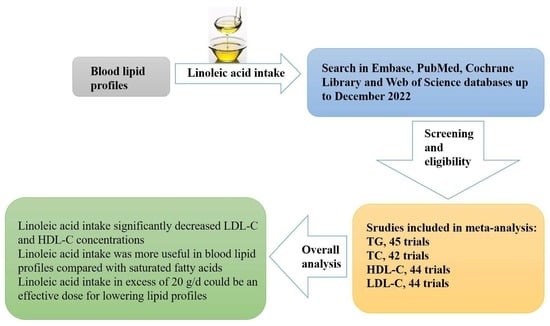
Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Studies
2.2. Eligibility Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Quality Assessment
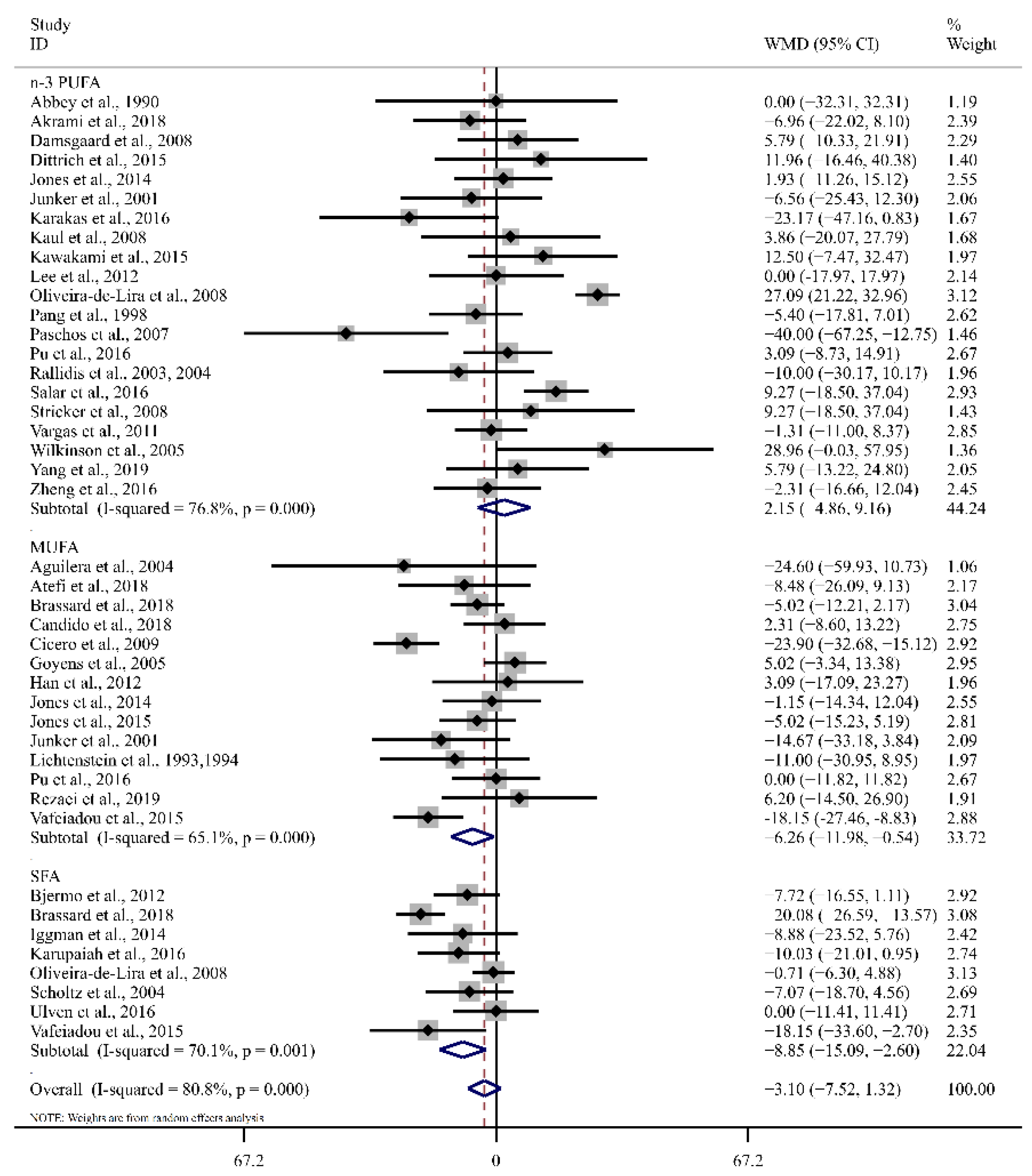
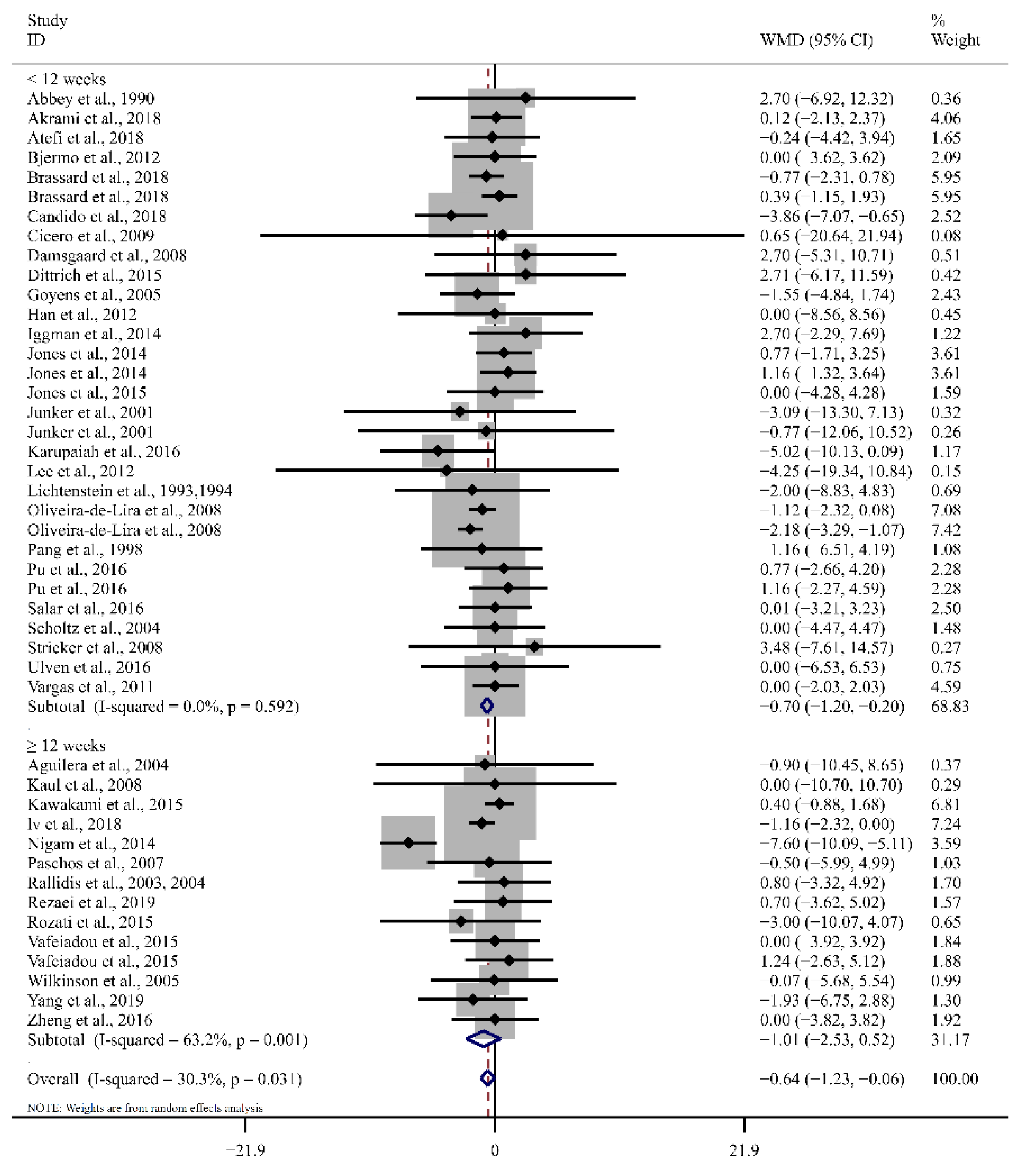
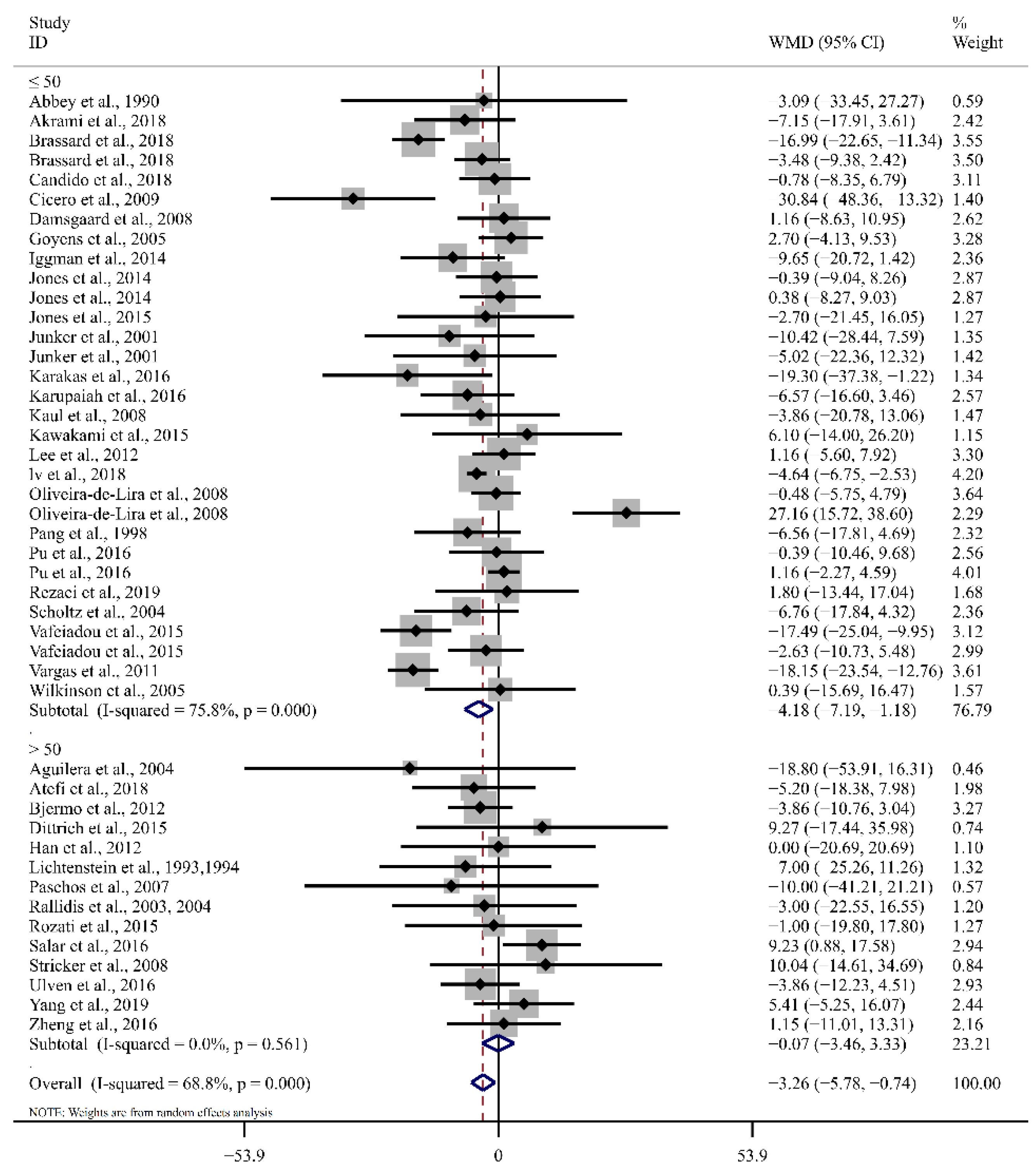
3.4. Effect of LA on Blood Lipid Profiles
3.5. Publication Bias and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benjamin, S.; Spener, F. Conjugated linoleic acids as functional food: An insight into their health benefits. Nutr. Metab. 2009, 6, 36. [Google Scholar] [CrossRef]
- Sahebkar, A. Effects of quercetin supplementation on lipid profile: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2017, 57, 666–676. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Ordovas, J.M.; Garcia-Rios, A.; Delgado-Lista, J.; Delgado-Casado, N.; Cruz-Teno, C.; Camargo, A.; Yubero-Serrano, E.M.; Rodriguez, F.; Perez-Jimenez, F.; et al. Consumption of diets with different type of fat influences triacylglycerols-rich lipoproteins particle number and size during the postprandial state. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21, 39–45. [Google Scholar] [CrossRef]
- Lands, B. Historical perspectives on the impact of n-3 and n-6 nutrients on health. Prog. Lipid Res. 2014, 55, 17–29. [Google Scholar] [CrossRef]
- Russo, G.L. Dietary n–6 and n–3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef]
- Harris, W.S. Linoleic acid and coronary heart disease. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 169–171. [Google Scholar] [CrossRef]
- Summers, L.K.M.; Fielding, B.A.; Bradshaw, H.A.; Ilic, V.; Beysen, C.; Clark, M.L.; Moore, N.R.; Frayn, K.N. Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 2002, 45, 369–377. [Google Scholar] [CrossRef]
- Grimsgaard, S.; Bønaa, K.H.; Jacobsen, B.K.; Bjerve, K.S. Plasma saturated and linoleic fatty acids are independently associated with blood pressure. Hypertension 1999, 34, 478. [Google Scholar] [CrossRef]
- Baylink, D.J.; Finkelman, R.D.; Mohan, S. Growth factors to stimulate bone formation. J. Bone Miner. Res. 1993, 8, S565–S572. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n–3 and n–6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary linoleic acid and risk of coronary heart disease: A systematic review and meta-analysis of prospective cohort studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.R.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Hibbeln, J.R.; Majchrzak, S.F.; Davis, J.M. n-6 fatty acid-specific and mixed polyunsaturate dietary interventions have different effects on CHD risk: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2010, 104, 1586–1600. [Google Scholar] [CrossRef]
- Li, J.; Guasch-Ferré, M.; Li, Y.; Hu, F.B. Dietary intake and biomarkers of linoleic acid and mortality: Systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 112, 150–167. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Jalilpiran, Y.; Karimi, E.; Aune, D.; Larijani, B.; Mozaffarian, D.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Linoleic Acid, Its Concentrations, and the Risk of Type 2 Diabetes: A Systematic Review and Dose-Response Meta-analysis of Prospective Cohort Studies. Diabetes Care 2021, 44, 2173–2181. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; D’Addato, S.; Fiorito, A.; Poli, A.; Gaddi, A.V. Plasma lipid effects of corn oil and extra-virgin olive oil in hypercholesterolaemic subjects: A randomised, controlled trial. Mediterr. J. Nutr. Metab. 2009, 1, 187–192. [Google Scholar] [CrossRef]
- Jones, P.J.; MacKay, D.S.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.; Connelly, P.W.; Lamarche, B.; Couture, P.; Kris-Etherton, P.M.; West, S.G.; et al. High-oleic canola oil consumption enriches LDL particle cholesteryl oleate content and reduces LDL proteoglycan binding in humans. Atherosclerosis 2015, 238, 231–238. [Google Scholar] [CrossRef]
- Oliveira-de-Lira, L.; Santos, E.M.C.; De Souza, R.F.; Matos, R.J.B.; Silva, M.C.d.; Oliveira, L.D.S.; Nascimento, T.G.d.; Schemly, P.A.d.L.S.; Souza, S.L.d. Supplementation-Dependent Effects of Vegetable Oils with Varying Fatty Acid Compositions on Anthropometric and Biochemical Parameters in Obese Women. Nutrients 2018, 10, 932. [Google Scholar] [CrossRef]
- Pang, D.; Allman-Farinelli, M.A.; Wong, T.; Barnes, R.; Kingham, K.M. Replacement of linoleic acid with α-linolenic acid does not alter blood lipids in normolipidaemic men. Br. J. Nutr. 1998, 80, 163–167. [Google Scholar] [CrossRef]
- Han, S.N.; Lichtenstein, A.H.; Ausman, L.M.; Meydani, S.N. Novel soybean oils differing in fatty acid composition alter immune functions of moderately hypercholesterolemic older adults. J. Nutr. 2012, 142, 2182–2187. [Google Scholar] [CrossRef]
- Pu, S.; Rodríguez-Pérez, C.; Ramprasath, V.R.; Segura-Carretero, A.; Jones, P.J. Dietary high oleic canola oil supplemented with docosahexaenoic acid attenuates plasma proprotein convertase subtilisin kexin type 9 (PCSK9) levels in participants with cardiovascular disease risk: A randomized control trial. Vasc. Pharmacol. 2016, 87, 60–65. [Google Scholar] [CrossRef]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 2018, CD011094. [Google Scholar] [CrossRef]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef]
- Hartweg, J.; Farmer, A.J.; Perera, R.; Holman, R.R.; Neil, H.A.W. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia 2007, 50, 1593–1602. [Google Scholar] [CrossRef]
- Pei, J.; Zhao, Y.; Huang, L.; Zhang, X.; Wu, Y. The Effect of n-3 Polyunsaturated Fatty Acids on Plasma Lipids and Lipoproteins in Patients With Chronic Renal Failure—A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2012, 22, 525–532. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3; Cochrane: London, UK, 2022. [Google Scholar]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Damsgaard, C.T.; Frøkiær, H.; Andersen, A.D.; Lauritzen, L. Fish oil in combination with high or low intakes of linoleic acid lowers plasma triacylglycerols but does not affect other cardiovascular risk markers in healthy men. J. Nutr. 2008, 138, 1061–1066. [Google Scholar] [CrossRef]
- Goyens, P.L.L.; Mensink, R.P. The dietary α-linolenic acid to linoleic acid ratio does not affect the serum lipoprotein profile in humans. J. Nutr. 2005, 135, 2799–2804. [Google Scholar] [CrossRef]
- Iggman, D.; Rosqvist, F.; Larsson, A.; Ärnlöv, J.; Beckman, L.; Rudling, M.; Risérus, U. Role of dietary fats in modulating cardiometabolic risk during moderate weight gain: A randomized double-blind overfeeding trial (LIPOGAIN Study). J. Am. Heart Assoc. 2014, 3, e001095. [Google Scholar] [CrossRef]
- Junker, R.; Kratz, M.; Neufeld, M.; Erren, M.; Nofer, J.-R.; Schulte, H.; Nowak-Göttl, U.; Assmann, G.; Wahrburg, U. Effects of Diets Containing Olive Oil, Sunflower Oil, or Rapeseed Oil on the Hemostatic System. Thromb. Haemost. 2001, 85, 280–286. [Google Scholar] [CrossRef]
- Karupaiah, T.; Chuah, K.-A.; Chinna, K.; Matsuoka, R.; Masuda, Y.; Sundram, K.; Sugano, M. Comparing effects of soybean oil- and palm olein-based mayonnaise consumption on the plasma lipid and lipoprotein profiles in human subjects: A double-blind randomized controlled trial with cross-over design. Lipids Health Dis. 2016, 15, 131. [Google Scholar] [CrossRef]
- Kaul, N.; Kreml, R.; Austria, J.A.; Richard, M.N.; Edel, A.L.; Dibrov, E.; Hirono, S.; Zettler, M.E.; Pierce, G.N. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J. Am. Coll. Nutr. 2008, 27, 51–58. [Google Scholar] [CrossRef]
- Kawakami, Y.; Yamanaka-Okumura, H.; Naniwa-Kuroki, Y.; Sakuma, M.; Taketani, Y.; Takeda, E. Flaxseed oil intake reduces serum small dense low-density lipoprotein concentrations in Japanese men: A randomized, double blind, crossover study. Nutr. J. 2015, 14, 39. [Google Scholar] [CrossRef]
- Lichtenstein, A.H.; Ausman, L.M.; Carrasco, W.; Jenner, J.L.; Gualtieri, L.J.; Goldin, B.R.; Ordovas, J.M.; Schaefer, E.J. Effects of canola, corn, and olive oils on fasting and postprandial plasma lipoproteins in humans as part of a National Cholesterol Education Program Step 2 diet. Arterioscler. Thromb. 1993, 13, 1533–1542. [Google Scholar] [CrossRef]
- Lv, C.; Wang, Y.; Zhou, C.; Ma, W.; Yang, Y.; Xiao, R.; Yu, H. Effects of dietary palm olein on the cardiovascular risk factors in healthy young adults. Food Nutr. Res. 2018, 62. [Google Scholar] [CrossRef]
- Ulven, S.M.; Leder, L.; Elind, E.; Ottestad, I.; Christensen, J.J.; Telle-Hansen, V.H.; Skjetne, A.J.; Raael, E.; Sheikh, N.A.; Holck, M.; et al. Exchanging a few commercial, regularly consumed food items with improved fat quality reduces total cholesterol and LDL-cholesterol: A double-blind, randomised controlled trial. Br. J. Nutr. 2016, 116, 1383–1393. [Google Scholar] [CrossRef]
- Wilkinson, P.; Leach, C.; Ah-Sing, E.E.; Hussain, N.; Miller, G.J.; Millward, D.J.; Griffin, B.A. Influence of α-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis 2005, 181, 115–124. [Google Scholar] [CrossRef]
- Abbey, M.; Clifton, P.; Kestin, M.; Belling, B.; Nestel, P. Effect of fish oil on lipoproteins, lecithin:cholesterol acyltransferase, and lipid transfer protein activity in humans. Arteriosclerosis 1990, 10, 85–94. [Google Scholar] [CrossRef]
- Dittrich, M.; Jahreis, G.; Bothor, K.; Drechsel, C.; Kiehntopf, M.; Blüher, M.; Dawczynski, C. Benefits of foods supplemented with vegetable oils rich in α-linolenic, stearidonic or docosahexaenoic acid in hypertriglyceridemic subjects: A double-blind, randomized, controlled trail. Eur. J. Nutr. 2015, 54, 881–893. [Google Scholar] [CrossRef]
- Jones, P.J.; Senanayake, V.K.; Pu, S.; Jenkins, D.J.; Connelly, P.W.; Lamarche, B.; Couture, P.; Charest, A.; Baril-Gravel, L.; West, S.G.; et al. DHA-enriched high–oleic acid canola oil improves lipid profile and lowers predicted cardiovascular disease risk in the canola oil multicenter randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 88–97. [Google Scholar] [CrossRef]
- Lee, S.P.S.; Dart, A.M.; Walker, K.Z.; O’Dea, K.; Chin-Dusting, J.P.F.; Skilton, M.R. Effect of altering dietary n-6:n-3 PUFA ratio on cardiovascular risk measures in patients treated with statins: A pilot study. Br. J. Nutr. 2012, 108, 1280–1285. [Google Scholar] [CrossRef]
- Paschos, G.K.; Zampelas, A.; Panagiotakos, D.B.; Katsiougiannis, S.; Griffin, B.A.; Votteas, V.; Skopouli, F.N. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur. J. Nutr. 2007, 46, 315–320. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Paschos, G.; Papaioannou, M.L.; Liakos, G.K.; Panagiotakos, D.B.; Anastasiadis, G.; Zampelas, A. The effect of diet enriched with α-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis 2004, 174, 127–132. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Paschos, G.; Liakos, G.K.; Velissaridou, A.H.; Anastasiadis, G.; Zampelas, A. Dietary α-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis 2003, 167, 237–242. [Google Scholar] [CrossRef]
- Bjermo, H.; Iggman, D.; Kullberg, J.; Dahlman, I.; Johansson, L.; Persson, L.; Berglund, J.; Pulkki, K.; Basu, S.; Uusitupa, M.; et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1003–1012. [Google Scholar] [CrossRef]
- Brassard, D.; Arsenault, B.J.; Boyer, M.; Bernic, D.; Tessier-Grenier, M.; Talbot, D.; Tremblay, A.; Levy, E.; Asztalos, B.; Jones, P.J.H.; et al. Saturated Fats from Butter but Not from Cheese Increase HDL-Mediated Cholesterol Efflux Capacity from J774 Macrophages in Men and Women with Abdominal Obesity. J. Nutr. 2018, 148, 573–580. [Google Scholar] [CrossRef]
- Candido, F.G.; Valente, F.X.; da Silva, L.E.; Leao Coelho, O.G.; Gouveia Peluzio, M.D.C.; Goncalves Alfenas, R.D.C. Consumption of extra virgin olive oil improves body composition and blood pressure in women with excess body fat: A randomized, double-blinded, placebo-controlled clinical trial. Eur. J. Nutr. 2018, 57, 2445–2455. [Google Scholar] [CrossRef]
- Rozati, M.; Barnett, J.; Wu, D.; Handelman, G.; Dallal, G.; Saltzman, E.; Wilson, T.; Li, L.; Wang, J.; Marcos, A.; et al. Cardiometabolic and immunological impacts of extra virgin olive oil consumption in overweight and obese older adults: A randomized controlled trial. Nutr. Metab. 2015, 29, 28. [Google Scholar] [CrossRef]
- Atefi, M.; Pishdad, G.R.; Faghih, S. Canola oil and olive oil impact on lipid profile and blood pressure in women with type 2 diabetes: A randomized, controlled trial. Prog. Nutr. 2018, 20, 102–109. [Google Scholar] [CrossRef]
- Salar, A.; Faghih, S.; Pishdad, G.R. Rice bran oil and canola oil improve blood lipids compared to sunflower oil in women with type 2 diabetes: A randomized, single-blind, controlled trial. J. Clin. Lipidol. 2016, 10, 299–305. [Google Scholar] [CrossRef]
- Zheng, J.-S.; Lin, M.; Fang, L.; Yu, Y.; Yuan, L.; Jin, Y.; Feng, J.; Wang, L.; Yang, H.; Chen, W.; et al. Effects of n-3 fatty acid supplements on glycemic traits in Chinese type 2 diabetic patients: A double-blind randomized controlled trial. Mol. Nutr. Food Res. 2016, 60, 2176–2184. [Google Scholar] [CrossRef]
- Nigam, P.; Bhatt, S.; Misra, A.; Chadha, D.S.; Vaidya, M.; Dasgupta, J.; Pasha, Q.M. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes Technol. Ther. 2014, 16, 255–261. [Google Scholar] [CrossRef]
- Rezaei, S.; Akhlaghi, M.; Sasani, M.R.; Barati Boldaji, R. Olive oil lessened fatty liver severity independent of cardiometabolic correction in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Nutrition 2019, 57, 154–161. [Google Scholar] [CrossRef]
- Akrami, A.; Nikaein, F.; Babajafari, S.; Faghih, S.; Yarmohammadi, H. Comparison of the effects of flaxseed oil and sunflower seed oil consumption on serum glucose, lipid profile, blood pressure, and lipid peroxidation in patients with metabolic syndrome. J. Clin. Lipidol. 2018, 12, 70–77. [Google Scholar] [CrossRef]
- Karakas, S.E.; Perroud, B.; Kind, T.; Palazoglu, M.; Fiehn, O. Changes in plasma metabolites and glucose homeostasis during omega-3 polyunsaturated fatty acid supplementation in women with polycystic ovary syndrome. BBA Clin. 2016, 5, 179–185. [Google Scholar] [CrossRef]
- Vargas, M.L.; Almario, R.U.; Buchan, W.; Kim, K.; Karakas, S.E. Metabolic and endocrine effects of long-chain versus essential omega-3 polyunsaturated fatty acids in polycystic ovary syndrome. Metab. Clin. Exp. 2011, 60, 1711–1718. [Google Scholar] [CrossRef]
- Yang, B.; Shi, M.-Q.; Li, Z.-H.; Shi, L.; Wang, A.-M.; Guo, X.-J.; Li, D. Effects of n-3 fatty acid supplements on cardiometabolic profiles in hypertensive patients with abdominal obesity in Inner Mongolia: A randomized controlled trial. Food Funct. 2019, 10, 1661–1670. [Google Scholar] [CrossRef]
- Vafeiadou, K.; Weech, M.; Altowaijri, H.; Todd, S.; Yaqoob, P.; Jackson, K.G.; Lovegrove, J.A. Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: Results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am. J. Clin. Nutr. 2015, 102, 40–48. [Google Scholar] [CrossRef]
- Stricker, H.; Duchini, F.; Facchini, M.; Mombelli, G. Canola oil decreases cholesterol and improves endothelial function in patients with peripheral arterial occlusive disease—A pilot study. Artery Res. 2008, 2, 67–73. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Nestares, M.T.; Ros, E.; Gil, A. Sunflower oil does not protect against LDL oxidation as virgin olive oil does in patients with peripheral vascular disease. Clin. Nutr. 2004, 23, 673–681. [Google Scholar] [CrossRef]
- Scholtz, S.C.; Pieters, M.; Oosthuizen, W.; Jerling, J.C.; Bosman, M.J.; Vorster, H.H. The effect of red palm olein and refined palm olein on lipids and haemostatic factors in hyperfibrinogenaemic subjects. Thromb. Res. 2004, 113, 13–25. [Google Scholar] [CrossRef]
- Helland, I.B.; Saugstad, O.D.; Saarem, K.; Van Houwelingen, A.C.; Nylander, G.; Drevon, C.A. Supplementation of n-3 fatty acids during pregnancy and lactation reduces maternal plasma lipid levels and provides DHA to the infants. J. Matern.-Fetal Neonatal Med. 2006, 19, 397–406. [Google Scholar] [CrossRef]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef]
- Su, H.; Liu, R.; Chang, M.; Huang, J.; Wang, X. Dietary linoleic acid intake and blood inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2017, 8, 3091–3103. [Google Scholar] [CrossRef]
- Lesna, I.K.; Suchanek, P.; Kovar, J.; Stavek, P.; Poledne, R. Replacement of dietary saturated FAs by PUFAs in diet and reverse cholesterol transport. J. Lipid Res. 2008, 49, 2414–2418. [Google Scholar] [CrossRef]
- Mensink, R.P.; Katan, M.B. Effect of a diet enriched with monounsaturated or polyunsaturated fatty acids on levels of low-density and high-density lipoprotein cholesterol in healthy women and men. N. Engl. J. Med. 1989, 321, 436–441. [Google Scholar] [CrossRef]
- Trautwein, E.A.; Rieckhoff, D.; Kunath-Rau, A.; Erbersdobler, H.F. Replacing saturated fat with PUFA-rich (sunflower oil) or MUFA-rich (rapeseed, olive and high-oleic sunflower oil) fats resulted in comparable hypocholesterolemic effects in cholesterol-fed hamsters. Ann. Nutr. Metab. 1999, 43, 159–172. [Google Scholar] [CrossRef]
- Kruse, M.; von Loeffelholz, C.; Hoffmann, D.; Pohlmann, A.; Seltmann, A.-C.; Osterhoff, M.; Hornemann, S.; Pivovarova, O.; Rohn, S.; Jahreis, G.; et al. Dietary rapeseed/canola-oil supplementation reduces serum lipids and liver enzymes and alters postprandial inflammatory responses in adipose tissue compared to olive-oil supplementation in obese men. Mol. Nutr. Food Res. 2015, 59, 507–519. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Zare, M.; Faghih, S. Effects of canola oil consumption on lipid profile: A systematic review and meta-analysis of randomized controlled clinical trials. J. Am. Coll. Nutr. 2019, 38, 185–196. [Google Scholar] [CrossRef]
- Ghobadi, S.; Hassanzadeh-Rostami, Z.; Mohammadian, F.; Nikfetrat, A.; Ghasemifard, N.; Raeisi Dehkordi, H.; Faghih, S. Comparison of blood lipid-lowering effects of olive oil and other plant oils: A systematic review and meta-analysis of 27 randomized placebo-controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2110–2124. [Google Scholar] [CrossRef]
- Pedersen, A.; Baumstark, M.W.; Marckmann, P.; Gylling, H.; Sandström, B. An olive oil-rich diet results in higher concentrations of LDL cholesterol and a higher number of LDL subfraction particles than rapeseed oil and sunflower oil diets. J. Lipid Res. 2000, 41, 1901–1911. [Google Scholar] [CrossRef]
- Yue, H.; Qiu, B.; Jia, M.; Liu, W.; Guo, X.-f.; Li, N.; Xu, Z.-x.; Du, F.-l.; Xu, T.; Li, D. Effects of α-linolenic acid intake on blood lipid profiles: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 2894–2910. [Google Scholar] [CrossRef]
- Vijaimohan, K.; Jainu, M.; Sabitha, K.E.; Subramaniyam, S.; Anandhan, C.; Shyamala Devi, C.S. Beneficial effects of alpha linolenic acid rich flaxseed oil on growth performance and hepatic cholesterol metabolism in high fat diet fed rats. Life Sci. 2006, 79, 448–454. [Google Scholar] [CrossRef]
- Asadi, F.; Shahriari, A.; Chahardah-Cheric, M. Effect of long-term optional ingestion of canola oil, grape seed oil, corn oil and yogurt butter on serum, muscle and liver cholesterol status in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2454–2457. [Google Scholar] [CrossRef]
- Gylling, H.; Miettinen, T.A. The effect of plant stanol- and sterol-enriched foods on lipid metabolism, serum lipids and coronary heart disease. Ann. Clin. Biochem. 2005, 42, 254–263. [Google Scholar] [CrossRef]
- Napoli, C.; Abete, P.; Corso, G.; Malorni, A.; Postiglione, A.; Ambrosio, G.; Cacciatore, F.; Rengo, F.; Palumbo, G. Increased low-density lipoprotein peroxidation in elderly men. Coron. Artery Dis. 1997, 8, 129–136. [Google Scholar] [CrossRef]
- Xu, Z.; Hua, N.; Godber, J.S. Antioxidant activity of tocopherols, tocotrienols, and γ-oryzanol components from rice bran against cholesterol oxidation accelerated by 2,2′-azobis(2-methylpropionamidine) dihydrochloride. J. Agric. Food Chem. 2001, 49, 2077–2081. [Google Scholar] [CrossRef]
- Perona, J.S.; Cañizares, J.; Montero, E.; Sánchez-Domínguez, J.M.; Catalá, A.; Ruiz-Gutiérrez, V. Virgin olive oil reduces blood pressure in hypertensive elderly subjects. Clin. Nutr. 2004, 23, 1113–1121. [Google Scholar] [CrossRef]
- Madigan, C.; Ryan, M.; Owens, D.; Collins, P.; Tomkin, G.H. Dietary unsaturated fatty acids in type 2 diabetes: Higher levels of postprandial lipoprotein on a linoleic acid-rich sunflower oil diet compared with an oleic acid-rich olive oil diet. Diabetes Care 2000, 23, 1472–1477. [Google Scholar] [CrossRef]
- Jantti, J.; Nikkari, T.; Solakivi, T.; Vapaatalo, H.; Isomaki, H. Evening primrose oil in rheumatoid arthritis: Changes in serum lipids and fatty acids. Ann. Rheum. Dis. 1989, 48, 124–127. [Google Scholar] [CrossRef]
- Mustad, V.A.; Ellsworth, J.L.; Cooper, A.D.; Kris-Etherton, P.M.; Etherton, T.D. Dietary linoleic acid increases and palmitic acid decreases hepatic LDL receptor protein and mRNA abundance in young pigs. J. Lipid Res. 1996, 37, 2310–2323. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Drouin-Chartier, J.-P.; Tremblay, A.J.; Lepine, M.-C.; Lemelin, V.; Lamarche, B.; Couture, P. Substitution of dietary ω-6 polyunsaturated fatty acids for saturated fatty acids decreases LDL apolipoprotein B-100 production rate in men with dyslipidemia associated with insulin resistance: A randomized controlled trial. Am. J. Clin. Nutr. 2018, 107, 26–34. [Google Scholar] [CrossRef]

| Reference | Country | Subjects Information | Age | BMI | Smoking | No. | M/F | Duration | Design | LA Dose (Source) |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbey et al. (1990) [39] | Australia | Hypercholesterolaemia | 47.4 | 26.1 | NR | 22 | 22/0 | 6 weeks | P | I: 14.3 g (Safflower oi) C: 4.3 g (Linseed oil) |
| Aguilera et al. (2004) [61] | Spain | Peripheral vascular disease | 65.1 | 26.7 | Mixed | 20 | 20/0 | 16 weeks | P | I: 4.23 g (Sunflower oil) C: 0.52 g (Olive oil) |
| Akrami et al. (2018) [55] | Iran | Metabolic syndrome | 48.6 | NR | Non-smoker | 52 | 33/19 | 7 weeks | P | I: 14.5 g (Sunflower oil) C: 4.03 g (Flaxseed oil) |
| Atefi et al. (2018) [50] | Iran | Type 2 diabetes mellitus | 58 | 28.5 | Non-smoker | 81 | 0/81 | 8 weeks | P | I: 3 g (Sunflower oil) C: 0 g (Olive oil) |
| Bjermo et al. (2012) [46] | Sweden | Abdominal obesity | 56.5 | 30.8 | NR | 61 | NR | 10 weeks | P | I: 36.5 g (Sunflower oil) C: 0 g (Butter) |
| Brassard et al. (2018) [47] | Canada | Abdominal obesity | 41 | 29.9 | Non-smoker | 46 | 21/25 | 4 weeks | CO | I: NR (Corn oil) C: NR (Butter) C: NR (Olive oil) |
| Candido et al. (2018) [48] | Brazil | Overweight or obese | 27 | 30.1 | Non-smoker | 41 | 0/41 | 9 weeks | P | I: 25 mL (Soybean oil) C: 25 mL (Olive oil) |
| Cicero et al. (2009) [16] | Italy | Moderate hypercholesterolaemia | 50 | 26.2 | NR | 22 | 11/11 | 45 days | P | I: 23.33 g (Corn oil) C: 9.05 g (Olive oil) |
| Damsgaard et al. (2008) [28] | Denmark | Healthy | 25 | 23.3 | Mixed | 33 | 33/0 | 8 weeks | P | I: 19.3 g (Sunflower oil) C: 12.3 g (Rapeseed oil) |
| Dittrich et al. (2015) [40] | Germany | Moderate hypertriacylglyceridemia | 56 | 28.2 | NR | 49 | 17/32 | 10 weeks | CO | I: 10 g (Sunflower oil) C: 3.1 g (Linseed oil) |
| Goyens et al. (2005) [29] | Netherlands | Healthy | 49.6 | 24.1 | Mixed | 36 | 14/22 | 6 weeks | P | I: 3.15 g (Sunflower oil, olive oil and rapeseed oil) C: 1.1 g (Olive and rapeseed oil) |
| Han et al. (2012) [20] | Korea | Moderately hypercholesterolemia | 63 | 26.7 | Non-smoker | 18 | 7/11 | 35 days | CO | I: 33.36 g (Soybean oil) C: 5.1 g (high oleic acid soybean oil) |
| Iggman et al. (2014) [30] | Sweden | Healthy | 26.9 | 20.2 | NR | 39 | 12/27 | 7 weeks | P | I: 35.36 g (Sunflower oil) C: 4.9 g (Palm oil) |
| Jones et al. (2014) [41] | Canada | Abdominal obesity (some with hyperlipidemia) | 46.5 | 29.8 | Non-smoker | 130 | 60/70 | 4 weeks | CO | I: 41.58 g (Corn and safflower oil) C: 8.82 g (High oleic canola oil) C: 22.5 g (Flaxseed oil) |
| Jones et al. (2015) [17] | Canada | Abdominal obesity (some with hyperlipidemia) | 45.8 | 30.4 | NR | 50 | 26/24 | 4 weeks | CO | I: 41.58 g (Corn and safflower oil) C: 8.82 g (High oleic canola oil) |
| Junker et al. (2001) [31] | Germany | Healthy | 26 | 23 | Non-smoker | 58 | 31/27 | 4 weeks | P | I: 50.75 g (Sunflower oil) C: 7.76 g (Olive oil) C: 18.35 g (Rapeseed oil) |
| Karakas et al. (2016) [56] | USA | Polycystic ovary syndrome | 29.2 | 34.1 | Non-smoker | 34 | 0/34 | 6 weeks | P | I: 2.57 g (Soybean oil) C: 0.97 g (Flaxseed oil) |
| Karupaiah et al. (2016) [32] | Malaysia | Healthy | 23.4 | 25.1 | NR | 34 | 16/18 | 4 weeks | CO | I: 7.97 g (Soybean oil) C: 1.85 g (Palm oil) |
| Kaul et al. (2008) [33] | Canada | Healthy | 34 | 24.3 | Non-smoker | 44 | 17/27 | 12 weeks | P | I: 1.36 g (Sunflower oil) C: 0.28 g (Flaxseed oil) |
| Kawakami et al. (2015) [34] | Japan | Healthy | 44.5 | 25.1 | Mixed | 15 | 15/0 | 12 weeks | CO | I: 5.45 g (Corn oil) C: 1.62 g (Flaxseed oil) |
| Lee et al. (2012) [42] | Australia | Hypercholesterolaemia | 47 | 24.9 | Non-smoker | 11 | 6/5 | 4 weeks | CO | I: 16.53 g (Safflower oil) C: 9.3 g (Canola oil) |
| Lichtenstein et al. (1993) [35] | USA | Healthy | 61 | 27.4 | Non-smoker | 15 | 7/8 | 32 days | CO | I: 36.14 g (Corn oil) C: 6.7 g (Olive oil) |
| Lv et al. (1993) [36] | China | Healthy | 21.6 | 21 | Non-smoker | 108 | 50/58 | 16 weeks | P | I: 10.34 g (Soybean oil) C: 2.9 g (Palm oil) |
| Nigam et al. (2014) [53] | India | Nonalcoholic fatty liver disease | 36.7 | 27.3 | Mixed | 60 | 60/0 | 24 weeks | P | I: 20 mL (Corn oil) C: 20 mL (Olive oil) |
| Oliveira-de-Lira et al. (2018) [18] | Brazil | Abdominal adiposity | 34.1 | 33.9 | Non-smoker | 75 | 0/75 | 8 weeks | P | I: 4.87 g (Safflower oil) C: 0.13 g (Coconut oil) |
| C: 0.6 g (Chia oil) | ||||||||||
| Pang et al. (1998) [19] | Australia | Healthy | 24.5 | 22.4 | NR | 29 | 29/0 | 6 weeks | P | I: 22.65 g (Safflower oil) C: 13.18 g (Linseed oil) |
| Paschos et al. (2007) [43] | Greece | Nondiabetic dyslipidemia | 52 | 28 | Non-smoker | 35 | 35/0 | 12 weeks | P | I: 11.2 g (Safflower oil) C: 2.07 g (Flaxseed oil) |
| Pu et al. (2016) [21] | Canada | Metabolic syndrome | 45.6 | 29.6 | NR | 84 | 35/49 | 30 days | CO | I: 41.4 g (Corn and safflower oil) C: 9 g (High oleic canola oil) C: 22.9 g (Flaxseed oil) |
| Rallidis et al. (2003, 2004) [44,45] | Greece | Dyslipidaemia | 51 | 28.4 | Mixed | 76 | 76/0 | 12 weeks | P | I: 11.2 g (Safflower oil) C: 2 g (Linseed oil) |
| Rezaei et al. (2019) [54] | Iran | Non-alcoholic fatty liver disease | 43.6 | 30.1 | Mixed | 66 | 29/37 | 12 weeks | P | I: 12.64 g (Sunflower oil) C: 3.08 g (Olive oil) |
| Rozati et al. (2015) [49] | USA | Overweight or obese | 72 | 29 | Non-smoker | 41 | 14/27 | 12 weeks | P | I: 46.61 g (10% corn oil and 90% soybean oil) C: 8.6 g (Olive oil) |
| Salar et al. (2006) [51] | Iran | Type 2 diabetes mellitus | 52.1 | 30.2 | Non-smoker | 99 | 58/41 | 8 weeks | P | I: 17.4 g (Sunflower oil) C: 6.39 g (Canola oil) |
| Scholtz et al. (2004) [62] | South Africa | Hyperfibrinogenaemia | 48.1 | 28.7 | Mixed | 56 | 36/20 | 4 weeks | P | I: 16 g (Sunflower oil) C: 3.2 g (Red palm oil) |
| Stricker et al. (2008) [60] | Switzerland | Chronic peripheral artery occlusive disease | 65 | NR | Mixed | 40 | 27/13 | 8 weeks | P | I: 16.24 g (Sunflower oil) C: 4.5 g (Canola oil) |
| Ulven et al. (2016) [37] | Norway | Healthy | 54.4 | 25 | Mixed | 99 | 58/41 | 8 weeks | P | I: 12.9 g (Sunflower oil) C: 4.1 g (Butter) |
| Vafeiadou et al. (2015) [59] | UK | Cardiovascular disease | 44 | 26.7 | Non-smoker | 195 | 85/110 | 16 weeks | P | I: 22.2 g (Safflower oil) C: 7.3 g (Butter) C: 10.1 g (Olive and rapeseed oil) |
| Vargas et al. (2011) [57] | USA | Polycystic ovary syndrome | 29.2 | 34.1 | Non-smoker | 34 | 0/34 | 6 weeks | P | I: 2.57 g (Soybean oil) C: 10.1 g (Flaxseed oil) |
| Wilkinson et al. (2005) [38] | UK | Healthy | 49 | 28.3 | Non-smoker | 38 | NR | 12 weeks | P | I: 28.35 g (Sunflower oil) C: NR (Flaxseed oil) |
| Yang et al. (2019) [58] | China | Hypertension | 57.5 | 26.8 | NR | 73 | 27/46 | 12 weeks | P | I: 2.14 g (Corn oil) C: 0 g (Flaxseed oil) |
| Zheng et al. (2016) [52] | China | Type 2 diabetes mellitus | 59.4 | 25.1 | NR | 108 | 35/73 | 180 days | P | I: 2.14 g (Corn oil) C: 0.62 g (Flaxseed oil) |
| TG | TC | |||||||
|---|---|---|---|---|---|---|---|---|
| Subgroup | N | WMD (95% CI) | p | I2 % | N | WMD (95% CI) | p | I2 % |
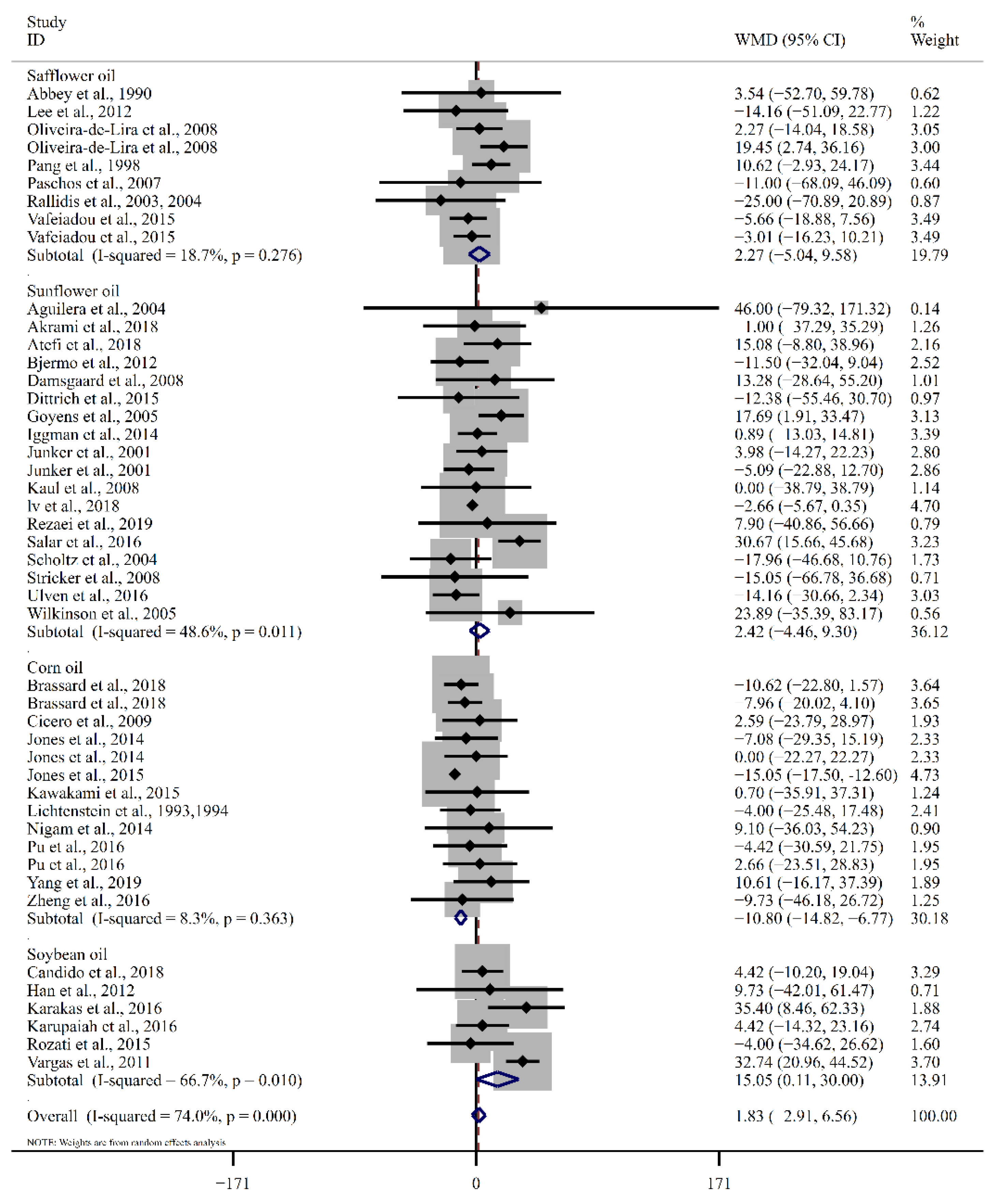
| Overall | 46 | 1.83 (−2.91, 6.57) | 0.45 | 74.0 | 43 | −3.10 (−7.52, 1.32) | 0.17 | 80.8 |
| Age | ||||||||
| ≤50 | 32 | 2.14 (−3.14, 7.43) | 0.43 | 78.2 | 30 | −3.13 (−8.50, 2.24) | 0.25 | 84.5 |
| >50 | 14 | 0.24 (−10.77, 11.25) | 0.97 | 47.2 | 13 | −2.57 (−10.09, 4.95) | 0.50 | 61.0 |
| Duration | ||||||||
| <12 weeks | 32 | 2.74 (−3.94, 9.42) | 0.42 | 80.5 | 32 | −2.53 (−7.49, 2.44) | 0.32 | 83.2 |
| ≥12 weeks | 14 | −2.63 (−5.42, 0.17) | 0.07 | 0.0 | 11 | −5.22 (−14.89, 4.45) | 0.29 | 64.4 |
| Intervention groups | ||||||||
| Safflower oil | 9 | 2.27 (−5.05, 9.58) | 0.54 | 18.7 | 9 | −6.13 (−19.98, 7.71) | 0.39 | 92.5 |
| Sunflower oil | 18 | 2.42 (−4.46, 9.30) | 0.49 | 48.6 | 17 | −0.17 (−5.52, 5.18) | 0.95 | 50.6 |
| Corn oil | 13 | −10.80 (−14.82, −6.77) | <0.01 | 8.3 | 12 | −5.03 (−11.51, 1.45) | 0.13 | 73.7 |
| Soybean oil | 6 | 15.05 (0.11, 30.00) | 0.05 | 66.7 | 5 | −3.84 (−10.86, 3.19) | 0.28 | 29.0 |
| Main fatty acid in comparison groups | ||||||||
| n-3 PUFA | 21 | 9.42 (1.40, 17.44) | 0.02 | 44.7 | 21 | 2.15 (−4.86, 9.16) | 0.55 | 76.8 |
| MUFA | 16 | −0.38 (−7.86, 7.09) | 0.92 | 62.4 | 14 | −6.26 (−11.98, −0.54) | 0.03 | 65.1 |
| SFA | 9 | −3.33 (−5.99, −0.68) | 0.01 | 0.0 | 8 | −8.85 (−15.09, −2.61) | <0.01 | 70.1 |
| Health status | ||||||||
| Normolipemic | 14 | −0.11 (−4.14, 3.93) | 0.96 | 9.0 | 13 | −1.51 (−6.44, 3.41) | 0.55 | 26.4 |
| Hyperlipidemia | 6 | −3.9 (−20.93, 13.13) | 0.65 | 0.0 | 6 | −11.25 (−25.59, 3.09) | 0.12 | 65.4 |
| Other disease | 26 | 2.78 (−4.94, 10.50) | 0.48 | 81.6 | 24 | −2.61 (−8.63, 3.42) | 0.40 | 86.3 |
| BMI | ||||||||
| <30 kg/m2 | 32 | −2.36 (−4.71, −0.02) | 0.05 | 0.0 | 30 | −5.88 (−10.04, −1.73) | <0.01 | 61.8 |
| ≥30 kg/m2 | 12 | 10.98 (−3.95, 25.91) | 0.15 | 91.5 | 11 | 2.52 (−6.45, 11.49) | 0.58 | 88.3 |
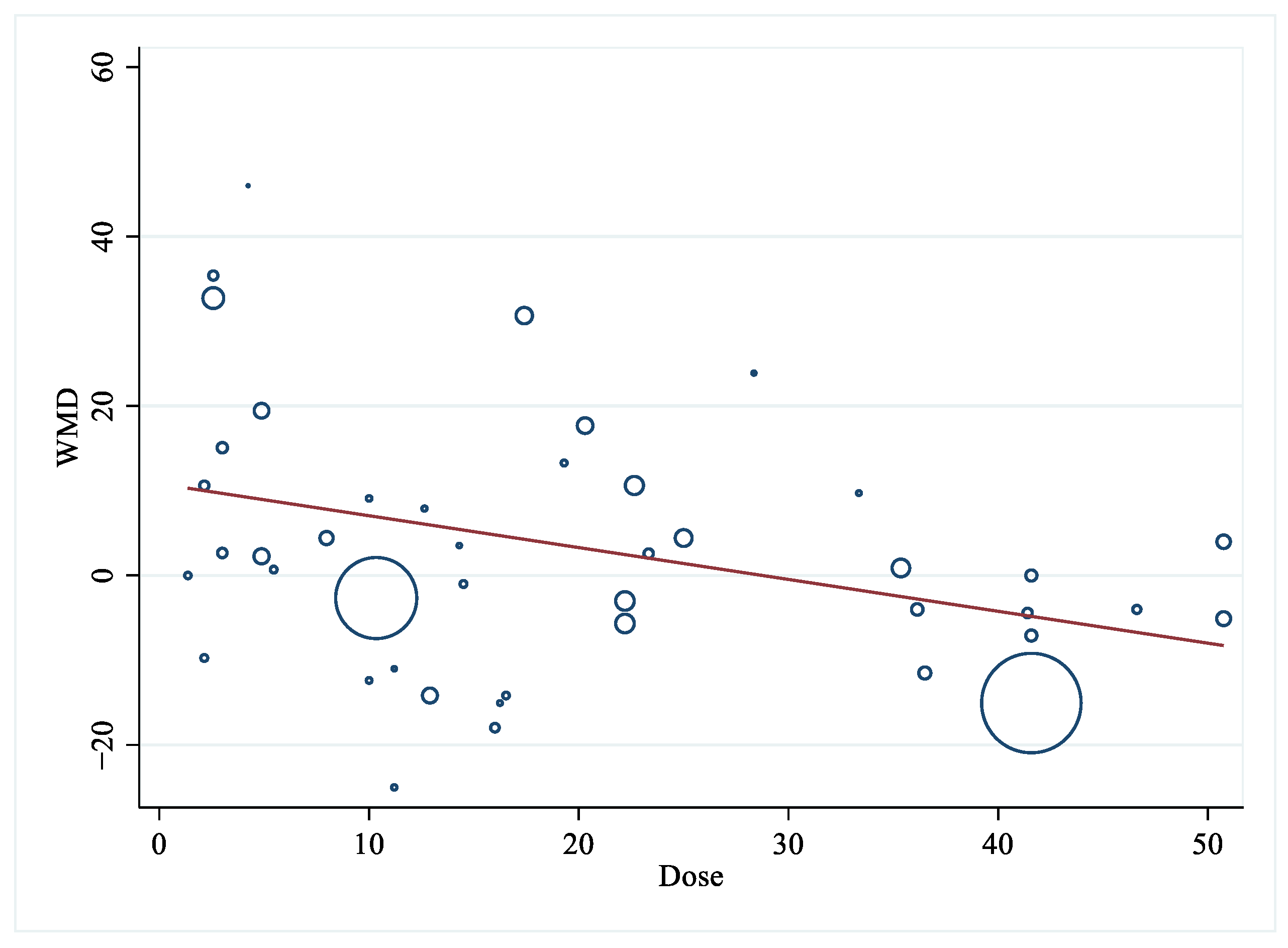
| Dose difference | ||||||||
| 0–10 g/d | 15 | 9.08 (−0.54, 18.69) | 0.06 | 55.4 | 14 | 0.81 (−7.59, 9.22) | 0.85 | 83.7 |
| 10–20 g/d | 12 | 3.20 (−8.44, 14.84) | 0.59 | 60.1 | 11 | −0.01 (−7.60, 7.58) | 0.99 | 60.1 |
| >20 g/d | 17 | −2.61 (−8.61, 3.40) | 0.40 | 56.3 | 16 | −6.96 (−12.07, −1.86) | <0.01 | 58.0 |
| Overall | 45 | −0.64 (−1.23, −0.06) | 0.03 | 30.3 | 45 | −3.26 (−5.78, −0.74) | 0.01 | 68.8 |
| Age | ||||||||
| ≤50 | 31 | −0.70 (−1.43, 0.04) | <0.01 | 49.9 | 30 | −4.19 (−7.19, −1.18) | <0.01 | 75.8 |
| >50 | 14 | −0.21 (−1.57, 1.14) | 0.99 | 0.0 | 14 | −0.07 (−3.46, 3.33) | 0.97 | 0.0 |
| Duration | ||||||||
| <12 weeks | 31 | −0.70 (−1.20, −0.20) | <0.01 | 0.0 | 32 | −3.27 (−6.55, 0.01) | 0.05 | 74.3 |
| ≥12 weeks | 14 | −1.01 (−2.53, 0.52) | 0.20 | 63.2 | 13 | −3.83 (−7.82, 0.17) | 0.06 | 36.0 |
| Intervention groups | ||||||||
| Safflower oil | 9 | −1.39 (−2.14, −0.64) | <0.01 | 0.0 | 9 | −1.11 (−9.12, 6.90) | 0.79 | 81.5 |
| Sunflower oil | 18 | −0.52 (−1.32, 0.29) | 0.21 | 0.0 | 18 | −2.56 (−5.20, −0.09) | 0.04 | 15.8 |
| Corn oil | 13 | −0.55 (−1.93, 0.84) | 0.44 | 69.2 | 12 | −3.55 (−8.75, 1.65) | 0.18 | 74.9 |
| Soybean oil | 5 | −2.14 (−4.49, −0.22) | 0.08 | 36.5 | 6 | −8.32 (−16.78, 0.15) | 0.05 | 71.4 |
| Main fatty acid in comparison groups | ||||||||
| n-3 PUFA | 20 | −0.48 (−1.10, 0.15) | 0.14 | 0.0 | 21 | −0.10 (−4.95, 4.76) | 0.97 | 75.1 |
| MUFA | 16 | −1.18 (−2.82, 0.47) | 0.16 | 61.5 | 15 | −2.41 (−5.38, 0.53) | 0.11 | 8.6 |
| SFA | 9 | −0.95 (−1.63, −0.27) | <0.01 | 0.0 | 9 | −7.65 (−11.79, −3.52) | <0.01 | 73.6 |
| Health status | ||||||||
| Normolipemic | 14 | −0.56 (−1.34, 0.21) | 0.15 | 0.0 | 14 | −3.74 (−5.46, −2.02) | <0.01 | 0.0 |
| Hyperlipidemia | 6 | 0.73 (−2.03, 3.48) | 0.61 | 0.0 | 6 | −7.71 (−20.51, 5.08) | 0.24 | 44.5 |
| BMI | ||||||||
| <30 kg/m2 | 32 | −0.30 (−0.89, 0.30) | 0.33 | 0.0 | 32 | −4.11 (−6.60, −1.61) | <0.01 | 54.5 |
| ≥30 kg/m2 | 11 | −1.39 (−2.74, −0.04) | 0.04 | 75.2 | 11 | −0.56 (−8.12, 7.01) | 0.89 | 85.9 |
| Dose difference | ||||||||
| 0–10 g/d | 14 | −1.37 (−2.72, −0.02) | 0.05 | 68.4 | 14 | −1.45 (−7.51, 4.6) | 0.64 | 81.9 |
| 10–20 g/d | 12 | −0.61 (−1.47, 0.25) | 0.16 | 0.0 | 12 | −0.58 (−4.11, 2.96) | 0.75 | 39.0 |
| >20 g/d | 17 | 0.02 (−1.01, 1.04) | 0.98 | 0.0 | 17 | −5.25 (−8.79, −1.72) | <0.01 | 38.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, H.; Jin, Q.; Wang, X. Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials. Foods 2023, 12, 2129. https://doi.org/10.3390/foods12112129
Wang Q, Zhang H, Jin Q, Wang X. Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials. Foods. 2023; 12(11):2129. https://doi.org/10.3390/foods12112129
Chicago/Turabian StyleWang, Qiong, Hui Zhang, Qingzhe Jin, and Xingguo Wang. 2023. "Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials" Foods 12, no. 11: 2129. https://doi.org/10.3390/foods12112129
APA StyleWang, Q., Zhang, H., Jin, Q., & Wang, X. (2023). Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials. Foods, 12(11), 2129. https://doi.org/10.3390/foods12112129