Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparations and Extraction Process
2.3. Response Surface Methodology (RSM) Optimization and Experiment Design
2.4. Determination of Total Polyphenol Content (TPC)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7. DPPH Radical Scavenging Activity
2.8. Ascorbic Acid Content
2.9. HPLC-Based Determination of the Chlorogenic Acid Content and Other Phenolic Compounds
2.10. Statistical Analysis
3. Results and Discussion
3.1. Extraction Optimization
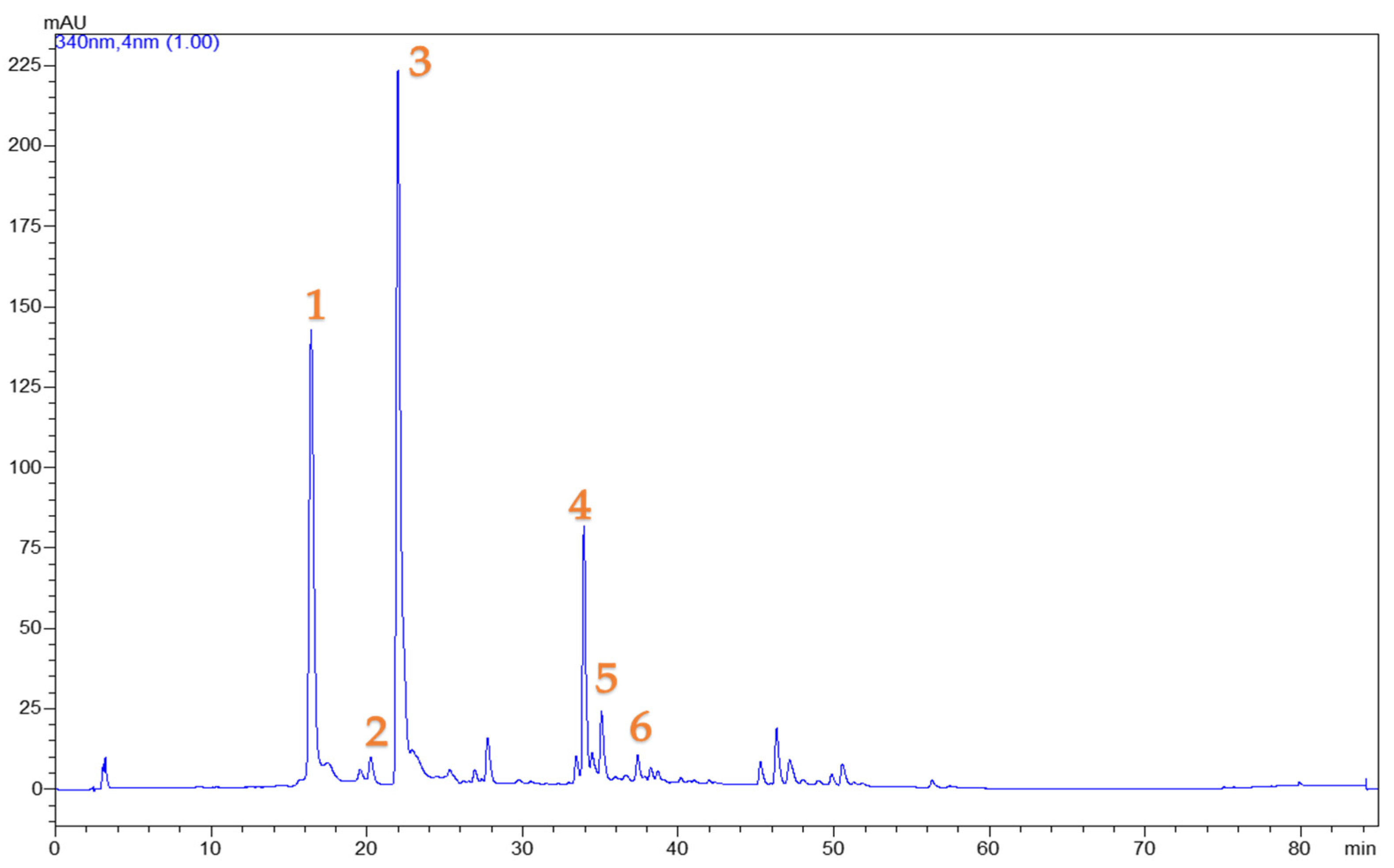
3.2. Chlorogenic Acid Content of the Extracts
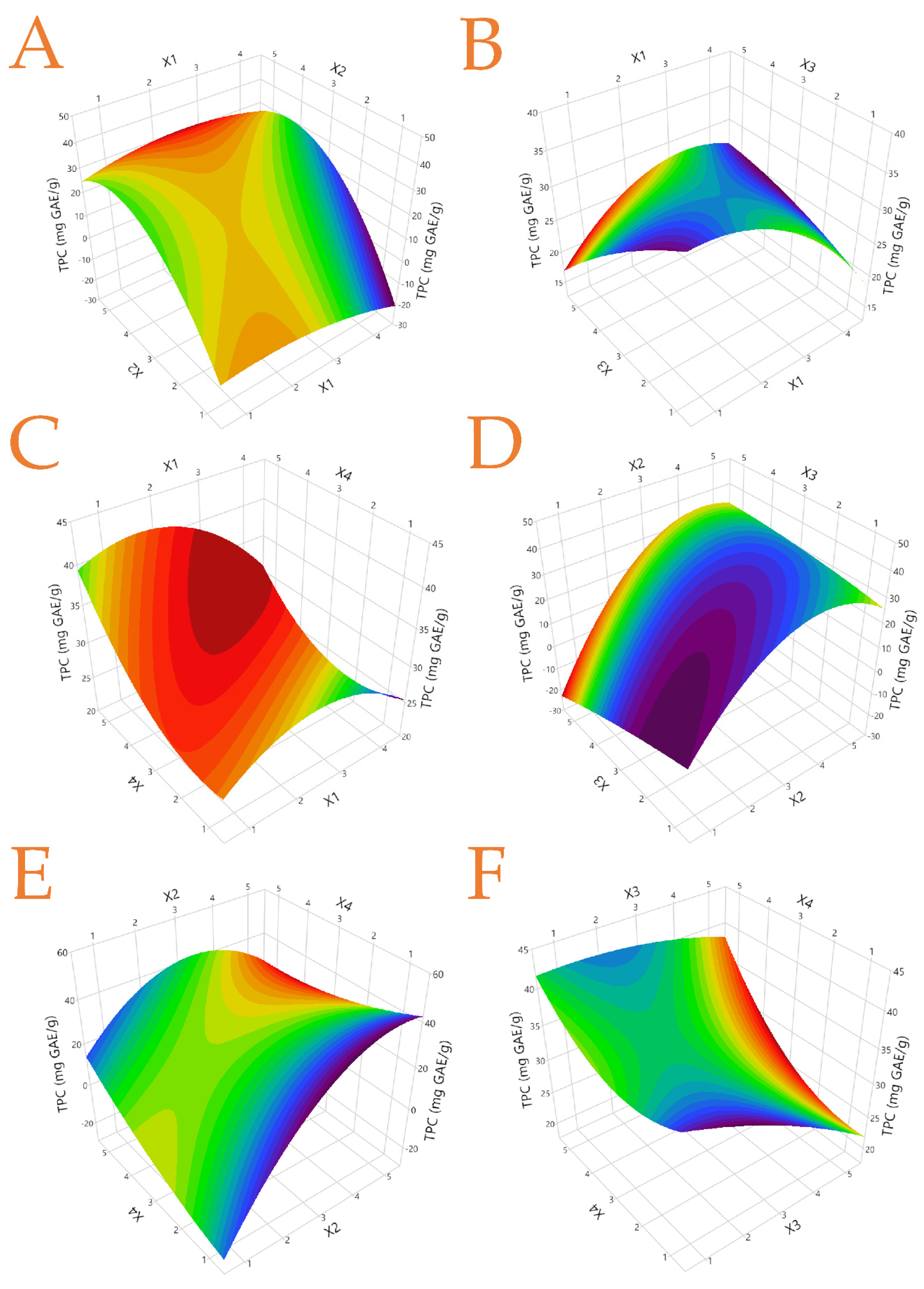
3.3. Total Polyphenol Content (TPC) and Flavonoids Content (TFC) of the Extracts
3.4. Antioxidant Properties of the Extracts
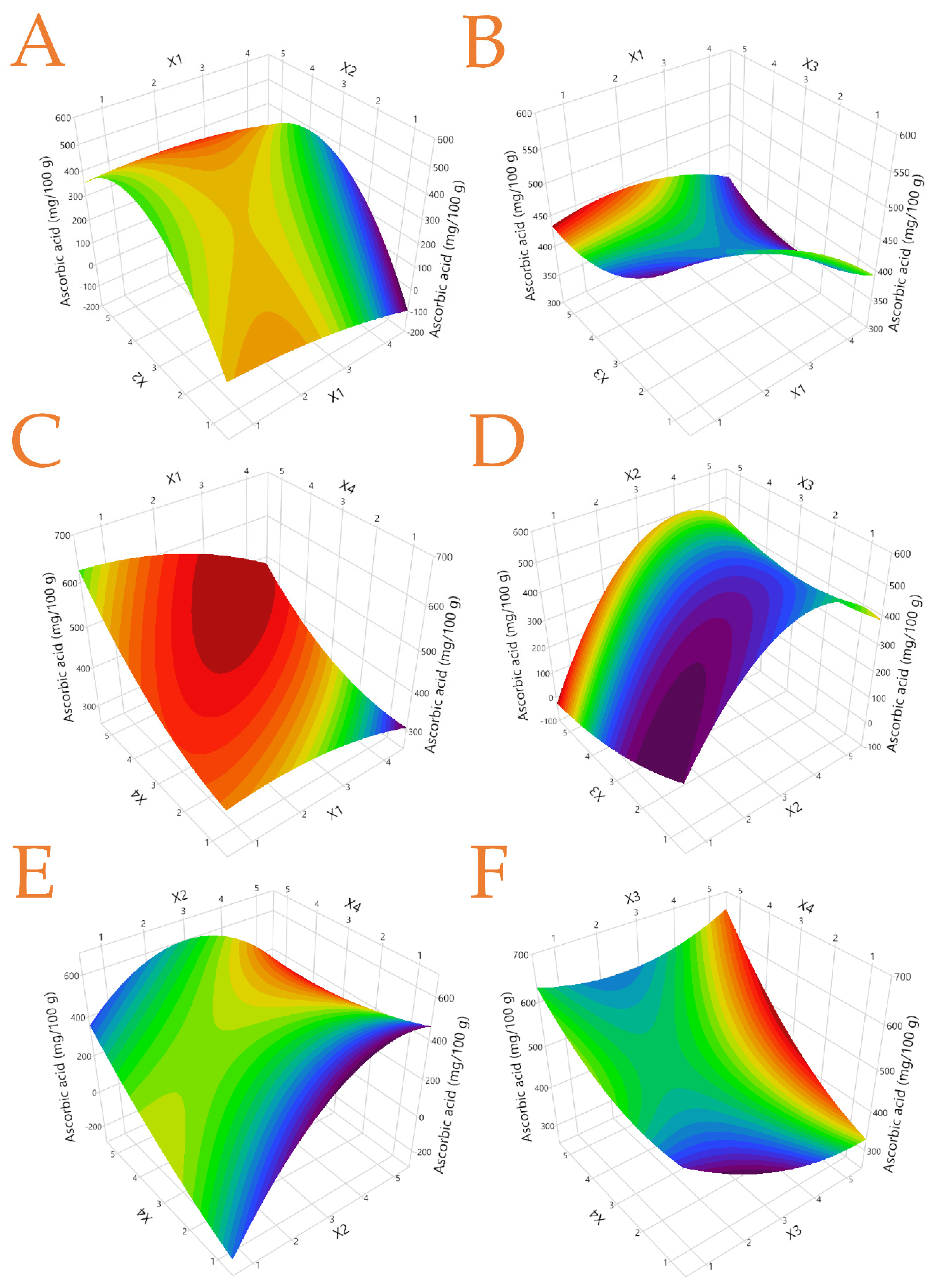
3.5. Ascorbic Acid Content of the Extracts
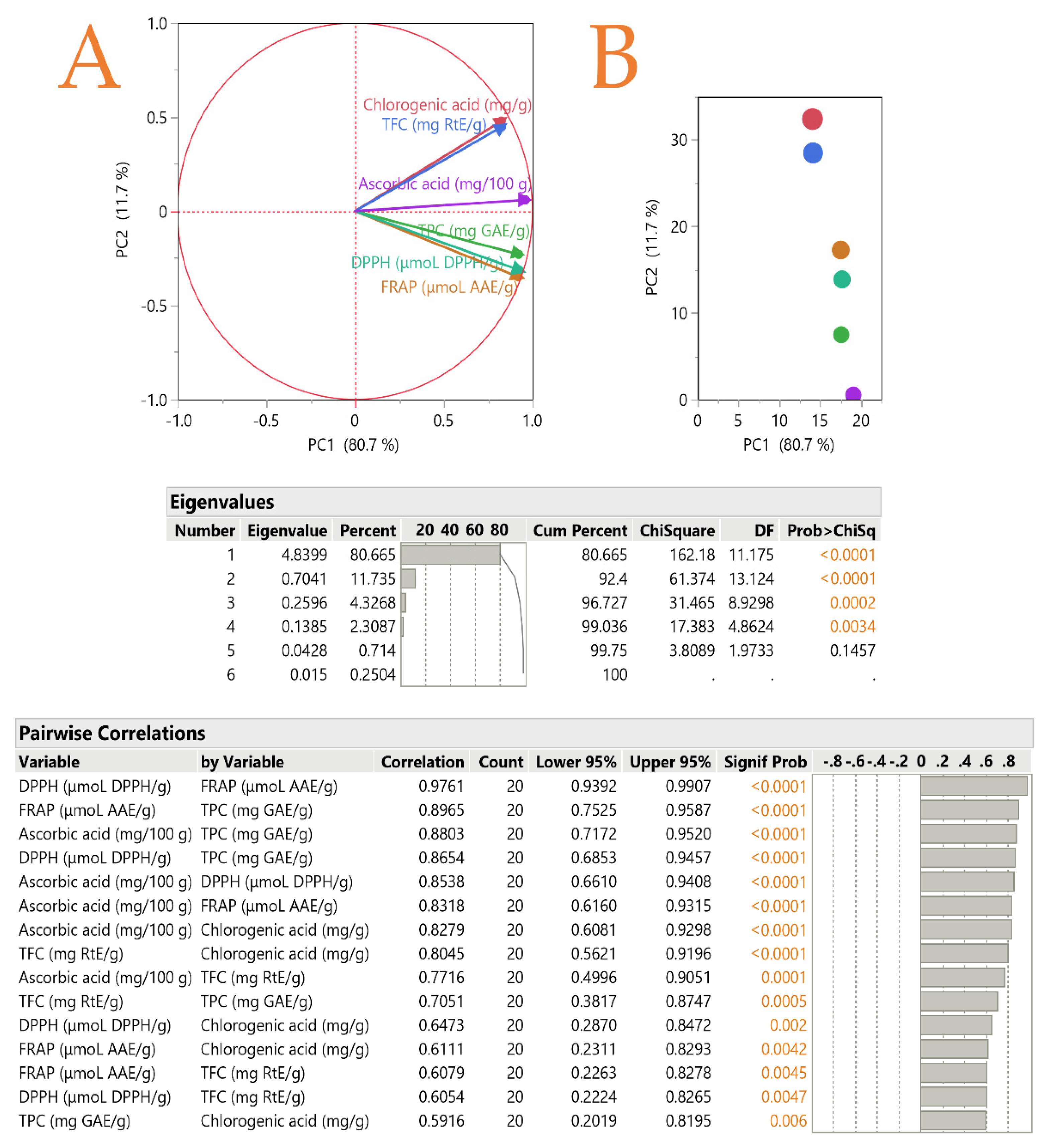
3.6. Principal Component Analysis (PCA)
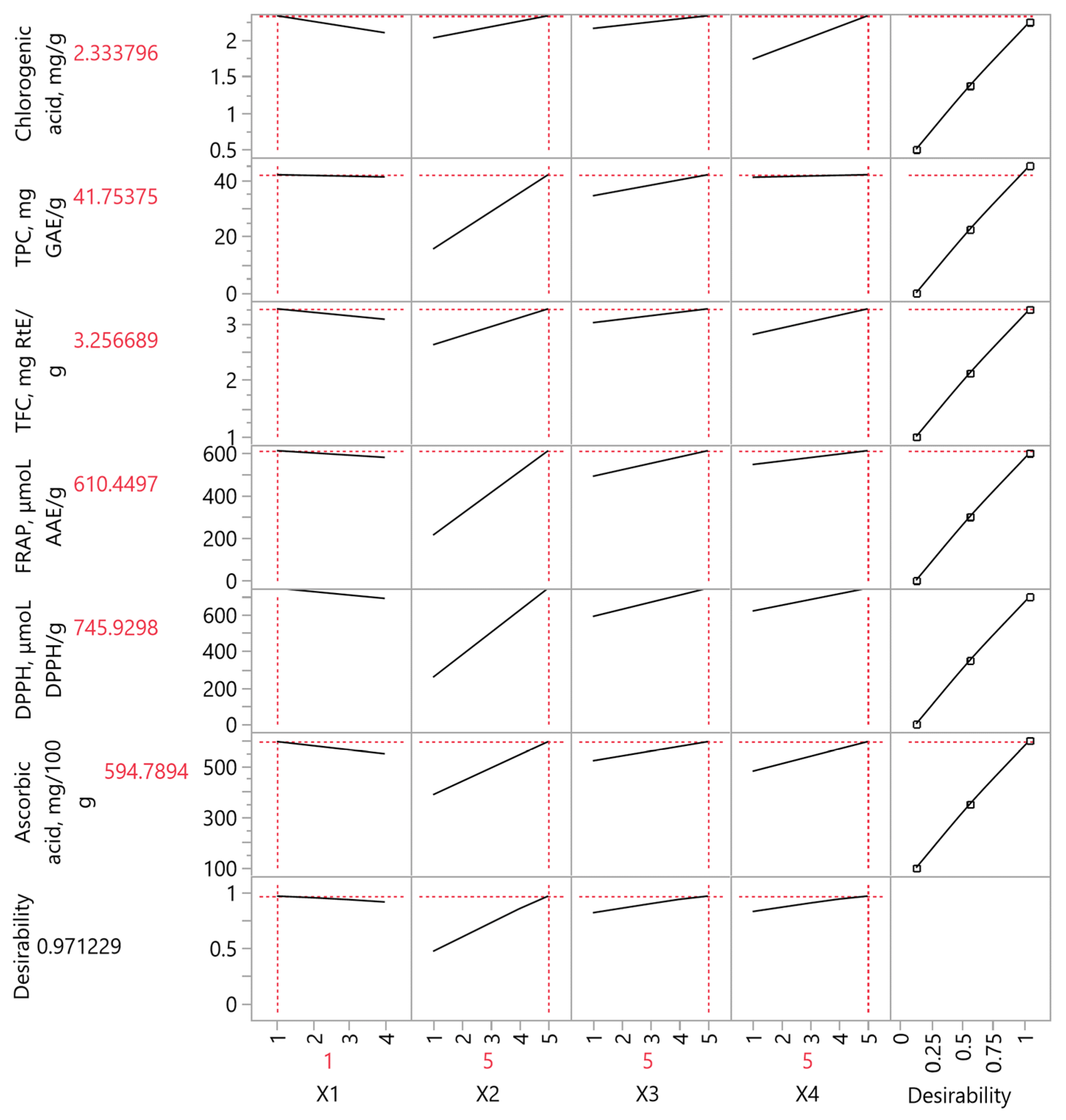
3.7. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khoubnasabjafari, M.; Jouyban, A. A Review of Phytochemistry and Bioactivity of Quince (Cydonia oblonga Mill.). J. Med. Plants Res. 2011, 5, 3577–3594. [Google Scholar]
- Zubair, M.; Kashmir, A.; Mahmood Sajid, S.; Waqas, M.; Nawaz, M.; Ahmad, Z. A Review on Quince (Cydonia oblonga): A Useful Medicinal Plant. Glob. Vet. 2015, 14, 517–524. [Google Scholar]
- Hedrick, U.P. Cyclopedia of Hardy Fruits; Macmillan Company: New York, NY, USA, 1992; Volume 1, pp. 105–112. [Google Scholar]
- Postman, J. Cydonia oblonga: The Unappreciated Quince. Arnoldia 2009, 67, 2–9. [Google Scholar]
- Ferreira, D.M.; de Oliveira, N.M.; Lopes, L.; Machado, J.; Oliveira, M.B. Potential Therapeutic Properties of the Leaf of Cydonia oblonga Mill. Based on Mineral and Organic Profiles. Plants 2022, 11, 2638. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Yousuf, S.; Ashraf, Q.S.; Mir, S.A.; Makroo, H.A.; Majid, D.; Barba, F.J.; Dar, B.N. Nutritional and Bioactive Composition, Nutraceutical Potential, Food and Packaging Applications of Cydonia oblonga and Its Byproducts: A Review. J. Food Compos. Anal. 2023, 115, 105000. [Google Scholar] [CrossRef]
- Patel, N.C.; Rathod, B.G.; Shah, V.N.; Mahajan, A.N. Cydonia Vulgaris Pers.: A Review on Diversity, Cultivation, Chemistry and Utilization. Pharm. Lett. 2011, 3, 51–61. [Google Scholar]
- Othman, S.; Añibarro-Ortega, M.; Dias, M.I.; Ćirić, A.; Mandim, F.; Soković, M.; Ferreira, I.C.F.R.; Pinela, J.; Barros, L. Valorization of Quince Peel into Functional Food Ingredients: A Path towards “Zero Waste” and Sustainable Food Systems. Heliyon 2022, 8, e11042. [Google Scholar] [CrossRef]
- Ercisli, S. A Short Review of the Fruit Germplasm Resources of Turkey. Genet. Resour. Crop Evol. 2004, 51, 419–435. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit (Pulp, Peel, and Seed) and Jam: Antioxidant Activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Seabra, R.M.; Silva, B.M. Phenolic Profile of Cydonia Oblonge Miller Leaves. J. Agric. Food Chem. 2007, 55, 7926–7930. [Google Scholar] [CrossRef]
- Oliveira, A.P.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Seabra, R.M.; Silva, B.M. Organic Acids Composition of Cydonia oblonga Miller Leaf. Food Chem. 2008, 111, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Chockalingam, V.; Suryakiran Kadali, S.D.V.; Gnanasambantham, P. Antiproliferative and Antioxidant Activity of Aegle Marmelos (Linn.) Leaves in Dalton’s Lymphoma Ascites Transplanted Mice. Indian J. Pharmacol. 2012, 44, 225–229. [Google Scholar] [CrossRef]
- Khademi, F.; Danesh, B.; Delazar, A.; Mohammad Nejad, D.; Ghorbani, M.; Soleimani Rad, J. Effects of Quince Leaf Extract on Biochemical Markers and Coronary Histopathological Changes in Rabbits. ARYA Atheroscler. 2013, 9, 223–231. [Google Scholar]
- Rajadurai, M.; Prince, P.S.M. Comparative Effects of Aegle Marmelos Extract and Alpha-Tocopherol on Serum Lipids, Lipid Peroxides and Cardiac Enzyme Levels in Rats with Isoproterenol-Induced Myocardial Infarction. Singap. Med. J. 2005, 46, 78–81. [Google Scholar]
- Abeliotis, K.; Lasaridi, K.; Costarelli, V.; Chroni, C. The Implications of Food Waste Generation on Climate Change: The Case of Greece. Sustain. Prod. Consum. 2015, 3, 8–14. [Google Scholar] [CrossRef]
- Sethi, G.; Bedregal LP, A.; Cassou, E.; Constantino, L.; Hou, X.; Jain, S.; Kneller, C. Banco Mundial Addressing Food Loss and Waste: A Global Problem with Local Solutions; World Bank: Washington, DC, USA, 2020; p. 128. [Google Scholar]
- Tang, Y.T. Accounting for the Environmental Impact of Food Waste on Water Resources and Climate Change. In Food Industry Wastes Assessment and Recuperation of Commodities; Academic Press: Cambridge, MA, USA, 2020; pp. 305–329. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimization of the Extraction Parameters for the Isolation of Bioactive Compounds from Orange Peel Waste. Sustainability 2022, 14, 13926. [Google Scholar] [CrossRef]
- Lundqvist, J.; Fraiture, C.; Molden, D.; Berndes, G.; Berntell, A.; Falkenmark, M. Saving Water: From Field to Fork: Curbing Losses and Wastage in the Food Chain; Draft for CDS, May 2008. SIWI Paper 13; Stockholm International Water Institute: Stockholm, Sweden, 2008. [Google Scholar]
- Reisch, L.A.; Sunstein, C.R.; Andor, M.A.; Doebbe, F.C.; Meier, J.; Haddaway, N.R. Mitigating Climate Change via Food Consumption and Food Waste: A Systematic Map of Behavioral Interventions. J. Clean. Prod. 2021, 279, 123717. [Google Scholar] [CrossRef]
- Pappas, V.M.; Lakka, A.; Palaiogiannis, D.; Athanasiadis, V.; Bozinou, E.; Ntourtoglou, G.; Makris, D.P.; Dourtoglou, V.G.; Lalas, S.I. Optimization of Pulsed Electric Field as Standalone “Green” Extraction Procedure for the Recovery of High Value-Added Compounds from Fresh Olive Leaves. Antioxidants 2021, 10, 1554. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Pappas, V.M.; Palaiogiannis, D.; Chatzimitakos, T.; Bozinou, E.; Makris, D.P.; Lalas, S.I. Pulsed Electric Field-Based Extraction of Total Polyphenols from Sideritis raiseri Using Hydroethanolic Mixtures. Oxygen 2022, 2, 91–98. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The Effect of Ultrasonication Pretreatment on the Production of Polyphenol-Enriched Extracts from Moringa Oleifera L. (Drumstick Tree) Using a Novel Bio-Based Deep Eutectic Solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef]
- Tzima, K.; Kallithraka, S.; Kotseridis, Y.; Makris, D.P. A Comparative Evaluation of Aqueous Natural Organic Acid Media for the Efficient Recovery of Flavonoids from Red Grape (Vitis vinifera) Pomace. Waste Biomass Valorization 2015, 6, 391–400. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Palaiogiannis, D.; Poulianiti, K.; Bozinou, E.; Lalas, S.I.; Makris, D.P. Extraction of Polyphenolic Antioxidants from Red Grape Pomace and Olive Leaves: Process Optimization Using a Tailor-Made Tertiary Deep Eutectic Solvent. Sustainability 2022, 14, 6864. [Google Scholar] [CrossRef]
- Dani, H.M.; Jagota, S.K. A New Calorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar]
- Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I. Optimized Isolation Procedure for the Extraction of Bioactive Compounds from Spent Coffee Grounds. Appl. Sci. 2023, 13, 2819. [Google Scholar] [CrossRef]
- Wedamulla, N.E.; Fan, M.; Choi, Y.J.; Kim, E.K. Citrus Peel as a Renewable Bioresource: Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Ferreres, F.; Domingues, A.L.; Seabra, R.M.; Ferreira, M.A. Phenolic Profile of Quince Fruit (Cydonia oblonga Miller) (Pulp and Peel). J. Agric. Food Chem. 2002, 50, 4615–4618. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Martins, R.C.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreira, M.A. Quince (Cydonia oblonga Miller) Fruit Characterization Using Principal Component Analysis. J. Agric. Food Chem. 2005, 53, 111–122. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic Acid Improves Glucose Tolerance, Lipid Metabolism, Inflammation and Microbiota Composition in Diabetic Db/Db Mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Jiang, Y.; Kusama, K.; Satoh, K.; Takayama, F.; Watanabe, S.; Sakagami, H. Induction of Cytotoxicity by Chlorogenic Acid in Human Oral Tumor Cell Lines. Phytomedicine 2000, 7, 483–491. [Google Scholar] [CrossRef]
- Noratto, G.; Porter, W.; Byrne, D.; Cisneros-Zevallos, L. Identifying Peach and Plum Polyphenols with Chemopreventive Potential against Estrogen-Independent Breast Cancer Cells. J. Agric. Food Chem. 2009, 57, 5219–5226. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Zhou, C.Y.; Qiu, C.H.; Lu, X.M.; Wang, Y.T. Chlorogenic Acid Induced Apoptosis and Inhibition of Proliferation in Human Acute Promyelocytic Leukemia HL-60 Cells. Mol. Med. Rep. 2013, 8, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering Plants with Increased Levels of the Antioxidant Chlorogenic Acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, H.; Yang, B.; Tao, H. Identification of Cyclodextrin Inclusion Complex of Chlorogenic Acid and Its Antimicrobial Activity. Food Chem. 2010, 120, 1138–1142. [Google Scholar] [CrossRef]
- Luo, H.J.; Wang, J.Z.; Chen, J.F.; Zou, K. Docking Study on Chlorogenic Acid as a Potential H5N1 Influenza A Virus Neuraminidase Inhibitor. Med. Chem. Res. 2011, 20, 554–557. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Xu, Y.; Zhang, B.; Xia, X. Antimicrobial Effect and Mode of Action of Chlorogenic Acid on Staphylococcus aureus. Eur. Food Res. Technol. 2014, 238, 589–596. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of Polyphenols, Antioxidant Capacity and Minerals for the Valorisation of Ancient Apple Cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Commisso, M.; Bianconi, M.; Poletti, S.; Negri, S.; Munari, F.; Ceoldo, S.; Guzzo, F. Metabolomic Profiling and Antioxidant Activity of Fruits Representing Diverse Apple and Pear Cultivars. Biology 2021, 10, 380. [Google Scholar] [CrossRef]
- Magalhães, A.S.; Silva, B.M.; Pereira, J.A.; Andrade, P.B.; Valentão, P.; Carvalho, M. Protective Effect of Quince (Cydonia oblonga Miller) Fruit against Oxidative Hemolysis of Human Erythrocytes. Food Chem. Toxicol. 2009, 47, 1372–1377. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Choi, J.; Miranda, C.L.; Lak, P.; Kioussi, C.; Bobe, G.; Gombart, A.F.; Raber, J.; Maier, C.S.; et al. Targeting the Liver-Brain Axis with Hop-Derived Flavonoids Improves Lipid Metabolism and Cognitive Performance in Mice. Mol. Nutr. Food Res. 2020, 64, 2000341. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus x domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Maihemuti, N.; Nuer, M.; Abudurousuli, K.; Simayi, J.; Talihati, Z.; Han, M.; Hailati, S.; Zhou, W.; Wumaier, A. Analysis of Major Polyphenolic Compounds of Cydonia oblonga Miller (Quince) Fruit Extract by UPLC-MS/MS and Its Effect on Adipogenesis in 3T3-L1 Cells. Separations 2022, 9, 167. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Joshi, V.K.; Rana, J.C. Nutritional Composition and Processed Products of Quince (Cydonia oblonga Mill.). Indian J. Nat. Prod. Resour. 2011, 2, 354–357. [Google Scholar]
- Hanan, E.; Sharma, V.; Ahmad, F.J. Nutritional Composition, Phytochemistry and Medicinal Use of Quince (Cydonia oblonga Miller) with Emphasis on Its Processed and Fortified Food. J. Food Process. Technol. 2020, 11, 1–13. [Google Scholar]
- Rasheed, M.; Hussain, I.; Rafiq, S.; Hayat, I.; Qayyum, A.; Ishaq, S.; Awan, M.S. Chemical Composition and Antioxidant Activity of Quince Fruit Pulp Collected from Different Locations. Int. J. Food Prop. 2018, 21, 2320–2327. [Google Scholar] [CrossRef]
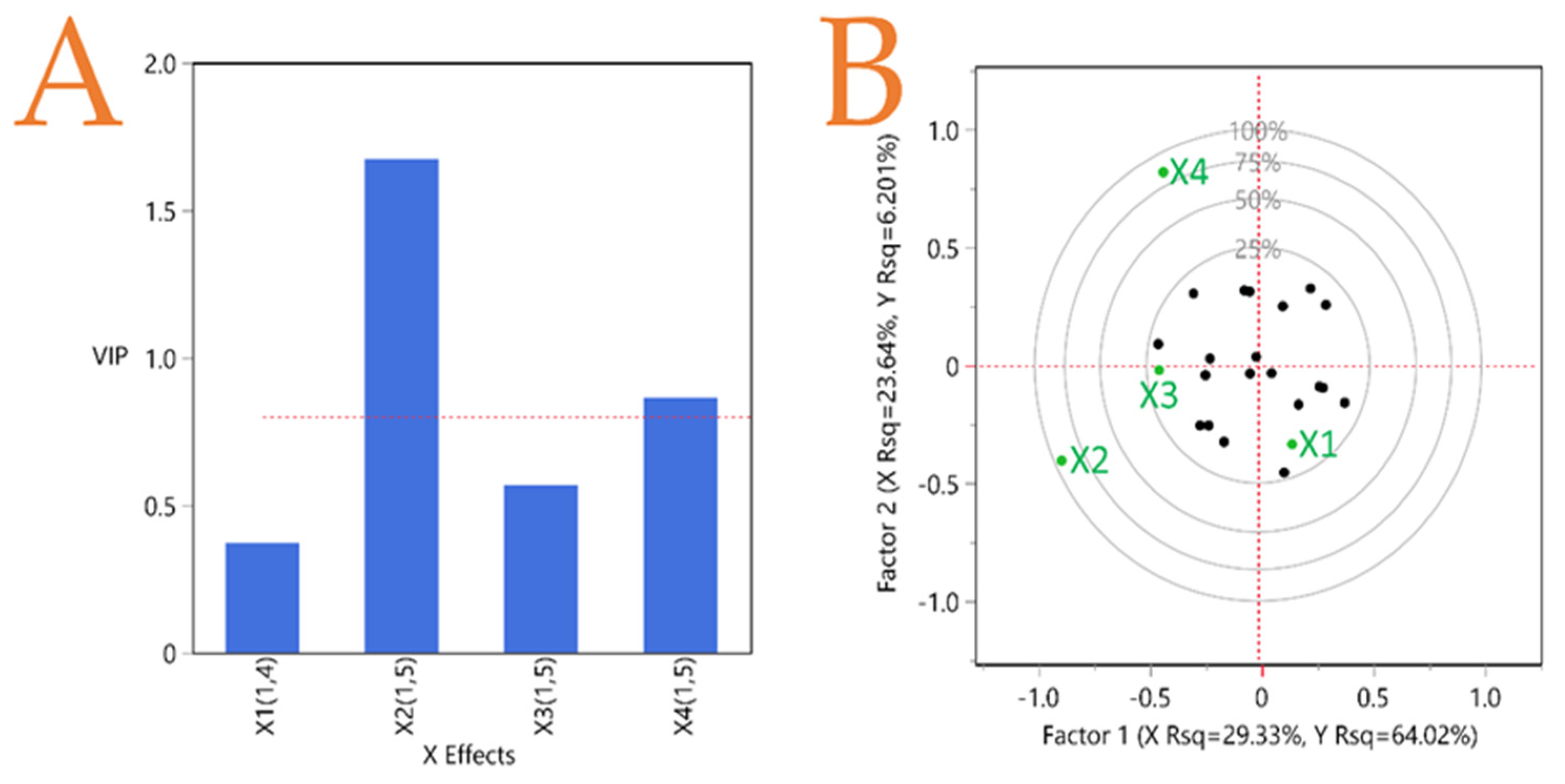
| Independent Variables | Code Units | Coded Variable Level | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Technique | X1 | ST | PEF + ST | US + ST | PEF + US + ST | – |
| C (%, v/v) | X2 | 0 | 25 | 50 | 75 | 100 |
| t (min) | X3 | 30 | 60 | 90 | 120 | 150 |
| T (°C) | X4 | 20 | 35 | 50 | 65 | 80 |
| Design Point | Independent Variables | Responses | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Chlorogenic Acid (mg/g) | TPC (mg GAE/g) | TFC (mg RtE/g) | FRAP (μmol AAE/g) | DPPH (μmol DPPH/g) | Ascorbic Acid (mg/100 g) | |
| 1 | 3 | 1 | 3 | 4 | 1.63 | 6.50 | 2.75 | 85.68 | 82.82 | 214.40 |
| 2 | 3 | 2 | 1 | 3 | 1.75 | 21.20 | 2.69 | 198.24 | 179.82 | 368.46 |
| 3 | 2 | 3 | 4 | 3 | 1.92 | 29.32 | 3.07 | 318.08 | 330.14 | 430.85 |
| 4 | 2 | 4 | 5 | 4 | 2.27 | 35.43 | 3.03 | 509.33 | 616.20 | 527.69 |
| 5 | 3 | 5 | 4 | 2 | 1.61 | 38.39 | 2.96 | 514.19 | 567.87 | 429.83 |
| 6 | 4 | 1 | 4 | 5 | 1.56 | 7.58 | 1.91 | 132.69 | 93.44 | 274.61 |
| 7 | 4 | 2 | 3 | 1 | 0.59 | 10.12 | 1.23 | 68.76 | 55.20 | 158.50 |
| 8 | 1 | 3 | 3 | 2 | 1.62 | 28.28 | 2.54 | 321.97 | 301.73 | 407.73 |
| 9 | 1 | 4 | 4 | 1 | 1.72 | 32.78 | 2.91 | 434.39 | 522.21 | 435.88 |
| 10 | 1 | 5 | 1 | 4 | 1.79 | 29.58 | 2.48 | 517.90 | 599.54 | 434.45 |
| 11 | 1 | 1 | 2 | 3 | 1.46 | 8.05 | 2.10 | 95.44 | 74.14 | 218.30 |
| 12 | 1 | 2 | 5 | 5 | 2.13 | 23.25 | 2.47 | 331.87 | 511.45 | 533.71 |
| 13 | 4 | 3 | 2 | 4 | 1.83 | 26.80 | 2.84 | 311.36 | 299.39 | 418.19 |
| 14 | 3 | 4 | 2 | 5 | 2.08 | 35.49 | 3.05 | 332.19 | 371.41 | 494.74 |
| 15 | 2 | 5 | 3 | 5 | 1.95 | 32.33 | 2.88 | 489.17 | 614.33 | 428.89 |
| 16 | 2 | 1 | 1 | 1 | 1.29 | 3.03 | 1.39 | 41.47 | 10.04 | 114.38 |
| 17 | 2 | 2 | 2 | 2 | 1.49 | 20.38 | 2.34 | 153.52 | 89.24 | 309.33 |
| 18 | 3 | 3 | 5 | 1 | 1.44 | 26.10 | 2.34 | 165.70 | 219.08 | 339.13 |
| 19 | 4 | 4 | 1 | 2 | 1.77 | 29.42 | 2.78 | 469.83 | 537.42 | 440.43 |
| 20 | 4 | 5 | 5 | 3 | 1.69 | 37.37 | 2.17 | 561.97 | 584.35 | 432.84 |
| Design Point | Independent Variables | Neochlorogenic Acid | Cryptochlorogenic Acid | Rutin | Quercetin 3-O-Galactoside | Kaempferol 3-O-β-Rutinoside | |||
|---|---|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | ||||||
| 1 | 3 | 1 | 3 | 4 | 1.35 | 0.19 | 0.71 | 0.11 | 0.07 |
| 2 | 3 | 2 | 1 | 3 | 1.29 | 0.21 | 0.76 | 0.12 | 0.08 |
| 3 | 2 | 3 | 4 | 3 | 1.34 | 0.21 | 0.77 | 0.12 | 0.08 |
| 4 | 2 | 4 | 5 | 4 | 0.95 | 0.23 | 0.81 | 0.13 | 0.08 |
| 5 | 3 | 5 | 4 | 2 | 0.67 | 0.17 | 0.70 | 0.11 | 0.07 |
| 6 | 4 | 1 | 4 | 5 | 1.16 | 0.16 | 0.58 | 0.09 | 0.06 |
| 7 | 4 | 2 | 3 | 1 | 0.54 | 0.12 | 0.43 | 0.05 | 0.06 |
| 8 | 1 | 3 | 3 | 2 | 1.18 | 0.19 | 0.81 | 0.14 | 0.08 |
| 9 | 1 | 4 | 4 | 1 | 0.68 | 0.15 | 0.78 | 0.13 | 0.08 |
| 10 | 1 | 5 | 1 | 4 | 0.70 | 0.15 | 0.61 | 0.11 | 0.07 |
| 11 | 1 | 1 | 2 | 3 | 1.12 | 0.16 | 0.58 | 0.08 | 0.06 |
| 12 | 1 | 2 | 5 | 5 | 1.51 | 0.20 | 0.75 | 0.13 | 0.08 |
| 13 | 4 | 3 | 2 | 4 | 1.32 | 0.21 | 0.69 | 0.11 | 0.07 |
| 14 | 3 | 4 | 2 | 5 | 0.90 | 0.21 | 0.81 | 0.13 | 0.08 |
| 15 | 2 | 5 | 3 | 5 | 0.75 | 0.19 | 0.80 | 0.13 | 0.09 |
| 16 | 2 | 1 | 1 | 1 | 1.09 | 0.16 | 0.61 | 0.09 | 0.06 |
| 17 | 2 | 2 | 2 | 2 | 1.05 | 0.19 | 0.69 | 0.10 | 0.07 |
| 18 | 3 | 3 | 5 | 1 | 1.08 | 0.18 | 0.79 | 0.13 | 0.09 |
| 19 | 4 | 4 | 1 | 2 | 0.83 | 0.19 | 0.61 | 0.10 | 0.07 |
| 20 | 4 | 5 | 5 | 3 | 0.68 | 0.19 | 0.68 | 0.11 | 0.08 |
| Responses | Second-Order Polynomial Equations (Models) | R2 | P | Equation |
|---|---|---|---|---|
| Chlorogenic acid | Y = 1.05 + 0.18X1 − 0.11X2 + 0.06X3 + 0.13X4 − 0.07X12 + 0.02X22 + 0.08X32 − 0.06X42 + 0.04X1X2 − 0.11X1X3 + 0.11X1X4 − 0.05X2X3 + 0.03X2X4 − 0.01X3X4 | 0.9454 | 0.0274 | (2) |
| TPC | Y = −16.06 + 0.39X1 + 28.88X2 − 8.76X3 + 3.27X4 − 1.33X12 − 3.34X22 − 0.21X32 + 0.73X42 + 0.67X1X2 + 1.41X1X3 − 0.38X1X4 + 1.29X2X3 − 2.08X2X4 + 0.47X3X4 | 0.9806 | 0.0024 | (3) |
| TFC | Y = −1.43 + 1.1X1 − 0.17X2 + 1.09X3 + 0.82X4 − 0.18X12 + 0.07X22 + 0.02X32 − 0.14X42 + 0.03X1X2 − 0.23X1X3 + 0.12X1X4 − 0.08X2X3 + 0.01X2X4 − 0.09X3X4 | 0.9892 | 0.0006 | (4) |
| FRAP | Y = 10.74 − 63.09X1 + 91.78X2 − 46.45X3 + 56X4 + 16.93X12 + 7.97X22 + 9.26X32 − 15.93X42 − 3.8X1X2 − 16.79X1X3 + 8.81X1X4 + 0.48X2X3 − 4.83X2X4 + 14.48X3X4 | 0.9861 | 0.0011 | (5) |
| DPPH | Y = −86.13 + 4.77X1 − 27.53X2 + 24.16X3 + 56.82X4 + 22.28X12 + 29.16X22 + 21.88X32 − 13.12X42 − 4.32X1X2 − 48.22X1X3 + 4.15X1X4 − 5.93X2X3 + 3.21X2X4 + 5.65X3X4 | 0.9816 | 0.0021 | (6) |
| Ascorbic acid | Y = −94.72 − 10.51X1 + 342.79X2 − 126.69X3 + 62.06X4 − 7.59X12 − 39.26X22 + 7.36X32 + 7.77X42 + 5.45X1X2 + 6.25X1X3 − 4.15X1X4 + 9.73X2X3 − 26.9X2X4 + 8.53X3X4 | 0.9776 | 0.0034 | (7) |
| Responses | Optimal Conditions | ||||
|---|---|---|---|---|---|
| Maximum Predicted Response | Technique (X1) | C (%, v/v) (X2) | t (min) (X3) | T (°C) (X4) | |
| Chlorogenic acid (mg/g) | 2.30 ± 0.41 | US + ST (3) | 75 (4) | 30 (1) | 65 (4) |
| TPC (mg GAE/g) | 39.36 ± 7.6 | US + ST (3) | 100 (5) | 150 (5) | 35 (2) |
| TFC (mg RtE/g) | 3.49 ± 0.26 | US + ST (3) | 75 (4) | 30 (1) | 65 (4) |
| FRAP (μmol AAE/g) | 602.21 ± 98.42 | US + ST (3) | 100 (5) | 150 (5) | 65 (4) |
| DPPH (μmol DPPH/g) | 708.06 ± 144.04 | PEF + ST (2) | 100 (5) | 120 (4) | 65 (4) |
| Ascorbic acid (mg/100 g) | 533.71 ± 86.12 | ST (1) | 25 (2) | 150 (5) | 80 (5) |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| Chlorogenic acid (mg/g) | 2.33 | 2.12 |
| TPC (mg GAE/g) | 41.75 | 43.99 |
| TFC (mg RtE/g) | 3.26 | 3.86 |
| FRAP (μmol AAE/g) | 610.45 | 627.73 |
| DPPH (μmol DPPH/g) | 745.93 | 699.61 |
| Ascorbic acid (mg/100 g) | 594.79 | 543.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods 2023, 12, 2099. https://doi.org/10.3390/foods12112099
Athanasiadis V, Chatzimitakos T, Bozinou E, Kotsou K, Palaiogiannis D, Lalas SI. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods. 2023; 12(11):2099. https://doi.org/10.3390/foods12112099
Chicago/Turabian StyleAthanasiadis, Vassilis, Theodoros Chatzimitakos, Eleni Bozinou, Konstantina Kotsou, Dimitrios Palaiogiannis, and Stavros I. Lalas. 2023. "Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology" Foods 12, no. 11: 2099. https://doi.org/10.3390/foods12112099
APA StyleAthanasiadis, V., Chatzimitakos, T., Bozinou, E., Kotsou, K., Palaiogiannis, D., & Lalas, S. I. (2023). Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response Surface Methodology. Foods, 12(11), 2099. https://doi.org/10.3390/foods12112099













