Chemistry behind Quality—Emission of Volatile Enantiomers from Mentha spp. Plant Tissue in Relationship to Odor Sensory Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. EOs Hydrodistillation and Sample Preparation
2.4. HS-SPME Arrow Extraction
2.5. GC-MS Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
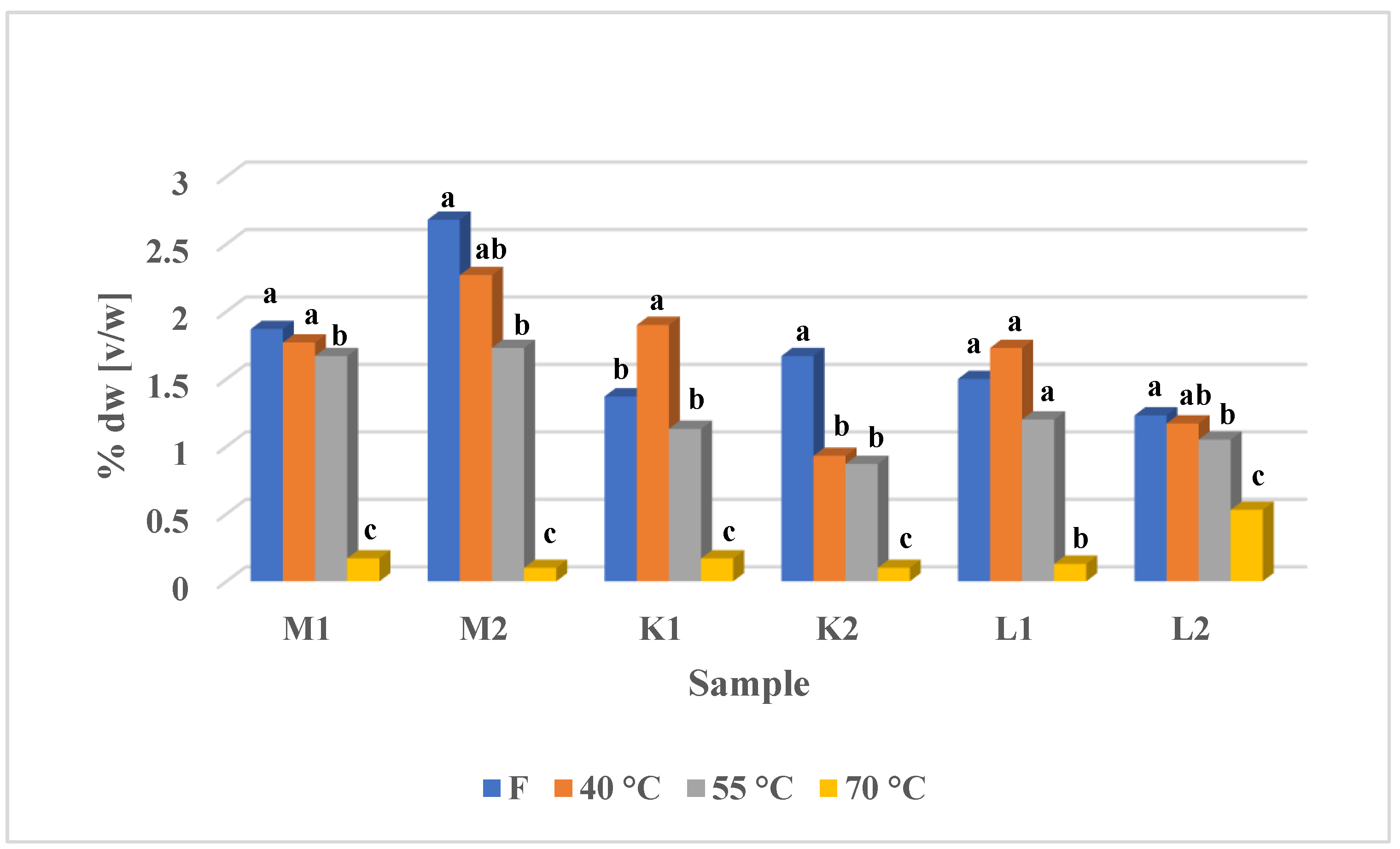
3.1. Essential Oil Yield
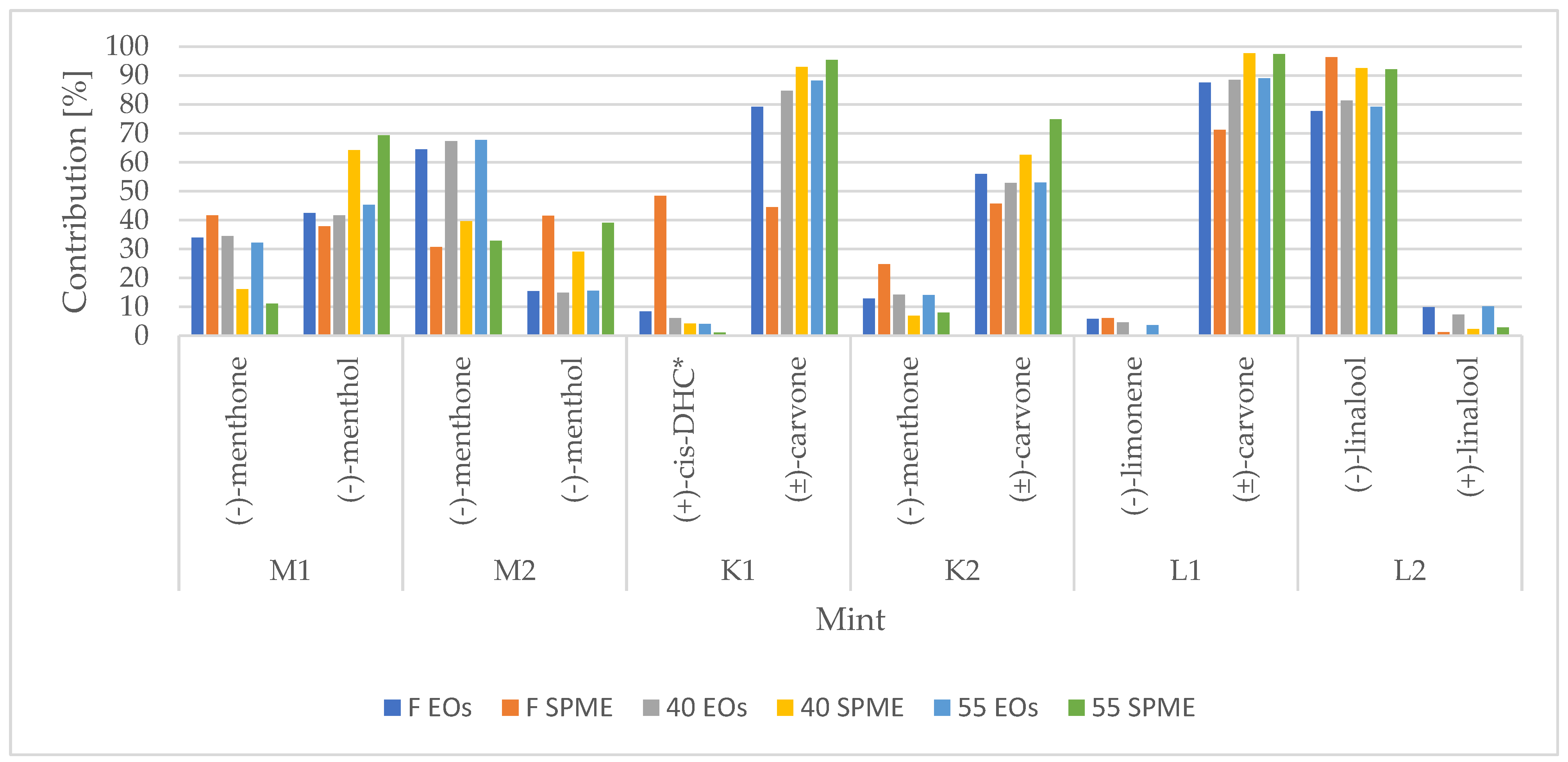
3.2. Enantioselective Analysis
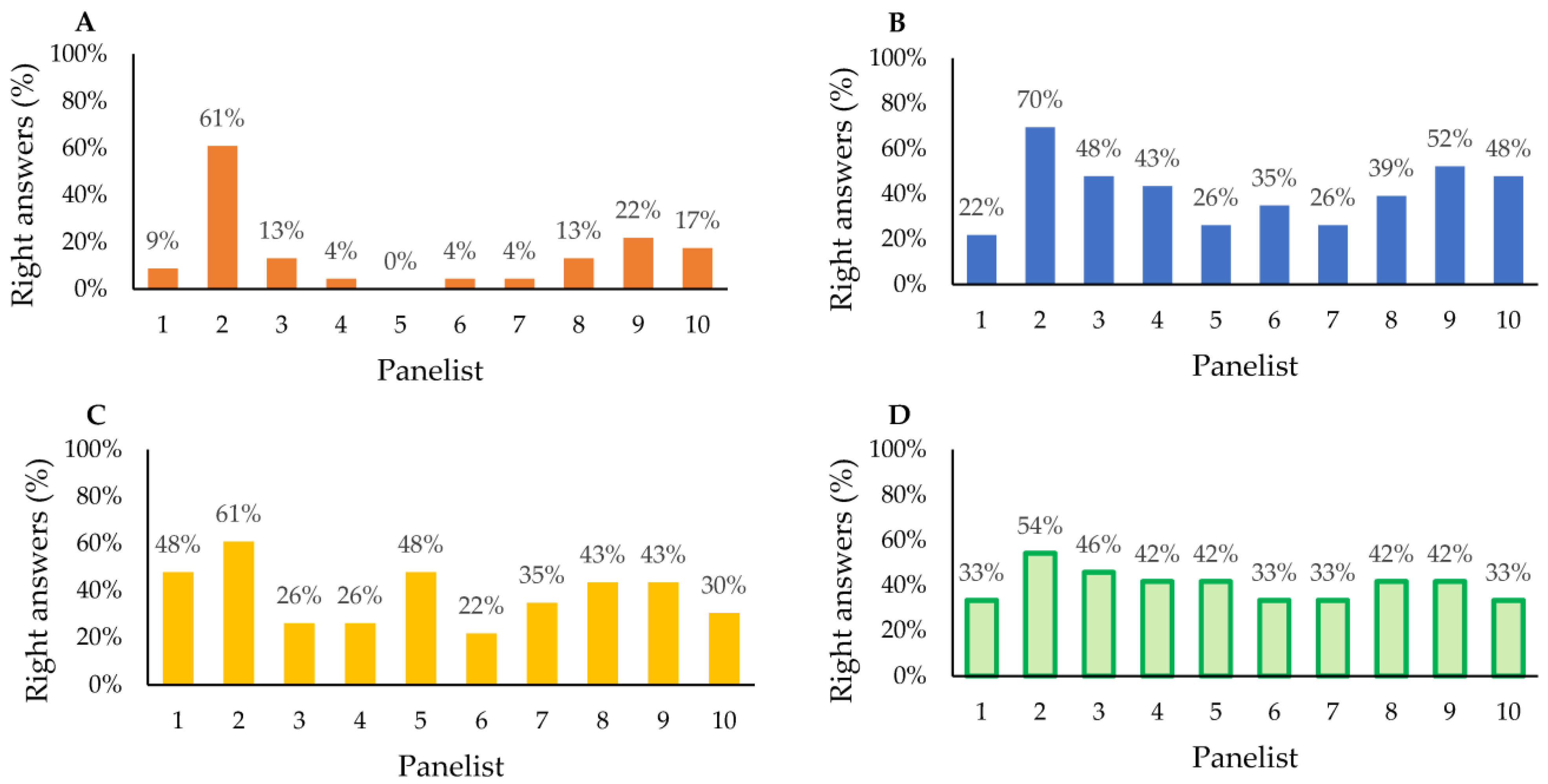
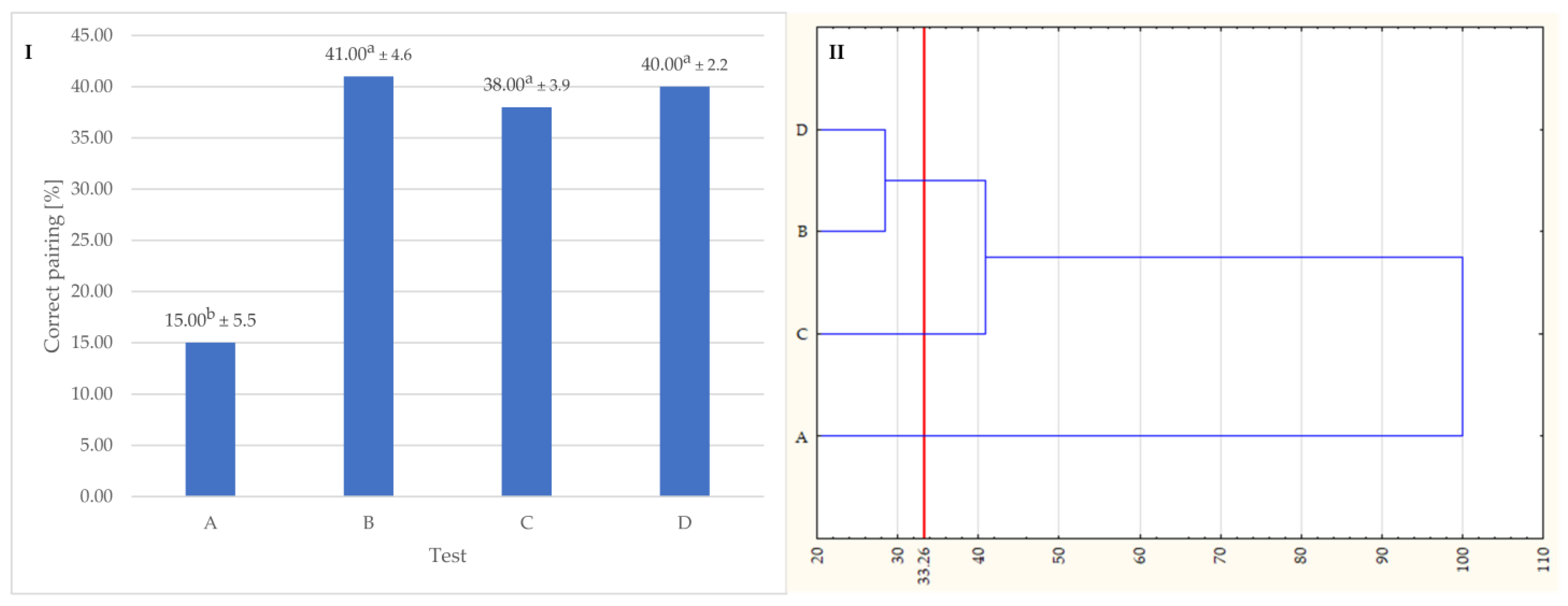
3.3. Sensory Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Başer, K.H.C.; Buchbauer, G. (Eds.) Handbook of Essential Oils. Science, Technology, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781466590472. [Google Scholar]
- Raghavan, S. Handbook of Spices, Seasoning and Flavorings; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9788578110796. [Google Scholar]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F.G. A Review of Drying Methods for Improving the Quality of Dried Herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, M.C.; Castillo, N.; Castro-Vázquez, L.; de Torres, C.; Pérez-Coello, M.S. Authenticity Evaluation of Different Mints Based on Their Volatile Composition and Olfactory Profile. J. Essent. Oil Bear. Plants 2008, 11, 1–16. [Google Scholar] [CrossRef]
- Łyczko, J.; Kiełtyka-Dadasiewicz, A.; Skrzyński, M.; Klisiewicz, K.; Szumny, A. Chemistry behind Quality—The Usability of Herbs and Spices Essential Oils Analysis in Light of Sensory Studies. Food Chem. 2023, 411, 135537. [Google Scholar] [CrossRef] [PubMed]
- Łyczko, J.; Masztalerz, K.; Lipan, L.; Lech, K.; Carbonell-Barrachina, Á.A.; Szumny, A. Chemical Determinants of Dried Thai Basil (O. Basilicum Var. Thyrsiflora) Aroma Quality. Ind. Crops Prod. 2020, 155, 112769. [Google Scholar] [CrossRef]
- Łyczko, J.; Jałoszyński, K.; Surma, M.; García-Garví, J.-M.; Carbonell-Barrachina, A.A.; Szumny, A. Determination of Various Drying Methods’ Impact on Odour Quality of True Lavender (Lavandula Angustifolia Mill.) Flowers. Molecules 2019, 24, 2900. [Google Scholar] [CrossRef]
- Ludwiczuk, A.; Kiełtyka-Dadasiewicz, A.; Sawicki, R.; Golus, J.; Ginalska, G. Essential Oils of Some Mentha Species and Cultivars, Their Chemistry and Bacteriostatic Activity. Nat. Prod. Commun. 2016, 11, 1015–1018. [Google Scholar] [CrossRef]
- Alavez-Rosas, D.; Nguyen, L.M.N.; Keefover-Ring, K. Retention Indices for Naturally-Occurring Chiral and Achiral Compounds on Common Gas Chromatography Chiral Stationary Phases. Results Chem. 2022, 4, 100659. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.V.; Carr, B.T. Sensory Evaluation Techniques, 5th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- 12. ISO 8586-1:1993 1993; International Standard Organization Sensory Analysis—General Guidance for the Selection, Training and Monitoring of As-sessors—Part 1: Selected Assessors. International Organization for Standardization: Geneva, Switzerland, 2008.
- Vallino, M.; Faccio, A.; Zeppa, G.; Dolci, P.; Cerutti, E.; Zaquini, L.; Faoro, F.; Balestrini, R. Impact of Drying Temperature on Tissue Anatomy and Cellular Ultrastructure of Different Aromatic Plant Leaves. Plant Biosyst. 2022, 156, 847–854. [Google Scholar] [CrossRef]
- Ghasemi Pirbalouti, A.; Salehi, S.; Craker, L.; Pirbalouti, A.G.; Salehi, S.; Craker, L. Effect of Drying Methods on Qualitative and Quantitative Properties of Essential Oil from the Aerial Parts of Coriander. J. Appl. Res. Med. Aromat. Plants 2017, 4, 35–40. [Google Scholar] [CrossRef]
- Sellami, I.H.; Wannes, W.A.; Bettaieb, I.; Berrima, S.; Chahed, T.; Marzouk, B.; Limam, F. Qualitative and Quantitative Changes in the Essential Oil of Laurus nobilis L. Leaves as Affected by Different Drying Methods. Food Chem. 2010, 126, 691–697. [Google Scholar] [CrossRef]
- Ahmed, A.; Ayoub, K.; Chaima, A.J.; Hanaa, L.; Abdelaziz, C. Effect of Drying Methods on Yield, Chemical Composition and Bioactivities of Essential Oil Obtained from Moroccan Mentha pulegium L. Biocatal. Agric. Biotechnol. 2018, 16, 638–643. [Google Scholar] [CrossRef]
- Chanotiya, C.S.; Pragadheesh, V.S.; Yadav, A.; Gupta, P.; Lal, R.K. Cyclodextrin-Based Gas Chromatography and GC/MS Methods for Determination of Chiral Pair Constituents in Mint Essential Oils. J. Essent. Oil Res. 2021, 33, 23–31. [Google Scholar] [CrossRef]
- Ruiz Del Castillo, M.L.; Blanch, G.P.; Herraiz, M. Natural Variability of the Enantiomeric Composition of Bioactive Chiral Terpenes in Mentha piperita. J. Chromatogr. A 2004, 1054, 87–93. [Google Scholar] [CrossRef]
- Kosakowska, O.; Węglarz, Z.; Bączek, K. Yield and Quality of ‘Greek Oregano’ (Origanum vulgare L. subsp. hirtum) Herb from Organic Production System in Temperate Climate. Ind. Crops Prod. 2019, 141, 111782. [Google Scholar] [CrossRef]
- Baczek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Weglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and Raw Material Quality from Organic Cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef]
- Asekun, O.T.; Grierson, D.S.; Afolayan, A.J. Effects of Drying Methods on the Quality and Quantity of the Essential Oil of Mentha longifolia L. subsp. Capensis. Food Chem. 2007, 101, 995–998. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Aly, M.S.A.; Alsuqub, A.N.A.; Khan, R.A. Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus Officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef]
- Rohloff, J.; Dragland, S.; Mordal, R.; Iversen, T.H. Effect of Harvest Time and Drying Method on Biomass Production, Essential Oil Yield, and Quality of Peppermint (Mentha × piperita L.). J. Agric. Food Chem. 2005, 53, 4143–4148. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Goli, S.A.H. Evaluation of Six Drying Treatments with Respect to Essential Oil Yield, Composition and Color Characteristics of Thymys Daenensis Subsp. Daenensis. Celak Leaves. Ind. Crops Prod. 2013, 42, 613–619. [Google Scholar] [CrossRef]
- Geithe, C.; Krautwurst, D. Chirality Matters—Enantioselective Orthologous Odorant Receptors for Related Terpenoid Structures. In Importance of Chirality to Flavor Compounds; American Chemical Society: Washington, DC, USA, 2015; pp. 159–181. [Google Scholar]
- Davies, N.W.; Larkman, T.; Marriott, P.J.; Khan, I.A. Determination of Enantiomeric Distribution of Terpenes for Quality Assessment of Australian Tea Tree Oil. J. Agric. Food Chem. 2016, 64, 4817–4819. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorsi, I.; Sciarrone, D.; Cotroneo, A.; Mondello, L.; Dugo, P.; Dugo, G. Enantiomeric Distribution of Key Volatile Components in Citrus Essential Oils. Rev. Bras. Farmacogn. 2011, 21, 841–849. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Avula, B.; Wang, Y.H.; Chittiboyina, A.G.; Parcher, J.F.; Khan, I.A. Quality Evaluation of Terpinen-4-ol-Type Australian Tea Tree Oils and Commercial Products: An Integrated Approach Using Conventional and Chiral GC/MS Combined with Chemometrics. J. Agric. Food Chem. 2015, 63, 2674–2682. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chittiboyina, A.G.; Parcher, J.F.; Ali, Z.; Ford, P.; Zhao, J.; Avula, B.; Wang, Y.H.; Khan, I.A. Piper Nigrum Oil—Determination of Selected Terpenes for Quality Evaluation. Planta Med. 2019, 85, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Chanotiya, C.S.; Yadav, A. Natural Variability in Enantiomeric Composition of Bioactive Chiral Terpenoids in the Essential Oil of Solidago canadensis L. from Uttarakhand, India. Nat. Prod. Commun. 2008, 3, 263–266. [Google Scholar] [CrossRef]
- Moè Llenbeck, S.; Koè Nig, T.; Schreier, P.; Schwab, W.; Rajaonarivony, J.; Ranarivelo, L. Chemical Composition and Analyses of Enantiomers of Essential Oils from Madagascar. Flavour Fragr. J. 1997, 12, 63–69. [Google Scholar] [CrossRef]
| Cultivar and Chemotype | Type of Treatment | Sample Code |
|---|---|---|
| Mentha × piperita L. ‘Multimentha’ (menthol pathway) | frozen | M1_F |
| dried at 40 °C | M1_40 | |
| dried at 55 °C | M1_55 | |
| dried at 70 °C | M1_70 | |
| Mentha × piperita L. ‘Swiss’ (menthol pathway) | frozen | M2_F |
| dried at 40 °C | M2_40 | |
| dried at 55 °C | M2_55 | |
| dried at 70 °C | M2_70 | |
| Mentha spicata L. ‘Moroccan’ (carvone pathway) | frozen | K1_F |
| dried at 40 °C | K1_40 | |
| dried at 55 °C | K1_55 | |
| dried at 70 °C | K1_70 | |
| Mentha spicata L. ‘Crispa’ (carvone pathway) | frozen | K2_F |
| dried at 40 °C | K2_40 | |
| dried at 55 °C | K2_55 | |
| dried at 70 °C | K2_70 | |
| Mentha × piperita L. ‘Grapefruit’ (linalool pathway) | frozen | L1_F |
| dried at 40 °C | L1_40 | |
| dried at 55 °C | L1_55 | |
| dried at 70 °C | L1_70 | |
| Mentha × piperita L. ‘Granada’ (linalool pathway) | frozen | L2_F |
| dried at 40 °C | L2_40 | |
| dried at 55 °C | L2_55 | |
| dried at 70 °C | L2_70 |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | * | 0.45 a | 0.05 b | 0.54 a | tr 2 | 0.49 a | tr |
| α-(+)-pinene | * | 0.33 a | 0.07 b | 0.58 a | tr | 0.53 a | tr |
| (+)-sabinene | * | 0.48 a | 0.18 b | 0.86 a | 0.09 b | 0.76 a | 0.08 b |
| (−)-sabinene | * | 0.16 a | 0.05 b | 0.28 a | tr | 0.24 a | tr |
| β-(+)-pinene | * | 0.54 a | 0.19 b | 0.87 a | tr | 0.81 a | tr |
| β-(−)-pinene | * | 0.52 a | 0.20 b | 0.89 a | 0.06 b | 0.83 a | 0.05 b |
| (−)-limonene | * | 4.69 a | 3.84 a | 5.88 a | 1.07 b | 5.33 a | 0.88 b |
| (+)-limonene | * | 0.16 b | 1.18 a | 0.18 b | 0.06 c | 0.18 b | 0.05 c |
| (−)-linalool | * | 0.26 b | 0.35 ab | 0.25 b | 0.40 a | 0.27 b | 0.39 a |
| (−)-menthone | * | 33.87 b | 41.56 a | 34.47 b | 16.10 c | 32.14 b | 11.10 d |
| (+)-isomenthone | * | 3.45 ab | 4.72 a | 3.00 ab | 2.29 b | 3.02 ab | 3.15 ab |
| (+)-terpinen-4-ol | NS | 0.20 | 0.18 | 0.16 | 0.25 | 0.22 | 0.28 |
| (−)-terpinen-4-ol | NS | 4.08 | 4.51 | 3.39 | 4.33 | 3.54 | 4.60 |
| (−)-menthol | * | 42.39 bc | 37.81 c | 41.56 bc | 64.14 a | 45.24 b | 69.28 a |
| (−)-menthyl acetate | * | 4.60 a | 1.18 b | 3.44 a | 3.11 a | 2.56 ab | 2.60 ab |
| α-(+)-terpineol | NS | 0.12 | 0.09 | 0.11 | 0.24 | 0.11 | 0.21 |
| α-(−)-terpineol | NS | 0.13 | 0.07 | 0.11 | 0.24 | 0.12 | 0.21 |
| (+)-piperitone | * | 3.40 b | 3.78 b | 3.27 b | 7.35 a | 3.47 b | 6.86 a |
| (−)-trans-caryophyllene | NS | 0.20 | tr | 0.15 | 0.14 | 0.13 | 0.13 |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | * | 0.39 a | tr 3 | 0.48 a | 0.10 b | 0.44 a | 0.05 b |
| α-(+)-pinene | * | 0.36 a | 0.10 b | 0.45 a | 0.08 b | 0.42 a | 0.05 b |
| (+)-sabinene | * | 0.52 a | 0.20 b | 0.66 a | 0.20 b | 0.52 a | 0.12 b |
| (−)-sabinene | * | 0.20 ab | 0.29 a | 0.25 a | 0.06 c | 0.19 b | tr |
| β-(+)-pinene | * | 0.60 a | tr | 0.72 a | 0.12 b | 0.67 a | 0.07 b |
| β-(−)-pinene | * | 0.50 a | 0.26 b | 0.62 a | 0.15 b | 0.57 a | 0.07 c |
| (−)-limonene | * | 0.86 a | tr | 0.44 b | 0.81 a | 0.39 b | 0.41 b |
| (+)-limonene | * | 0.06 b | 2.47 a | tr | 0.11 b | tr | 0.08 b |
| (−)-linalool | * | 0.39 c | 0.32 c | 0.38 c | 0.65 b | 0.40 c | 0.81 a |
| (−)-menthone | * | 64.40 a | 30.64 c | 67.28 a | 39.56 b | 67.74 a | 32.77 c |
| (+)-isomenthone | * | 5.14 a | 4.38 ab | 4.74 ab | 4.34 b | 5.08 ab | 5.07 ab |
| (+)-terpinen-4-ol | * | 0.43 b | 6.61 a | 0.25 b | 0.31 b | 0.36 b | 0.43 b |
| (−)-terpinen-4-ol | * | 4.10 b | 6.12 a | 1.82 d | 2.68 c | 1.75 d | 3.20 c |
| (−)-menthol | * | 15.43 c | 41.47 a | 14.87 c | 29.04 b | 15.55 c | 39.02 a |
| (−)-menthyl acetate | NS | 3.55 | 3.00 | 3.17 | 7.35 | 2.58 | 4.61 |
| (±)-carvone 2 | * | 0.16 b | 0.43 b | 0.68 b | 6.19 a | 0.04 b | 4.02 a |
| (+)-piperitone | * | 2.49 c | 3.67 b | 2.76 c | 7.89 a | 2.87 c | 8.35 a |
| (−)-trans-caryophyllene | * | 0.42 b | tr | 0.42 b | 0.35 c | 0.39 bc | 0.76 a |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | * | 0.54 a | tr 3 | 0.53 a | 0.05 b | 0.42 a | 0.05 b |
| α-(+)-pinene | * | 0.31 a | tr | 0.31 a | 0.03 b | 0.23 a | tr |
| (+)-sabinene | * | 0.49 a | tr | 0.41 a | 0.06 b | 0.34 a | tr |
| (−)-sabinene | * | 0.28 b | 2.62 a | 0.24 b | tr | 0.20 b | tr |
| β-(+)-pinene | * | 0.57 b | 0.71 a | 0.52 b | 0.05 d | 0.45 c | 0.05 d |
| β-(−)-pinene | * | 0.61 a | tr | 0.55 ab | 0.06 c | 0.48 b | 0.06 c |
| (−)-limonene | * | 6.79 a | 2.02 bc | 5.13 ab | 1.12 c | 4.10 ab | 0.45 c |
| (−)-linalool | NS | 0.31 | tr | 0.20 | 0.23 | 0.22 | 0.24 |
| (−)-menthone | * | 0.23 c | tr | 0.18 c | 0.70 b | 0.17 c | 1.48 a |
| (+)-terpinen-4-ol | NS | 0.12 | 0.16 | 0.05 | tr | 0.05 | tr |
| (−)-terpinen-4-ol | NS | 0.09 | 0.17 | tr | 0.05 | tr | 0.09 |
| (+)-cis-dihydrocarvone | * | 8.35 b | 48.38 a | 6.14 c | 4.24 c | 4.10 c | 1.13 d |
| (−)-menthol | NS | 0.14 | tr | 0.12 | 0.11 | 0.12 | 0.19 |
| (+)-dihydrocarveol | * | 1.72 b | 2.96 a | 0.41 b | 0.22 b | 0.30 b | 0.46 b |
| (±)-carvone 2 | * | 79.11 c | 44.44 d | 84.73 bc | 92.88 a | 88.26 ab | 95.38 a |
| (−)-dihydrocarveol | * | 0.21 a | tr | 0.14 b | tr | 0.11 bc | 0.08 c |
| (−)-trans-caryophyllene | * | 0.16 d | 0.50 a | 0.30 c | 0.10 d | 0.41 b | 0.26 cd |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | * | 0.89 a | tr 3 | 1.00 a | 0.08 b | 0.93 a | 0.07 b |
| α-(+)-pinene | * | 1.19 b | tr | 1.42 a | 0.10 c | 1.30 ab | 0.10 c |
| (+)-sabinene | * | 1.84 c | 3.48 a | 2.05 b | 0.25 d | 2.00 b | 0.21 d |
| (−)-sabinene | * | 0.48 bc | 3.52 a | 0.54 b | 0.05 c | 0.51 b | 0.05 c |
| β-(+)-pinene | * | 1.57 a | tr | 1.87 a | 0.14 b | 1.84 a | 0.12 b |
| β-(−)-pinene | * | 2.02 a | tr | 2.41 a | 0.20 b | 2.39 a | 0.19 b |
| (−)-limonene | * | 9.57 a | tr | 7.16 b | 1.44 c | 6.26 b | 0.95 cd |
| (−)-linalool | * | 1.29 b | 1.21 b | 1.45 ab | 0.97 c | 1.58 a | 1.27 b |
| (−)-menthone | * | 12.84 b | 24.67 a | 14.23 b | 6.89 c | 14.02 b | 7.94 c |
| (+)-terpinen-4-ol | NS | 0.37 | 0.27 | 0.24 | 0.18 | 0.25 | 0.18 |
| (−)-terpinen-4-ol | * | 1.55 a | 1.21 ab | 1.38 ab | 1.04 b | 1.42 ab | 1.21 ab |
| (+)-cis-dihydrocarvone | * | 0.19 b | 13.53 a | 0.15 b | 15.32 a | 0.22 b | 0.56 b |
| (−)-cis-dihydrocarvone | NS | 0.07 | tr | 0.15 | tr | 0.14 | 0.06 |
| (−)-menthol | * | 4.27 bc | 3.68 c | 4.72 abc | 5.53 ab | 5.08 abc | 5.82 a |
| (−)-menthyl acetate | * | 1.47 b | 0.62 b | 2.81 a | 0.75 b | 3.12 a | 1.30 b |
| (+)-menthyl acetate | NS | 0.45 | tr | 0.44 | 0.69 | 0.51 | 0.67 |
| (+)-dihydrocarveol | NS | 0.12 | tr | 0.11 | tr | 0.13 | tr |
| (±)-carvone 2 | * | 55.90 c | 45.65 d | 52.83 c | 62.49 b | 52.98 c | 74.80 a |
| (+)-piperitone | * | 1.87 bc | 1.46 c | 2.09 bc | 2.89 a | 2.08 bc | 2.45 ab |
| (−)-dihydrocarveol | * | 0.81 ab | 0.31 b | 1.13 a | 0.49 b | 0.94 ab | 0.96 ab |
| (−)-trans-caryophyllene | * | 1.26 b | 0.39 c | 1.82 ab | 0.51 c | 2.28 a | 1.06 b |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | NS | 0.52 | 0.44 | 0.66 | tr 3 | 0.62 | tr |
| α-(+)-pinene | NS | 0.39 | 0.32 | 0.48 | tr | 0.47 | tr |
| (+)-sabinene | NS | 0.73 | 0.71 | 0.83 | tr | 0.77 | tr |
| (−)-sabinene | NS | 0.23 | 0.22 | 0.27 | tr | 0.24 | tr |
| β-(+)-pinene | NS | 0.62 | 0.49 | 0.71 | tr | 0.70 | tr |
| β-(−)-pinene | * | 0.75 b | 0.50 c | 0.87 a | tr | 0.84 a | 0.06 d |
| (−)-limonene | * | 5.79 a | 6.11 a | 4.60 ab | 0.24 c | 3.63 b | 0.22 c |
| (+)-terpinen-4-ol | * | 0.27 a | 0.06 b | 0.09 b | 0.06 b | 0.09 b | 0.07 b |
| (−)-terpinen-4-ol | NS | 0.16 | tr | 0.07 | 0.08 | 0.06 | 0.07 |
| (+)-cis-dihydrocarvone | * | 0.74 b | 17.08 a | 1.09 b | 0.09 b | 1.26 b | 0.11 b |
| (−)-menthol | NS | 0.29 | 0.28 | 0.25 | 0.37 | 0.28 | 0.38 |
| (−)-menthyl acetate | NS | 0.36 | 0.44 | 0.37 | 0.27 | 0.36 | 0.38 |
| (+)-menthyl acetate | NS | tr | tr | tr | 0.29 | tr | 0.31 |
| (+)-dihydrocarveol | * | 0.67 a | tr | 0.26 c | tr | 0.53 b | 0.07 d |
| (−)-borneol | * | 0.78 b | 1.99 a | 0.72 bc | 0.57 c | 0.85 b | 0.52 c |
| (±)-carvone 2 | * | 87.60 ab | 71.23 b | 88.53 ab | 97.70 a | 89.08 ab | 97.46 a |
| (−)-trans-caryophyllene | NS | 0.12 | 0.11 | 0.18 | 0.17 | 0.22 | 0.13 |
| Compound | ANOVA 1 | [%] | |||||
|---|---|---|---|---|---|---|---|
| F | 40 | 55 | |||||
| EOs | SPME | EOs | SPME | EOs | SPME | ||
| α-(−)-pinene | NS | 0.05 | tr 2 | 0.09 | 0.05 | 0.06 | 0.05 |
| α-(+)-pinene | * | 0.26 c | 0.08 d | 0.62 a | 0.07 d | 0.41 b | 0.11 d |
| (+)-sabinene | * | 0.40 bc | 0.25 d | 0.94 a | 0.28 cd | 0.65 b | 0.35 c |
| (−)-sabinene | NS | 0.05 | tr | 0.14 | tr | 0.09 | 0.06 |
| β-(+)-pinene | * | 0.35 c | 0.12 d | 0.76 a | 0.08 d | 0.55 b | 0.12 d |
| β-(−)-pinene | * | 0.45 c | 0.16 d | 0.99 a | 0.10 d | 0.70 b | 0.15 d |
| (−)-limonene | * | 0.21 b | 0.14 b | 0.16 b | 0.58 a | 0.13 b | 0.56 a |
| (+)-limonene | NS | 0.39 | 0.28 | 0.39 | 0.39 | 0.34 | 0.37 |
| (−)-linalool | * | 77.68 d | 96.31 a | 81.26 c | 92.53 ab | 79.22 cd | 92.16 b |
| (+)-linalool | * | 9.93 a | 1.26 c | 7.25 b | 2.38 c | 10.12 a | 2.81 c |
| α-(+)-terpineol | NS | 2.73 | 0.62 | 2.08 | 1.38 | 2.16 | 1.26 |
| α-(−)-terpineol | * | 7.50 a | 0.78 c | 5.31 b | 2.15 c | 5.57 b | 2.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyczko, J.; Kiełtyka-Dadasiewicz, A.; Issa-Issa, H.; Skrzyński, M.; Galek, R.; Carbonell-Barrachina, Á.A.; Szumny, A. Chemistry behind Quality—Emission of Volatile Enantiomers from Mentha spp. Plant Tissue in Relationship to Odor Sensory Quality. Foods 2023, 12, 2057. https://doi.org/10.3390/foods12102057
Łyczko J, Kiełtyka-Dadasiewicz A, Issa-Issa H, Skrzyński M, Galek R, Carbonell-Barrachina ÁA, Szumny A. Chemistry behind Quality—Emission of Volatile Enantiomers from Mentha spp. Plant Tissue in Relationship to Odor Sensory Quality. Foods. 2023; 12(10):2057. https://doi.org/10.3390/foods12102057
Chicago/Turabian StyleŁyczko, Jacek, Anna Kiełtyka-Dadasiewicz, Hanán Issa-Issa, Mariusz Skrzyński, Renata Galek, Ángel A. Carbonell-Barrachina, and Antoni Szumny. 2023. "Chemistry behind Quality—Emission of Volatile Enantiomers from Mentha spp. Plant Tissue in Relationship to Odor Sensory Quality" Foods 12, no. 10: 2057. https://doi.org/10.3390/foods12102057
APA StyleŁyczko, J., Kiełtyka-Dadasiewicz, A., Issa-Issa, H., Skrzyński, M., Galek, R., Carbonell-Barrachina, Á. A., & Szumny, A. (2023). Chemistry behind Quality—Emission of Volatile Enantiomers from Mentha spp. Plant Tissue in Relationship to Odor Sensory Quality. Foods, 12(10), 2057. https://doi.org/10.3390/foods12102057












