Effects of Four Different Dietary Fibre Supplements on Weight Loss and Lipid and Glucose Serum Profiles during Energy Restriction in Patients with Traits of Metabolic Syndrome: A Comparative, Randomized, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Participants
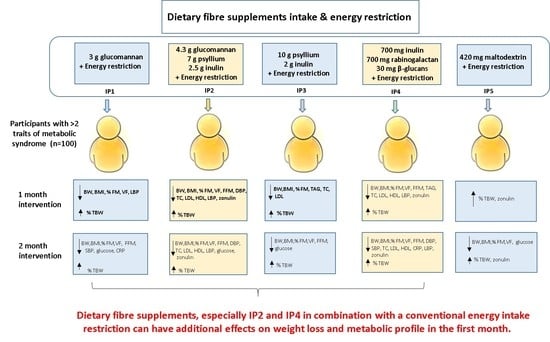
2.3. Intervention
2.4. Anthropometric Measurements
2.5. Biochemical Analysis
2.6. Product Evaluation, Changes in Appetite and Adverse Events
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Participants
3.2. Adherence
3.3. Effects of Fibre Supplementation during Energy-Restricted Diet on Anthropometric Parameters
3.4. Effects of Fibre Supplementation during Energy-Restricted Diet on Metabolic Outcomes
3.5. Effects of Fibre Supplementation during Energy-Restricted Diet on Inflammatory Parameters and Gut Permeability
3.6. Product Evaluation
3.7. Dietary Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Pagotto, U.; Vanuzzo, D.; Vicennati, V.; Pasquali, R. Pharmacological therapy of obesity. G. Ital. Cardiol. 2008, 9, 83S–93S. [Google Scholar]
- Fried, M.; Hainer, V.; Basdevant, A.; Buchwald, H.; Deitel, M.; Finer, N.; Greve, J.W.M.; Horber, F.; Mathus-Vliegen, E.; Scopinaro, N.; et al. Interdisciplinary European guidelines on surgery of severe obesity. Obes. Facts 2008, 1, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Artham, S.M.; Lavie, C.J.; Milani, R.V.; Ventura, H.O. The obesity paradox: Impact of obesity on the prevalence and prognosis of cardiovascular diseases. Postgrad. Med. 2008, 120, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Kushner, R.F.; Ryan, D.H. Assessment and lifestyle management of patients with obesity: Clinical recommendations from systematic reviews. JAMA 2014, 312, 943–952. [Google Scholar] [CrossRef]
- Corella, D.; Qi, L.; Sorlí, J.V.; Godoy, D.; Portolés, O.; Coltell, O.; Greenberg, A.S.; Ordovas, J.M. Obese subjects carrying the 11482G > A polymorphism at the perilipin locus are resistant to weight loss after dietary energy restriction. J. Clin. Endocrinol. Metab. 2005, 90, 5121–5126. [Google Scholar] [CrossRef]
- Phares, D.A.; Halverstadt, A.A.; Shuldiner, A.R.; Ferrell, R.E.; Douglass, L.W.; Ryan, A.S.; Goldberg, A.P.; Hagberg, J.M. Association between body fat response to exercise training and multilocus ADR genotypes. Obes. Res. 2004, 12, 807–815. [Google Scholar] [CrossRef]
- MacLean, P.S.; Wing, R.R.; Davidson, T.; Epstein, L.; Goodpaster, B.; Hall, K.D.; Levin, B.E.; Perri, M.G.; Rolls, B.J.; Rosenbaum, M.; et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity 2015, 23, 7–15. [Google Scholar] [CrossRef]
- Apovian, C.M.; Aronne, L.J.; Bessesen, D.H.; McDonnell, M.E.; Murad, M.H.; Pagotto, U.; Ryan, D.H.; Still, C.D.; Endocrine, S. Pharmacological management of obesity: An endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2015, 100, 342–362. [Google Scholar] [CrossRef]
- Patel, D. Pharmacotherapy for the management of obesity. Metab. Clin. Exp. 2015, 64, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Jovanovski, E.; Mazhar, N.; Komishon, A.; Khayyat, R.; Li, D.; Blanco Mejia, S.; Khan, T.; Jenkins, A.L.; Smircic-Duvnjak, L.; Sievenpiper, J.L.; et al. Can dietary viscous fiber affect body weight independently of an energy-restrictive diet? A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 111, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Malnick, S.D.H.; Knobler, H. The medical complications of obesity. QJM Mon. J. Assoc. Physicians 2006, 99, 565–579. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Mann, J.; Cummings, J.; Winter, N.; Mete, E.; Te Morenga, L. Carbohydrate quality and human health: A series of systematic reviews and meta-analyses. Lancet 2019, 393, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Thompson, S.V.; Hannon, B.A.; An, R.; Holscher, H.D. Effects of isolated soluble fiber supplementation on body weight, glycemia, and insulinemia in adults with overweight and obesity: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2017, 106, 1514–1528. [Google Scholar] [CrossRef]
- Fuglsang-Nielsen, R.; Rakvaag, E.; Langdahl, B.; Knudsen, K.E.B.; Hartmann, B.; Holst, J.J.; Hermansen, K.; Gregersen, S. Effects of whey protein and dietary fiber intake on insulin sensitivity, body composition, energy expenditure, blood pressure, and appetite in subjects with abdominal obesity. Eur. J. Clin. Nutr. 2021, 75, 611–619. [Google Scholar] [CrossRef]
- Lyon, M.R.; Reichert, R.G. The effect of a novel viscous polysaccharide along with lifestyle changes on short-term weight loss and associated risk factors in overweight and obese adults: An observational retrospective clinical program analysis. Altern. Med. Rev. A J. Clin. Ther. 2010, 15, 68–75. [Google Scholar]
- Cairella, M.; Marchini, G. Evaluation of the action of glucomannan on metabolic parameters and on the sensation of satiation in overweight and obese patients. La Clinica Terapeutica 1995, 146, 269–274. [Google Scholar]
- Hess, A.L.; Benítez-Páez, A.; Blædel, T.; Larsen, L.H.; Iglesias, J.R.; Madera, C.; Sanz, Y.; Larsen, T.M.; MyNewGut, C. The effect of inulin and resistant maltodextrin on weight loss during energy restriction: A randomised, placebo-controlled, double-blinded intervention. Eur. J. Nutr. 2020, 59, 2507–2524. [Google Scholar] [CrossRef]
- Qin, Y.-Q.; Wang, L.-Y.; Yang, X.-Y.; Xu, Y.-J.; Fan, G.; Fan, Y.-G.; Ren, J.-N.; An, Q.; Li, X. Inulin: Properties and health benefits. Food Funct. 2023, 14, 2948–2968. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, F. Dietary fructans and serum triacylglycerols: A meta-analysis of randomized controlled trials. J. Nutr. 2007, 137, 2552S–2556S. [Google Scholar] [CrossRef] [PubMed]
- McRorie, J.W.; McKeown, N.M. Understanding the Physics of Functional Fibers in the Gastrointestinal Tract: An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, A.D.; Salter, J.; Dettmar, P.W.; Chaplin, M.F. Dietary fibre, physicochemical properties and their relationship to health. J. R. Soc. Promot. Health 2000, 120, 242–247. [Google Scholar] [CrossRef]
- Yao, M.; Roberts, S.B. Dietary energy density and weight regulation. Nutr. Rev. 2001, 59, 247–258. [Google Scholar] [CrossRef]
- Holt, S.; Brand, J.; Soveny, C.; Hansky, J. Relationship of satiety to postprandial glycaemic, insulin and cholecystokinin responses. Appetite 1992, 18, 129–141. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Davis, P.A.; Schneeman, B.O. Plasma cholecystokinin is associated with subjective measures of satiety in women. Am. J. Clin. Nutr. 2002, 76, 659–667. [Google Scholar] [CrossRef]
- Anderson, J.W.; Randles, K.M.; Kendall, C.W.C.; Jenkins, D.J.A. Carbohydrate and fiber recommendations for individuals with diabetes: A quantitative assessment and meta-analysis of the evidence. J. Am. Coll. Nutr. 2004, 23, 5–17. [Google Scholar] [CrossRef]
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and functional gastrointestinal disorders. Am. J. Gastroenterol. 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Birketvedt, G.S.; Shimshi, M.; Erling, T.; Florholmen, J. Experiences with three different fiber supplements in weight reduction. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2005, 11, PI5–PI8. [Google Scholar]
- Castro-Sánchez, F.H.; Da, O.-A.; Valenzuela-Rubio, N.G.; Domínguez-Rodríguez, M.; Fierros-Valdez, J.A.; Vergara-Jiménez, M.J. Inulin Effect on Weight Loss and Associated Parameters with the Development of Cardiovascular Disease in Obese Dyslipidemic Subjects. Austin J. Nutr. Metab. 2017, 4, 5. [Google Scholar]
- NIJZ. Referenčne Vrednosti za Energijski Vnos ter Vnos Hranil. Tabelarična Priporočila za Otroke (od 1. Leta Starosti Naprej), Mladostnike, Odrasle, Starejše Odrasle, Nosečnice ter Doječe Matere. Dopolnjena Izdaja 2020. Available online: https://nijz.si/wp-content/uploads/2020/04/referencne_vrednosti_2020_3_2.cleaned.pdf (accessed on 18 May 2023).
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary fiber and weight regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.A.; Ludwig, D.S. Dietary fiber and body-weight regulation. Observations and mechanisms. Pediatr. Clin. N. Am. 2001, 48, 969–980. [Google Scholar] [CrossRef] [PubMed]
- Onakpoya, I.; Posadzki, P.; Ernst, E. The efficacy of glucomannan supplementation in overweight and obesity: A systematic review and meta-analysis of randomized clinical trials. J. Am. Coll. Nutr. 2014, 33, 70–78. [Google Scholar] [CrossRef]
- Sood, N.; Baker, W.L.; Coleman, C.I. Effect of glucomannan on plasma lipid and glucose concentrations, body weight, and blood pressure: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2008, 88, 1167–1175. [Google Scholar] [CrossRef]
- Efsa Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to konjac mannan (glucomannan) and reduction of body weight (ID 854, 1556, 3725), reduction of post-prandial glycaemic responses (ID 1559), maintenance of normal blood glucose concentrations (ID 835, 3724), maintenance of normal (fasting) blood concentrations of triglycerides (ID 3217), maintenance of normal blood cholesterol concentrations (ID 3100, 3217), maintenance of normal bowel function (ID 834, 1557, 3901) and decreasing potentially pathogenic gastro-intestinal microorganisms (ID 1558) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1798. [Google Scholar] [CrossRef]
- Darooghegi Mofrad, M.; Mozaffari, H.; Mousavi, S.M.; Sheikhi, A.; Milajerdi, A. The effects of psyllium supplementation on body weight, body mass index and waist circumference in adults: A systematic review and dose-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 859–872. [Google Scholar] [CrossRef]
- Liatis, S.; Tsapogas, P.; Chala, E.; Dimosthenopoulos, C.; Kyriakopoulos, K.; Kapantais, E.; Katsilambros, N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef]
- Reyna, N.Y.; Cano, C.; Bermúdez, V.J.; Medina, M.T.; Souki, A.J.; Ambard, M.; Nuñez, M.; Ferrer, M.A.; Inglett, G.E. Sweeteners and beta-glucans improve metabolic and anthropometrics variables in well controlled type 2 diabetic patients. Am. J. Ther. 2003, 10, 438–443. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Cuda, C.; Luhovyy, B.L.; Anderson, G.H. Beta glucan: Health benefits in obesity and metabolic syndrome. J. Nutr. Metab. 2012, 2012, 851362. [Google Scholar] [CrossRef]
- Marciani, L.; Gowland, P.A.; Spiller, R.C.; Manoj, P.; Moore, R.J.; Young, P.; Fillery-Travis, A.J. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 280, G1227–G1233. [Google Scholar] [CrossRef] [PubMed]
- Hamman, R.F.; Wing, R.R.; Edelstein, S.L.; Lachin, J.M.; Bray, G.A.; Delahanty, L.; Hoskin, M.; Kriska, A.M.; Mayer-Davis, E.J.; Pi-Sunyer, X.; et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care 2006, 29, 2102–2107. [Google Scholar] [CrossRef] [PubMed]
- Cloetens, L.; Ulmius, M.; Johansson-Persson, A.; Akesson, B.; Onning, G. Role of dietary beta-glucans in the prevention of the metabolic syndrome. Nutr. Rev. 2012, 70, 444–458. [Google Scholar] [CrossRef]
- Keenan, J.M.; Pins, J.J.; Frazel, C.; Moran, A.; Turnquist, L. Oat ingestion reduces systolic and diastolic blood pressure in patients with mild or borderline hypertension: A pilot trial. J. Fam. Pract. 2002, 51, 369. [Google Scholar] [PubMed]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T.M.S.; Tosh, S.M.; Gibbs, A.L.; Brand-Miller, J.; Duncan, A.M.; Hart, V.; Lamarche, B.; Thomson, B.A.; Duss, R.; Wood, P.J. Physicochemical properties of oat β-glucan influence its ability to reduce serum LDL cholesterol in humans: A randomized clinical trial. Am. J. Clin. Nutr. 2010, 92, 723–732. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Wilson, P.W.F.; Jacques, P.F. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am. J. Clin. Nutr. 2002, 76, 390–398. [Google Scholar] [CrossRef]
- Pal, S.; Khossousi, A.; Binns, C.; Dhaliwal, S.; Ellis, V. The effect of a fibre supplement compared to a healthy diet on body composition, lipids, glucose, insulin and other metabolic syndrome risk factors in overweight and obese individuals. Br. J. Nutr. 2011, 105, 90–100. [Google Scholar] [CrossRef]
- Brown, L.; Rosner, B.; Willett, W.W.; Sacks, F.M. Cholesterol-lowering effects of dietary fiber: A meta-analysis. Am. J. Clin. Nutr. 1999, 69, 30–42. [Google Scholar] [CrossRef]
- Tiwari, U.; Cummins, E. Meta-analysis of the effect of β-glucan intake on blood cholesterol and glucose levels. Nutrition 2011, 27, 1008–1016. [Google Scholar] [CrossRef]
- Nilsson, L.M.; Abrahamsson, A.; Sahlin, S.; Gustafsson, U.; Angelin, B.; Parini, P.; Einarsson, C. Bile acids and lipoprotein metabolism: Effects of cholestyramine and chenodeoxycholic acid on human hepatic mRNA expression. Biochem. Biophys. Res. Commun. 2007, 357, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Raymond, K. Beta-glucans in the treatment of diabetes and associated cardiovascular risks. Vasc. Health Risk Manag. 2008, 4, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration, HHS. Food labeling: Health claims; soluble dietary fiber from certain foods and coronary heart disease. Interim final rule. Fed. Regist. 2002, 67, 61773–61783. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Merz, C.N.B.; Brewer, H.B.; Clark, L.T.; Hunninghake, D.B.; Pasternak, R.C.; Smith, S.C.; Stone, N.J.; Coordinating Committee of the National Cholesterol Education Program. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J. Am. Coll. Cardiol. 2004, 44, 720–732. [Google Scholar] [CrossRef]
- Ma, Y.; Griffith, J.A.; Chasan-Taber, L.; Olendzki, B.C.; Jackson, E.; Stanek, E.J.; Li, W.; Pagoto, S.L.; Hafner, A.R.; Ockene, I.S. Association between dietary fiber and serum C-reactive protein. Am. J. Clin. Nutr. 2006, 83, 760–766. [Google Scholar] [CrossRef]
- Ajani, U.A.; Ford, E.S.; Mokdad, A.H. Dietary fiber and C-reactive protein: Findings from national health and nutrition examination survey data. J. Nutr. 2004, 134, 1181–1185. [Google Scholar] [CrossRef]
- Jensen, M.K.; Koh-Banerjee, P.; Franz, M.; Sampson, L.; Grønbaek, M.; Rimm, E.B. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation. Am. J. Clin. Nutr. 2006, 83, 275–283. [Google Scholar] [CrossRef]
- Yeh, S.-L.; Wu, T.-C.; Chan, S.-T.; Hong, M.-J.; Chen, H.-L. Fructo-oligosaccharide attenuates the production of pro-inflammatory cytokines and the activation of JNK/Jun pathway in the lungs of d-galactose-treated Balb/cJ mice. Eur. J. Nutr. 2014, 53, 449–456. [Google Scholar] [CrossRef]
- Xie, L.-M.; Ge, Y.-Y.; Huang, X.; Zhang, Y.-Q.; Li, J.-X. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int. J. Clin. Exp. Med. 2015, 8, 1363–1369. [Google Scholar]
- Gonlachanvit, S.; Coleski, R.; Owyang, C.; Hasler, W. Inhibitory actions of a high fibre diet on intestinal gas transit in healthy volunteers. Gut 2004, 53, 1577–1582. [Google Scholar] [CrossRef]
- Yang, J.; Wang, H.-P.; Zhou, L.; Xu, C.-F. Effect of dietary fiber on constipation: A meta analysis. World J. Gastroenterol. 2012, 18, 7378–7383. [Google Scholar] [CrossRef] [PubMed]
- Seljak, B.K.; Valenčič, E.; Hristov, H.; Hribar, M.; Lavriša, Ž.; Kušar, A.; Žmitek, K.; Krušič, S.; Gregorič, M.; Blaznik, U.; et al. Inadequate Intake of Dietary Fibre in Adolescents, Adults, and Elderlies: Results of Slovenian Representative SI. Menu Study. Nutrients 2021, 13, 3826. [Google Scholar] [CrossRef] [PubMed]





| Company Product Name | Form | Administration | Ingredients—In Total |
|---|---|---|---|
| Product BSMS (IP1) | Soft chew | 6 soft chews/day (with water): 2 soft chews 30 min before main meal (breakfast, lunch, dinner). | 3 g glucomannan |
| Product BVCF (IP2) | Powder | 1 bag/day (with water) 30 min before breakfast. | 4.3 g glucomannan 7 g psyllium 2.5 g inulin 1 g apple fibre |
| Set BPRX (IP3) | Powder capsule | 1 bag/day (with water) 30 min before breakfast. 3 capsules/day (with water or tea): one at breakfast, one at lunch, and one at dinner. | 10 g psyllium 2 g inulin 1.5 g apple fibre 500 mg apple pectin seed extracts (pumpkin (300 mg), cumin (150 mg), anise (150 mg)), leaf extracts (artichoke (150 mg), peppermint (150 mg)), root of pellitory (500 mg), garlic extract odourless (300 mg), curcuma rhizome extract (150 mg) |
| Product BLMB (IP4) | Liquid | 2 × 20 mL/day (diluted in water) 30 min before breakfast and 30 min. | 700 mg inulin 700 mg arabinogalactan 30 mg β-glucans |
| Placebo product (PP) | Capsule | 3 capsules/day (with water): one at breakfast, one at lunch, and one at dinner. | 420 mg maltodextrin |
| IP1 | IP2 | IP3 | IP4 | PP | p | |
|---|---|---|---|---|---|---|
| N (F/M) | 20 (16/4) | 20 (17/3) | 20 (17/3) | 20 (16/4) | 20 (17/3) | |
| Age (years) | 48.8 ± 8.6 | 50.1 ± 5.1 | 51.5 ± 6.7 | 46.3 ± 5.3 | 50.7 ± 6.0 | 0.109 |
| Body weight (kg) | 86.9 ± 10.7 | 87.7 ± 16.9 | 82.0 ± 8.8 | 85.9 ± 15.1 | 77.4 ± 8.1 | 0.122 |
| BMI (kg/m2) | 29.1 ± 3.5 | 30.0 ± 4.3 | 29.8 ± 2.9 | 29.4 ± 4.2 | 28.0 ± 2.2 | 0.095 |
| Fat mass (%) | 33.8 ± 8.2 | 37.4 ± 5.7 | 35.4 ± 5.1 | 30.9 ± 7.5 | 30.0 ± 8.1 | 0.080 |
| IP1 Mean ± SD | IP2 Mean ± SD | IP3 Mean ± SD | IP4 Mean ± SD | PP Mean ± SD | p | |
|---|---|---|---|---|---|---|
| N (F/M) | 17 (14/3) | 15 (14/1) | 16 (15/1) | 17 (15/2) | 15 (14/1) | |
| ANTHROPOMETRIC PARAMETERS | ||||||
| Body weight (kg) | ||||||
| Baseline | 87.5 ± 11.4 | 91.8 ± 18.5 | 82.3 ± 9.2 | 84.9 ± 14.8 | 76.9 ± 9.8 | 0.160 |
| After 4 weeks | 85.8 ± 11.5 b | 88.5 ± 17.8 b | 80.2 ± 8.9 b | 81.9 ± 13.7 b | 75.9 ± 8.9 | 0.019 c |
| After 8 weeks | 84.2 ± 11.1 b | 86.7 ± 18.4 b | 78.8 ± 9.0 b | 80.5 ± 13.7 b | 74.4 ± 7.8 b | 0.054 |
| BMI (kg/m2) | ||||||
| Baseline | 29.3 ± 3.7 | 31.7 ± 3.9 | 30.1 ± 2.9 | 29.4 ± 4.2 | 27.2 ± 2.8 | 0.029 a |
| After 4 weeks | 28.7 ± 3.8 b | 30.5 ± 3.7 b | 29.3 ± 2.8 b | 28.4 ± 4.0 b | 25.8 ± 2.4 | 0.012 c |
| After 8 weeks | 28.2 ± 3.6 b | 29.9 ± 3.9 b | 28.8 ± 2.8 b | 27.9 ± 4.0 b | 25.3 ± 2.4 b | 0.044 c |
| Fat mass (%) | ||||||
| Baseline | 33.8 ± 8.2 | 38.5 ± 5.7 | 36.1 ± 5.3 | 31.1 ± 7.7 | 30.6 ± 5.7 | 0.073 |
| After 4 weeks | 32.0 ± 8.1 b | 37.6 ± 6.1 b | 34.8 ± 5.4 b | 30.2 ± 7.8 b | 29.8 ± 8.9 | 0.804 |
| After 8 weeks | 31.8 ± 8.6 b | 36.9 ± 5.6 b | 33.4 ± 5.4 b | 29.0 ± 7.4 b | 29.4 ± 8.5 b | 0.190 |
| Fat-free mass (kg) | ||||||
| Baseline | 58.4 ± 10.2 | 56.0 ± 11.3 | 52.4 ± 5.8 | 57.9 ± 10.2 | 53.3 ± 9.3 | 0.508 |
| After 4 weeks | 58.2 ± 10.1 | 54.8 ± 10.8 b | 52.1 ± 5.5 | 56.9 ± 9.9 b | 53.2 ± 9.1 | 0.032 c |
| After 8 weeks | 57.3 ± 10.2 b | 54.3 ± 11.4 b | 51.7 ± 5.2 b | 56.9 ± 9.7 b | 52.5 ± 8.2 | 0.025 c |
| Visceral fat rating | ||||||
| Baseline | 9.5 ± 3.3 | 9.7 ± 3.3 | 9.1 ± 2.4 | 8.1 ± 2.5 | 7.2 ± 1.8 | 0.232 |
| After 4 weeks | 9.0 ± 3.2 b | 9.1 ± 3.2 b | 8.8 ± 2.2 | 7.6 ± 2.3 b | 7.1 ± 1.8 | 0.209 |
| After 8 weeks | 8.9 ± 3.4 b | 8.8 ± 3.3 b | 8.4 ± 2.4 b | 7.3 ± 2.3 b | 6.5 ± 1.9 b | 0.583 |
| Total body water (%) | ||||||
| Baseline | 47 ± 6 | 43 ± 4 | 45 ± 4 | 49 ± 5 | 49 ± 6 | 0.079 |
| After 4 weeks | 48 ± 6 b | 44 ± 4 b | 46 ± 4 b | 50 ± 5 b | 50 ± 6 b | 0.842 |
| After 8 weeks | 48 ± 6 b | 44 ± 4 b | 46 ± 4 b | 50 ± 5 b | 50 ± 6 b | 0.722 |
| BLOOD PRESSURE | ||||||
| Systolic blood pressure | ||||||
| Baseline | 129 ± 9 | 128 ± 14 | 127 ± 14 | 129 ± 17 | 121 ± 24 | 0.831 |
| After 4 weeks | 125 ± 14 | 124 ± 14 | 126 ± 12 | 120 ± 11 | 126 ± 15 | 0.280 |
| After 8 weeks | 123 ± 11 b | 126 ± 15 | 120 ± 17 | 120 ± 14 b | 121 ± 14 | 0.217 |
| Diastolic blood pressure | ||||||
| Baseline | 81 ± 6 | 83 ± 5 | 81 ± 7 | 83 ± 7 | 82 ± 15 | 0.948 |
| After 4 weeks | 81 ± 10 | 78 ± 6 b | 80 ± 6 | 76 ± 8 | 76 ± 8 | 0.297 |
| After 8 weeks | 80 ± 5 | 78 ± 5 b | 78 ± 8 | 76 ± 7 b | 74 ± 11 | 0.559 |
| IP1 Mean ± SD | IP2 Mean ± SD | IP3 Mean ± SD | IP4 Mean ± SD | PP Mean ± SD | p | |
|---|---|---|---|---|---|---|
| N (F/M) | 17 (14/3) | 15 (14/1) | 16 (15/1) | 17 (15/2) | 15 (14/1) | |
| BIOCHEMICAL PARAMETERS | ||||||
| Triacylglycerols (mmol/L) | ||||||
| Reference range | 0.6–1.7 mmol/L | |||||
| Baseline | 1.06 ± 0.61 | 1.52 ± 0.54 | 1.39 ± 0.34 | 1.39 ± 0.42 | 1.48 ± 0.79 | 0.316 |
| After 4 weeks | 0.97 ± 0.40 | 1.32 ± 0.47 | 1.12 ± 0.29 a | 1.10 ± 0.28 a | 1.29 ± 0.59 | 0.878 |
| After 8 weeks | 1.05 ± 0.53 | 1.58 ± 0.73 | 1.17 ± 0.38 | 1.18 ± 0.49 | 1.21 ± 0.32 | 0.596 |
| Total cholesterol (mmol/L) | ||||||
| Reference range | 4.0–5.2 mmol/L | |||||
| Baseline | 4.93 ± 1.16 | 5.56 ± 0.51 | 5.32 ± 0.46 | 5.74 ± 1.28 | 5.82 ± 1.31 | 0.247 |
| After 4 weeks | 4.59 ± 1.05 | 4.80 ± 0.44 a | 4.86 ± 0.77 a | 5.19 ± 1.26 a | 5.67 ± 0.99 | 0.165 |
| After 8 weeks | 4.77 ± 1.21 | 5.23 ± 0.59 a | 5.01 ± 0.33 | 5.22 ± 1.10 a | 5.35 ± 0.91 | 0.479 |
| LDL cholesterol (mmol/L) | ||||||
| Reference range | 2.0–3.3 mmol/L | |||||
| Baseline | 3.79 ± 1.21 | 4.58 ± 0.60 | 4.14 ± 0.46 | 4.66 ± 1.47 | 4.53 ± 1.55 | 0.337 |
| After 4 weeks | 3.45 ± 1.21 | 3.83 ± 0.59 a | 3.70 ± 0.58 a | 4.13 ± 1.45 a | 4.41 ± 1.17 | 0.157 |
| After 8 weeks | 3.66 ± 1.32 | 4.25 ± 0.68 a | 3.92 ± 0.45 | 4.18 ± 1.25 a | 4.11 ± 1.19 | 0.508 |
| HDL cholesterol (mmol/L) | ||||||
| Reference range | >1.4 mmol/L (M) >1.6 mmol/L (F) | |||||
| Baseline | 1.63 ± 0.45 | 1.49 ± 0.33 | 1.69 ± 0.41 | 1.66 ± 0.32 | 1.79 ± 0.52 | 0.583 |
| After 4 weeks | 1.59 ± 0.40 | 1.36 ± 0.27 a | 1.65 ± 0.41 | 1.59 ± 0.24 a | 1.82 ± 0.52 | 0.229 |
| After 8 weeks | 1.54 ± 0.45 | 1.33 ± 0.27 a | 1.57 ± 0.39 | 1.53 ± 0.26 a | 1.74 ± 0.39 | 0.745 |
| Glucose (mmol/L) | ||||||
| Reference range | 3.3–6.1 mmol/L | |||||
| Baseline | 5.65 ± 0.49 | 5.48 ± 0.49 | 5.76 ± 1.22 | 5.28 ± 0.55 | 5.54 ± 0.46 | 0.499 |
| After 4 weeks | 5.35 ± 0.47 | 5.26 ± 0.47 | 5.50 ± 1.00 | 5.22 ± 0.39 | 5.46 ± 0.63 | 0.821 |
| After 8 weeks | 5.05 ± 0.38 a | 4.99 ± 0.58 a | 5.34 ± 1.02 a | 5.01 ± 0.38 | 5.06 ± 0.49 a | 0.637 |
| IP1 Mean ± SD | IP2 Mean ± SD | IP3 Mean ± SD | IP4 Mean ± SD | PP Mean ± SD | p | |
|---|---|---|---|---|---|---|
| N (F/M) | 17 (14/3) | 15 (14/1) | 16 (15/1) | 17 (15/2) | 15 (14/1) | |
| INFLAMMATORY MARKERS | ||||||
| CRP (mg/L) | ||||||
| Baseline | 1.82 ± 1.31 | 2.25 ± 1.58 | 1.02 ± 0.38 | 1.54 ± 1.60 | 1.45 ± 0.98 | 0.345 |
| After 4 weeks | 1.71 ± 1.70 | 2.39 ± 1.97 | 1.16 ± 1.27 | 1.20 ± 1.03 | 1.40 ± 0.96 | 0.671 |
| After 8 weeks | 1.64 ± 1.35 a | 1.63 ± 1.50 a | 1.09 ± 0.69 | 1.22 ± 1.24 a | 1.68 ± 1.36 | 0.041 b |
| IL-6 (pg/mL) | ||||||
| Baseline | 2.18 ± 1.07 | 4.51 ± 2.88 | 1.59 ± 0.26 | 1.61 ± 0.75 | 2.13 ± 1.45 | 0.293 |
| After 4 weeks | 2.96 ± 2.41 | 2.24 ± 1.16 | 1.49 ± 0.75 | 1.34 ± 0.66 | 1.98 ± 1.15 | 0.082 |
| After 8 weeks | 2.88 ± 1.52 | 2.97 ± 1.49 | 1.88 ± 0.59 | 2.49 ± 1.77 | 3.36 ± 3.77 | 0.395 |
| TNF-α (pg/mL) | ||||||
| Baseline | 0.21 ± 0.06 | 0.23 ± 0.07 | 0.19 ± 0.05 | 0.21 ± 0.04 | 0.23 ± 0.07 | 0.861 |
| After 4 weeks | 0.17 ± 0.02 | 0.21 ± 0.03 | 0.17 ± 0.04 | 0.18 ± 0.04 | 0.22 ± 0.05 | 0.965 |
| After 8 weeks | 0.23 ± 0.07 | 0.19 ± 0.06 | 0.19 ± 0.04 | 0.20 ± 0.07 | 0.26 ± 0.09 | 0.689 |
| GUT PERMEABILITY | ||||||
| LBP (µg/mL) | ||||||
| Baseline | 10.6 ± 1.6 | 11.2 ± 4.1 | 10.2 ± 5.8 | 10.8 ± 1.3 | 11.4 ± 4.2 | 0.990 |
| After 4 weeks | 12.2 ± 2.0 a | 9.5 ± 4.9 a | 6.5 ± 6.0 | 7.5 ± 2.2 a | 9.7 ± 5.1 | 0.419 |
| After 8 weeks | 10.1 ± 2.0 | 12.9 ± 3.9 | 7.9 ± 4.1 | 11.9 ± 5.6 | 10.3 ± 5.0 | 0.501 |
| IP1 | IP2 | IP3 | IP4 | PP | |
|---|---|---|---|---|---|
| Overall likeability | 7.1 | 4.8 | 6.8 | 7.3 | 6.3 |
| Taste acceptability | 3.9 | 2.9 | 3.7 | 3.9 | 4.0 |
| Texture acceptability | 3.9 | 1.8 | 3.1 | 4.3 | 3.8 |
| SIDE EFFECTS | IP1 | IP2 | IP3 | IP4 | PP |
|---|---|---|---|---|---|
| Gastrointestinal discomfort | 15% | 55% | 20% | 27% | - |
| Flatulence | 15% | 55% | 20% | - | - |
| Stool change | - | 15% | - | - | - |
| Abdominal discomfort | 15% | 13% | 17% | 7% | - |
| Bloating | - | - | - | - | - |
| Cramping | - | - | - | - | - |
| Feeling too full | 15% | 13% | 17% | 7% | - |
| Meal replacement | - | - | - | - | - |
| Changed bowel habits | - | 13% | 20% | 27% | - |
| Urgency of bowel movements | - | - | - | - | - |
| Problems with defecation | - | - | 20% | - | - |
| Dehydration | - | - | - | - | - |
| Nausea | - | - | - | - | - |
| Diarrhoea | - | 6% | - | - | - |
| Constipation | - | 13% | - | 27% | - |
| Easier bowel movement | - | - | - | - | - |
| DECREASED APPETITE | 15% | 13% | 17% | 7% | - |
| IP1 Mean ± SD | IP2 Mean ± SD | IP3 Mean ± SD | IP4 Mean ± SD | PP Mean ± SD | p | |
|---|---|---|---|---|---|---|
| Energy intake (kJ/day) | ||||||
| Baseline a | 7734 ± 1651 | 8353 ± 1201 | 7938 ± 1785 | 7707 ± 1683 | 7504 ± 1715 | 0.991 |
| After 8 weeks b | 6155 ± 728 | 6911 ± 2008 | 6148 ± 1176 | 6227 ± 1009 | 6485 ± 1079 | 0.998 |
| CHO intake (% E) | ||||||
| Baseline a | 43 ± 4 | 42 ± 13 | 43 ± 8 | 44 ± 13 | 44 ± 5 | 0.892 |
| After 8 weeks b | 47 ± 4 | 50 ± 10 | 47 ± 6 | 45 ± 8 | 48 ± 6 c | 0.229 |
| Fat intake (% E) | ||||||
| Baseline a | 39 ± 3 | 37 ± 17 | 35 ± 14 | 36 ± 6 | 36 ± 8 | 0.945 |
| After 8 weeks b | 30 ± 7 c | 32 ± 5 | 30 ± 6 | 30 ± 7 | 27 ± 7 c | 0.680 |
| Protein intake (% E) | ||||||
| Baseline a | 18 ± 5 | 19 ± 6 | 18 ± 5 | 19 ± 8 | 17 ± 5 | 0.975 |
| After 8 weeks b | 22 ± 4 c | 21 ± 4 | 23 ± 5 c | 24 ± 6 | 24 ± 6 | 0.688 |
| Fibre intake (% E) | ||||||
| Baseline a | 21 ± 4 | 25 ± 5 | 23 ± 6 | 19 ± 5 | 19 ± 7 | 0.705 |
| After 8 weeks b | 25 ± 6 | 28 ± 7 | 22 ± 6 | 20 ± 6 | 25 ± 5 | 0.922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jenko Pražnikar, Z.; Mohorko, N.; Gmajner, D.; Kenig, S.; Petelin, A. Effects of Four Different Dietary Fibre Supplements on Weight Loss and Lipid and Glucose Serum Profiles during Energy Restriction in Patients with Traits of Metabolic Syndrome: A Comparative, Randomized, Placebo-Controlled Study. Foods 2023, 12, 2122. https://doi.org/10.3390/foods12112122
Jenko Pražnikar Z, Mohorko N, Gmajner D, Kenig S, Petelin A. Effects of Four Different Dietary Fibre Supplements on Weight Loss and Lipid and Glucose Serum Profiles during Energy Restriction in Patients with Traits of Metabolic Syndrome: A Comparative, Randomized, Placebo-Controlled Study. Foods. 2023; 12(11):2122. https://doi.org/10.3390/foods12112122
Chicago/Turabian StyleJenko Pražnikar, Zala, Nina Mohorko, Dejan Gmajner, Saša Kenig, and Ana Petelin. 2023. "Effects of Four Different Dietary Fibre Supplements on Weight Loss and Lipid and Glucose Serum Profiles during Energy Restriction in Patients with Traits of Metabolic Syndrome: A Comparative, Randomized, Placebo-Controlled Study" Foods 12, no. 11: 2122. https://doi.org/10.3390/foods12112122
APA StyleJenko Pražnikar, Z., Mohorko, N., Gmajner, D., Kenig, S., & Petelin, A. (2023). Effects of Four Different Dietary Fibre Supplements on Weight Loss and Lipid and Glucose Serum Profiles during Energy Restriction in Patients with Traits of Metabolic Syndrome: A Comparative, Randomized, Placebo-Controlled Study. Foods, 12(11), 2122. https://doi.org/10.3390/foods12112122