in food processing. Food Technol. 2016, 12, 16. [Google Scholar]
- Jhandai, P.; Jadhav, V.J.; Gupta, R. Bio-preservation of Foods: A Review. Eur. J. Nutr. Food Saf. 2019, 11, 164–174. [Google Scholar] [CrossRef]
- Ravindra, M.R. Vacuum processing of food–A Mini Review. MOJ Food Process. Technol. 2018, 6, 283–290. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice. In Food Processing Technology, 2nd ed.; parts 1–4; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- EL Sabagh, A.; Islam, M.S.; Skalicky, M.; Raza, M.A.; Singh, K.; Hossain, M.A.; Hossain, A.; Mahboob, W.; Iqbal, M.A.; Ratnasekera, D.; et al. Salinity Stress in Wheat (Triticum aestivum L.) in the Changing Climate: Adaptation and Management Strategies. Front. Agron. 2021, 3, 661932. [Google Scholar] [CrossRef]
- Wieser, H.; Koehler, P.; Scherf, K.A. Chapter 2—Chemical Composition of Wheat Grains. In Wheat—An Exceptional Crop; Wieser, H., Koehler, P., Scherf, K.A., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 13–45. [Google Scholar]
- Uthayakumaran, S.; Wrigley, C. Chapter 5—Wheat: Grain-Quality Characteristics and Management of Quality Requirements. In Cereal Grains: Assessing and Managing Quality, 2nd ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Wrigley, C., Batey, I., Miskelly, D., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 91–134. [Google Scholar]
- Colmenero, F.J. Chemistry and Physics of Comminuted Products Nonmeat Proteins. In Encyclopedia of Meat Sciences, 2nd ed.; Dikeman, M., Devine, C., Eds.; Academic Press: Oxford, UK, 2014; pp. 289–295. [Google Scholar]
- Carson, G.R.; Edwards, N.M. Chapter 4—Criteria of Wheat and Flour Quality. In American Associate of Cereal Chemists International, 4th ed.; Khan, K., Ed.; AACC International Press: Washington, DC, USA, 2009; pp. 97–118. [Google Scholar]
- Posner, E.S. Chapter 5—Wheat Flour Milling. In American Associate of Cereal Chemists International, 4th ed.; Khan, K., Ed.; AACC International Press: Washington, DC, USA, 2009; pp. 119–152. [Google Scholar]
- Békés, F.; Wrigley, C.W. The Protein Chemistry of Cereal Grains. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Scherf, K.A.; Köhler, P. Wheat and gluten: Technological and health aspects. Ernahr. Umsch. 2016, 63, 166–175. [Google Scholar]
- Cato, L.; Mullan, D. Chapter 8—Wheat quality: Wheat breeding and quality testing in Australia. In Woodhead Publishing Series in Food Science, Technology and Nutrition, 3rd ed.; Woodhead Publishing: Cambridge, UK, 2020; pp. 221–259. [Google Scholar]
- Cracknell, R.L. Wheat: The Crop. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 478–485. [Google Scholar]
- Gras, P.W.; Anderssen, R.S.; Keentok, M.; Békés, F.; Appels, R. Gluten protein functionality in wheat flour processing: A review. Aust. J. Agric. Res. 2001, 52, 1311. [Google Scholar] [CrossRef]
- Goesaert, H.; Brijs, K.; Veraverbeke, W.; Courtin, C.; Gebruers, K.; Delcour, J. Wheat flour constituents: How they impact bread quality, and how to impact their functionality. Trends Food Sci. Technol. 2005, 16, 12–30. [Google Scholar] [CrossRef]
- Ooms, N.; Delcour, J.A. How to impact gluten protein network formation during wheat flour dough making. Curr. Opin. Food Sci. 2019, 25, 88–97. [Google Scholar] [CrossRef]
- Jekle, M.; Becker, T. Wheat Dough Microstructure: The Relation between Visual Structure and Mechanical Behavior. Crit. Rev. Food Sci. Nutr. 2015, 55, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Dogan, C.E.; Cebi, N.; Develioglu, A.; Olgun, E.O.; Sagdic, O. Detection of cystine and cysteine in wheat flour using a robust LC-MS/MS method. J. Cereal Sci. 2018, 84, 49–54. [Google Scholar] [CrossRef]
- Li, M.; Lee, T.-C. Effect of Cysteine on the Functional Properties and Microstructures of Wheat Flour Extrudates. J. Agric. Food Chem. 1996, 44, 1871–1880. [Google Scholar] [CrossRef]
- Stauffer, C.E. Principles of dough formation. In Technology of Breadmaking; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Rombouts, I.; Lagrain, B.; Brijs, K.; Delcour, J.A. Cross-linking of wheat gluten proteins during production of hard pretzels. Amino Acids 2012, 42, 2429–2438. [Google Scholar] [CrossRef]
- Alldrick, A.J. Chemical contamination of cereals. In Chemical Contaminants and Residues in Food; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wieser, H. The use of redox agents in breadmaking. In Breadmaking: Improving Quality, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Cauvain, S.P. The use of redox agents in breadmaking. In Breadmaking: Improving Quality, 3rd ed.; Woodhead Publishing: Cambridge, UK, 2020. [Google Scholar]
- Obadi, M.; Zhu, K.-X.; Peng, W.; Sulieman, A.A.; Mahdi, A.A.; Mohammed, K.; Zhou, H.-M. Shelf life characteristics of bread produced from ozonated wheat flour. J. Texture Stud. 2018, 49, 492–502. [Google Scholar] [CrossRef]
- Sandhu, H.P.; A Manthey, F.; Simsek, S. Quality of bread made from ozonated wheat (Triticum aestivum L.) flour. J. Sci. Food Agric. 2011, 91, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Liu, G.; Huang, X.; Ding, W. Effects of ozone treatment on medium hard wheat (Triticum aestivum L.) flour quality and performance in steamed bread making. CyTA J. Food 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Rice, R.G.; Graham, D.M. U.S. FDA Regulatory Approval of Ozone as an Antimicrobial Agent—What Is Allowed and What Needs to Be Understood. Ozone News. Available online: https://technozone.in/img/pdf/FDA-e.pdf (accessed on 10 January 2023).
- Dutta, D.; Nath, A.; Mukhim, K.; Swer, T.; Verma, N.; Deka, B.C.; Gangwar, B. A Review on Application of Ozone in the Food Processing and Packaging. J. Food Prod. Dev. Packag. 2014, 1, 7–21. [Google Scholar]
- Takeuchi, K. OZONE, 2nd ed.; Worsfold, P., Townshend, A., Eds.; Elsevier: Oxford, UK, 2005; pp. 462–471. [Google Scholar]
- Yehia, A. Characteristics of the dielectric barrier corona discharges. AIP Adv. 2019, 9, 045214. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Z.; Ye, T.; Xu, Z.; Xia, W. Corona Inducing Dielectric Barrier Discharge. Ispc_20. 2011. Available online: https://www.ispc-conference.org/ispcproc/ispc20/290.pdf (accessed on 1 March 2023).
- Penetrante, B.M.; Hsiao, M.C.; Merritt, B.T.; Vogtlin, G.E.; Wallman, P.H.; Neiger, M.; Wolf, O.; Hammer, T.; Broer, S. Pulsed corona and dielectric-barrier discharge processing of NO in N2. Appl. Phys. Lett. 1996, 68, 3719–3721. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Khanal, R. Introduction to the Plasma State of Matter. Himal. Phys. 2013, 4, 60–64. [Google Scholar] [CrossRef]
- Rezaei, F.; Vanraes, P.; Nikiforov, A.; Morent, R.; De Geyter, N. Applications of Plasma-Liquid Systems: A Review. Materials 2019, 12, 2751. [Google Scholar] [CrossRef]
- Zhang, B. Principles, Methods, Formation Mechanisms, and Structures of Nanomaterials Prepared via Gas-Phase Processes. In Physical Fundamentals of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Frank-Kamenetskii, D.A. Plasma Thermodynamics BT-Plasma: The Fourth State of Matter; Frank-Kamenetskii, D.A., Ed.; Macmillan Education: London, UK, 1972; pp. 25–34. [Google Scholar]
- O’Neill, T.; Rynk, R.; Black, G.; Bokowa, A.F. Odor management and community relations. In The Composting Handbook; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Holubová, L.; Kyzek, S.; Ďurovcová, I.; Fabová, J.; Horváthová, E.; Ševčovičová, A.; Gálová, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Patil, S.; Bourke, P.; Cullen, P.J. Principles of Nonthermal Plasma Decontamination. In Cold Plasma in Food and Agriculture: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Bahrami, N.; Bayliss, D.; Chope, G.; Penson, S.; Perehinec, T.; Fisk, I.D. Cold plasma: A new technology to modify wheat flour functionality. Food Chem. 2016, 202, 247–253. [Google Scholar] [CrossRef]
- Scholtz, V.; Šerá, B.; Khun, J.; Šerý, M.; Julak, J. Effects of Nonthermal Plasma on Wheat Grains and Products. J. Food Qual. 2019, 2019, 7917825. [Google Scholar] [CrossRef]
- Li, X.; Wen, Y.; Zhang, J.; Ma, D.; Zhang, J.; An, Y.; Song, X.; Ren, X.; Zhang, W.; Xuejie, L.; et al. Effects of non-thermal plasma treating wheat kernel on the physicochemical properties of wheat flour and the quality of fresh wet noodles. Int. J. Food Sci. Technol. 2022, 57, 1544–1553. [Google Scholar] [CrossRef]
- Wannicke, N. Potential on non-thermal plasma techniques for decontamination of wheat grains for agricultural use. Cereal Technol. 2018, 4, 244–255. [Google Scholar] [CrossRef]
- Montenegro, F.M.; Júnior, A.M.; Berteli, M.N.; Campelo, P.H.; Pedrosa, M.T.; Clerici, S. Effects of microwave-generated non-thermal plasma treatment applied to wheat flour and bran. Soc. Dev. 2021, 10, e12810817035. [Google Scholar] [CrossRef]
- Misra, N.N.; Kaur, S.; Tiwari, B.K.; Kaur, A.; Singh, N.; Cullen, P. Atmospheric pressure cold plasma (ACP) treatment of wheat flour. Food Hydrocoll. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M. Non-Thermal Processing Technologies for the Grain Industry; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the Impact of Nonthermal Plasma on Food Constituents and Microstructure—A Review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- Khan, M.J.; Jovicic, V.; Zbogar-Rasic, A.; Delgado, A. Enhancement of Wheat Flour and Dough Properties by Non-Thermal Plasma Treatment of Wheat Flour. Appl. Sci. 2022, 12, 7997. [Google Scholar] [CrossRef]
- U.S. Food and Drug. Water Activity (aw) in Foods. Available online: https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-technical-guides/water-activity-aw-foods (accessed on 15 November 2022).
- Millar, S.J. The Development of Near Infrared (NIR) Spectroscopy Calibrations for the Prediction of Wheat and Flour Quality; HGCA Project Report; 1 January 2000–31 May 2003; Rothamsted Reaearch: Harpenden, UK, 2003. [Google Scholar]
- Dowell, F.E.; Maghirang, E.B.; Xie, F.; Lookhart, G.L.; Pierce, R.O.; Seabourn, B.W.; Bean, S.R.; Wilson, J.D.; Chung, O.K. Predicting Wheat Quality Characteristics and Functionality Using Near-Infrared Spectroscopy. Cereal Chem. 2006, 83, 529–536. [Google Scholar] [CrossRef]
- Oikonomou, N.A.; Bakalis, S.; Rahman, M.S.; Krokida, M.K. Gluten Index for Wheat Products: Main Variables in Affecting the Value and Nonlinear Regression Model. Int. J. Food Prop. 2015, 18, 1–11. [Google Scholar] [CrossRef]
- Foote, D. The Sedimentation Test of Wheat Quality; No. May; University of Nebraska-Lincoln: Lincoln, NE, USA, 2014. [Google Scholar]
- Dunnewind, B.; Sliwinski, E.; Grolle, K.; Vliet, T. The Kieffer Dough and Gluten Extensibility Rig—An Experimental Evaluation. J. Texture Stud. 2003, 34, 537–560. [Google Scholar] [CrossRef]
- Sissons, M.J.; Soh, H.N.; A Turner, M. Role of gluten and its components in influencing durum wheat dough properties and spaghetti cooking quality. J. Sci. Food Agric. 2007, 87, 1874–1885. [Google Scholar] [CrossRef]
- Kiszonas, A.M.; Engle, D.A.; Pierantoni, L.A.; Morris, C.F. Relationships between Falling Number, α-amylase activity, milling, cookie, and sponge cake quality of soft white wheat. Cereal Chem. 2018, 95, 373–385. [Google Scholar] [CrossRef]
- Pojić, M.; Hadnađev, M.; Hadnađev, T.D. Gelatinization properties of wheat flour as determined by empirical and fundamental rheometric method. Eur. Food Res. Technol. 2013, 237, 299–307. [Google Scholar] [CrossRef]
- CIE. International Comission on Illumination. Available online: http://cie.co.at/about-cie (accessed on 6 July 2022).
- Delcour, J.A.; Hoseney, R.C. Principles of Cereal Science and Technology; Amer Assn of Cereal Chemists: Egan, MN, USA, 2010. [Google Scholar]
- Calvin, O. Starch and modified starch in bread making: A review. Afr. J. Food Sci. 2016, 10, 344–351. [Google Scholar] [CrossRef]
- Rose, D.J.; Bianchini, A.; Martinez, B.; Flores, R.A. Methods for Reducing Microbial Contamination of Wheat Flour and Effects on Functionality. Cereal Foods World 2012, 57, 104–109. [Google Scholar] [CrossRef]
- Cordier, J.-L. 17-Enterobacteriaceae. In Woodhead Publishing Series in Food Science, Technology and Nutrition; Motarjemi, Y., Adams, M., Eds.; Woodhead Publishing: Cambridge, UK, 2006; pp. 450–475. [Google Scholar]
- Domian, E.; Poszytek, K. Wheat flour flowability as affected by water activity, storage time and consolidation. Int. Agrophysics 2005, 19, 119–124. [Google Scholar]
- Nasir, M.; Butt, M.S.; Faqir, M.A.; Sharif, K.; Minhas, R. Effect of Moisture on the Shelf Life of Wheat Flour. Int. J. Agric. Biol. 2003, 5, 458–459. [Google Scholar]
- Los, A.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Bourke, P. Investigation of mechanisms involved in germination enhancement of wheat (Triticum aestivum) by cold plasma: Effects on seed surface chemistry and characteristics. Plasma Process. Polym. 2019, 16, 1800148. [Google Scholar] [CrossRef]
- Labuza, T.P.; McNally, L.; Gallagher, D.; Hawkes, J.; Hurtado, F. Stability of Intermediate Moisture Foods. 1. Lipid Oxidation. J. Food Sci. 1972, 37, 154–159. [Google Scholar] [CrossRef]
- Kadkol, G.P.; Sissons, M. Durum Wheat: Overview. In Encyclopedia of Food Grains, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Czuchajowska, Z.; Paszczyñska, B. Is wet gluten good for baking? Cereal Chem. 1996, 73, 483–489. [Google Scholar]
- Ionescu, V.; Stoenescu, G. Comparative evaluation of wet gluten quantity and quality through Different Methods. Food Technol. 2010, 34, 49–53. [Google Scholar]
- Menkovska, M.; Mangova, M.; Dimitrov, K. Effect of Cold Plasma on Wheat Flour and Bread Making Quality. Maced. J. Anim. Sci. 2014, 4, 27–30. [Google Scholar] [CrossRef]
- Xhabiri, G.Q. Rheological Qualities of Dough from Mixture of Flour and Wheat Bran and Possible Correlation between Bra Bender and Mixolab Chopin Equipments. MOJ Food Process. Technol. 2016, 2, 121–128. [Google Scholar] [CrossRef]
- Okuda, R.; Tabara, A.; Okusu, H.; Seguchi, M. Measurement of Water Absorption in Wheat Flour by Mixograph Test. Food Sci. Technol. Res. 2016, 22, 841–846. [Google Scholar] [CrossRef]
- Van Der Borght, A.; Goesaert, H.; Veraverbeke, W.S.; Delcour, J.A. Fractionation of wheat and wheat flour into starch and gluten: Overview of the main processes and the factors involved. J. Cereal Sci. 2005, 41, 221–237. [Google Scholar] [CrossRef]
- Maningat, C.C.; Seib, P.A. Understanding the Physicochemical and Functional Properties of Wheat Starch in Various Foods. Cereal Chem. 2010, 87, 305–314. [Google Scholar] [CrossRef]
- Ma, Y.; Wu, D.; Guo, L.; Yao, Y.; Yao, X.; Wang, Z.; Wu, K.; Cao, X.; Gao, X. Effects of Quinoa Flour on Wheat Dough Quality, Baking Quality, and in vitro Starch Digestibility of the Crispy Biscuits. Front. Nutr. 2022, 9, 846808. [Google Scholar] [CrossRef]
- Almeida, E.L.; Kil Chang, Y. Effect of proving time on the quality of frozen pre-baked French style rolls elaborated with the addition of wholegrain flour and enzymes. J. Food Sci. Technol. 2014, 51, 3390–3396. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, R.M.; Rocha, F.; Moreira, M.A.; Castro, I.A. Effect of Proofing Time and Wheat Flour Strength on Bleaching, Sensory Characteristics, and Volume of French Breads with Added Soybean Lipoxygenase. Cereal Chem. 2007, 84, 443–449. [Google Scholar] [CrossRef]
- V. DLG e. Deutsche Landwirtschafts-Gesellschaft–German Agricultural Society. Available online: https://www.dlg.org/en/ (accessed on 8 December 2022).
- Pareyt, B.; Delcour, J.A. The Role of Wheat Flour Constituents, Sugar, and Fat in Low Moisture Cereal Based Products: A Review on Sugar-Snap Cookies. Crit. Rev. Food Sci. Nutr. 2008, 48, 824–839. [Google Scholar] [CrossRef]
- Manley, D. Technology of Biscuits, Crackers, and Cookies, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Hui, Y.H.; Corke, H.; De Leyn, I.; Nip, W.K.; Cross, N. Bakery Products: Science and Technology; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]

| Samples | Wet Gluten (%) | Dry Gluten (%) | GI | |
|---|---|---|---|---|
| Untreated Flour | Control | 23.9 ± 0.5 b | 10.3 ± 0.2 b | 89.4 ± 3.3 b |
| Treated Flour | 1 min | 23.8 ± 0.5 b | 10.3 ± 0.1 b | 89.6 ± 5.5 b |
| 3 min | 24.3 ± 1.5 b | 10.5 ± 0.0 b | 90.9 ± 3.3 b | |
| 5 min | 22.1± 0.4 a | 09.9 ± 0.1 a | 97.2 ± 1.6 a | |
| Samples | Farinograph (%) | |
|---|---|---|
| Untreated Flour | Control | 60.5 ± 0.1 a |
| Treated Flour | 1 min | 60.5 ± 0.2 a |
| 3 min | 60.4 ± 0.2 a | |
| 5 min | 60.4 ± 0.1 a | |
| Samples | Stability (min) | Softening (BU) | |
|---|---|---|---|
| Untreated Flour | Control | 7.13 ± 0.1 d | 53 ± 0.1 d |
| Treated Flour | 1 min | 6.96 ± 0.2 c | 55 ± 0.2 c |
| 3 min | 7.60 ± 0.2 b | 52 ± 0.1 b | |
| 5 min | 8.26 ± 0.15 a | 49 ± 0.4 a | |
| Samples | Stretch Resistance (Rmax) (g) | Extensibility (E) (mm) | Ratio (Rmax/E) | |
|---|---|---|---|---|
| Untreated Flour | Control | 24.6 ± 2.6 b | 48.4 ± 5.2 b | 0.5 ± 0.1 b |
| Treated Flour | 1 min | 26.3 ± 1.5 b | 45.3 ± 4.7 b | 0.6 ± 0.1 b |
| 3 min | 36.0 ± 3.8 a | 32.2 ± 3.2 a | 1.1 ± 0.2 a | |
| 5 min | 24.3 ± 1.6 b | 45.8 ± 4.7 b | 0.5 ± 0.1 b | |
| Samples | L—Lightness | b—Yellowness | a—Greenness | E*—Uniformity | ΔE—Color Difference | |
|---|---|---|---|---|---|---|
| Untreated Flour | Control | 79.7 ± 0.7 c | 17.4 ± 0.23 c | 0.73 ± 0.25 a | 81.6 | 0.0 |
| Treated Flour | 1 min | 80.2 ± 0.8 c | 16.8 ± 0.20 b | 1.33 ± 0.08 b | 81.9 | 0.9 |
| 3 min | 84.3 ± 2.6 b | 17.1 ± 0.74 c | 1.21 ± 0.03 c | 86.1 | 4.6 | |
| 5 min | 84.8 ± 1.0 a | 16.6 ± 0.28 a | 1.20 ± 0.51 d | 86.4 | 5.1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.J.; Jovicic, V.; Zbogar-Rasic, A.; Zettel, V.; Delgado, A.; Hitzmann, B. Influence of Non-Thermal Plasma Treatment on Structural Network Attributes of Wheat Flour and Respective Dough. Foods 2023, 12, 2056. https://doi.org/10.3390/foods12102056
Khan MJ, Jovicic V, Zbogar-Rasic A, Zettel V, Delgado A, Hitzmann B. Influence of Non-Thermal Plasma Treatment on Structural Network Attributes of Wheat Flour and Respective Dough. Foods. 2023; 12(10):2056. https://doi.org/10.3390/foods12102056
Chicago/Turabian StyleKhan, Muhammad Jehanzaib, Vojislav Jovicic, Ana Zbogar-Rasic, Viktoria Zettel, Antonio Delgado, and Bernd Hitzmann. 2023. "Influence of Non-Thermal Plasma Treatment on Structural Network Attributes of Wheat Flour and Respective Dough" Foods 12, no. 10: 2056. https://doi.org/10.3390/foods12102056







