Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Lingonberry Sample Material
2.1.1. Harvest of Wild Berry Samples
2.1.2. Chemicals
2.1.3. Methanolic Extraction of Lingonberries
2.2. Analysis of Sugars and Organic Acids
2.3. Analysis of Anthocyanins, Flavonols, Cinnamic Acid Derivatives and Procyanidins
2.4. Analysis of Volatile Organic Compounds
2.5. Statistical Analysis
3. Results and Discussion
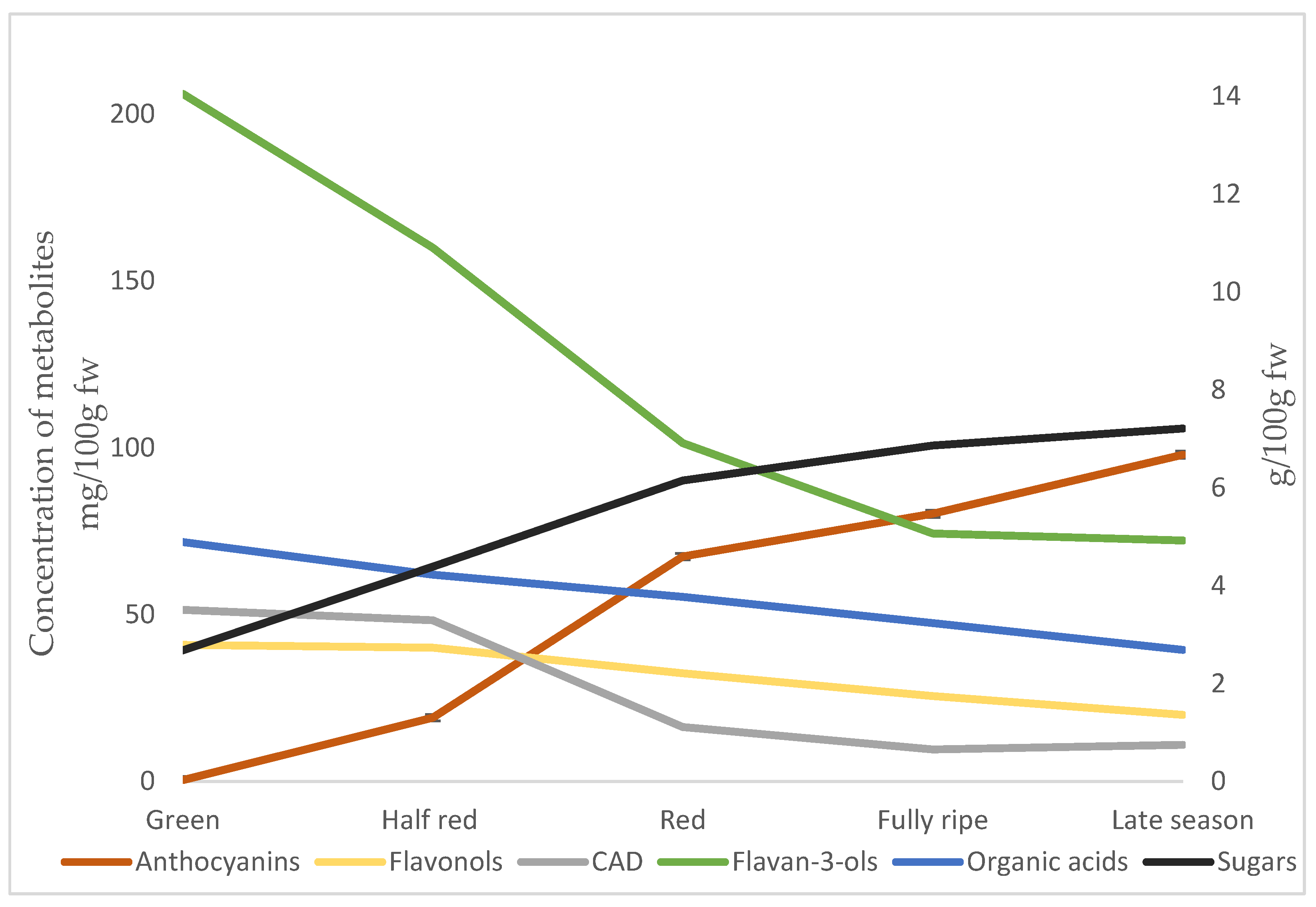
3.1. Sugars and Organic Acids
3.2. Phenolic Compounds
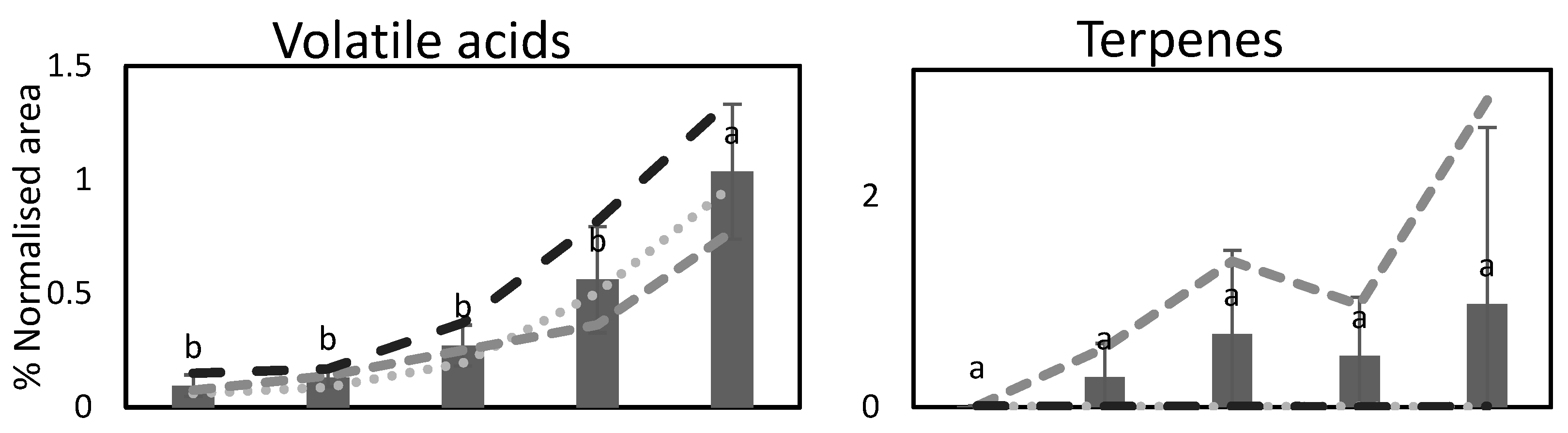
3.3. Volatile Compounds
3.4. General Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hjalmarsson, I.; Ortiz, R. Lingonberry: Botany and horticulture. Hortic. Rev. 2001, 27, 79–123. [Google Scholar] [CrossRef]
- Salo, K. Metsä: Monikäyttö ja Ekosysteemipalvelut; Luonnonvarakeskus (Luke): Helsinki, Finland, 2015; Available online: https://urn.fi/URN:978-952-326-123-5 (accessed on 15 February 2023).
- Paassilta, M.M.; Moisio, S.; Jaakola, L.; Häggman, H. Voice of the Nordic Wild Berry Industry. A Survey among the Companies; Oulu University Press: Oulu, Finland, 2009; pp. 1–84. [Google Scholar]
- Laaksonen, O.; Knaapila, A.; Niva, T.; Deegan, K.C.; Sandell, M. Sensory properties and consumer characteristics contributing to liking of berries. Food Qual. Prefer. 2016, 53, 117–126. [Google Scholar] [CrossRef]
- Viljakainen, S.; Visti, A.; Laakso, S. Concentrations of organic acids and soluble sugars in juices from Nordic berries. Acta Agric. Scand.—B Soil Plant Sci. 2002, 52, 101–109. [Google Scholar] [CrossRef]
- Viljanen, K.; Heiniö, R.-L.; Juvonen, R.; Kössö, T.; Puupponen-Pimiä, R. Relation of sensory perception with chemical composition of bioprocessed lingonberry. Food Chem. 2014, 157, 148–156. [Google Scholar] [CrossRef]
- Bujor, O.-C.; Ginies, C.; Popa, V.I.; Dufour, C. Phenolic compounds and antioxidant activity of lingonberry (Vaccinium vitis-idaea L.) leaf, stem and fruit at different harvest periods. Food Chem. 2018, 252, 356–365. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Sater, H.M.; Bizzio, L.N.; Tieman, D.M.; Muñoz, P.D. A review of the fruit volatiles found in blueberry and other Vaccinium species. J. Agric. Food Chem. 2020, 68, 5777–5786. [Google Scholar] [CrossRef]
- Kowalska, K. Lingonberry (Vaccinium vitis-idaea L.) fruit as a source of bioactive compounds with health-promoting effects—A review. Int. J. Mol. Sci. 2021, 22, 5126. [Google Scholar] [CrossRef]
- Shamilov, A.A.; Bubenchikova, V.N.; Chemikov, M.V.; Pozdnyakov, D.I.; Garsiya, E.R. Vaccinium vitis-idaea L.: Chemical contents, pharmacological activities. Pharm. Sci. 2020, 26, 344–362. [Google Scholar] [CrossRef]
- Ryyti, R.; Hämäläinen, M.; Peltola, R.; Moilanen, E. Beneficial effects of lingonberry (Vaccinium vitis-idaea L.) supplementation on metabolic and inflammatory adverse effects induced by high-fat diet in a mouse model of obesity. PLoS ONE 2020, 15, e0232605. [Google Scholar] [CrossRef]
- Lima, R.d.C.L.; Böcker, U.; McDougall, G.J.; Allwood, J.W.; Afseth, N.K.; Wubshet, S.G. Magnetic ligand fishing using immobilized DPP-IV for identification of antidiabetic ligands in lingonberry extract. PLoS ONE 2021, 16, e0247329. [Google Scholar] [CrossRef]
- Cosme, F.; Pinto, T.; Aires, A.; Morais, M.C.; Bacelar, E.; Anjos, R.; Ferreira-Cardoso, J.; Oliveira, I.; Vilela, A.; Gonçalves, B. Red fruits composition and their health benefits—A review. Foods 2022, 11, 644. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudone, L. Vaccinium vitis-idaea L. Fruits: Chromatographic Analysis of Seasonal and Geographical Variation in Bioactive Compounds. Foods 2021, 10, 2243. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Matos, M.; Carnide, V.; Silva, A.P.; Gonçalves, B. Variation of chemical constituents, antioxidant activity, and endogenous plant hormones throughout different ripening stages of highbush blueberry (Vaccinium corymbosum L.) cultivars produced in centre of Portugal. J. Food Biochem. 2017, 41, e12414. [Google Scholar] [CrossRef]
- Dare, A.P.; Günther, C.S.; Grey, A.C.; Guo, G.; Demarais, N.J.; Cordiner, S.; McGhie, T.K.; Boldingh, H.; Hunt, M.; Deng, C.; et al. Resolving the developmental distribution patterns of polyphenols and related primary metabolites in bilberry (Vaccinium myrtillus) fruit. Food Chem. 2022, 374, 131703. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Haggman, H.; Jaakola, L. On the developmental and environmental regulation of secondary metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.O.; Molina-Torres, J.; Angoa-Pérez, M.V.; Cárdenas-Valdovinos, J.G.; García-Ruíz, I.; Ceja-Díaz, J.A.; Mena-Violante, H.G. Phenolic compound content and the antioxidant and antimicrobial activity of wild blueberries (Vaccinium stenophyllum steud.) fruits extracts during ripening. Horticulturae 2021, 8, 15. [Google Scholar] [CrossRef]
- Jaakola, L.; Maatta, K.; Pirttila, A.M.; Torronen, R.; Karenlampi, S.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Forney, C.F.; Kalt, W.; Jordan, M.A.; Vinqvist-Tymchuk, M.R.; Fillmore, S.A. Blueberry and cranberry fruit composition during development. J. Berry Res. 2012, 2, 169–177. [Google Scholar] [CrossRef]
- González-Barreiro, C.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. Wine aroma compounds in grapes: A critical review. Crit. Rev. Food Sci. Nutr. 2015, 55, 202–218. [Google Scholar] [CrossRef]
- Amundsen, M.; Jaakola, L.; Aaby, K.; Martinussen, I.; Kelanne, N.; Tuominen, S.; Laaksonen, O.; Yang, B.; Hykkerud, A. Effect of ripening temperature on the chemical composition of lingonberries (Vaccinium vitis-idaea L.) of northern and southern origin. Food Res. Int. 2023, 167, 112738. [Google Scholar] [CrossRef]
- Lussana, C.; Tveito, O.E.; Dobler, A.; Tunheim, K. seNorge_2018, daily precipitation, and temperature datasets over Norway. Earth Syst. Sci. Data 2019, 11, 1531–1551. [Google Scholar] [CrossRef]
- Aaby, K.; Amundsen, M. Evaluation of extraction methods for determination of phenolic compounds, organic acids and sugars in lingonberries (Vaccinium vitis-idaea). In Proceedings of the XII International Vaccinium Symposium 1357, Debert, NS, Canada, 30 August–1 September 2021; pp. 215–222. [Google Scholar]
- Davik, J.; Aaby, K.; Buti, M.; Alsheikh, M.; Šurbanovski, N.; Martens, S.; Røen, D.; Sargent, D.J. Major-effect candidate genes identified in cultivated strawberry (Fragaria × ananassa Duch.) for ellagic acid deoxyhexoside and pelargonidin-3-O-malonylglucoside biosynthesis, key polyphenolic compounds. Hortic. Res. 2020, 7, 125. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Sønsteby, A.; Aaby, K.; Martinsen, B.K.; Heide, O.M.; Wold, A.B.; Remberg, S.F. Ascorbate pool, sugars and organic acids in black currant (Ribes nigrum L.) berries are strongly influenced by genotype and post-flowering temperature. J. Sci. Food Agric. 2017, 97, 1302–1309. [Google Scholar] [CrossRef]
- Aaby, K.; Grimmer, S.; Holtung, L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. LWT-Food Sci. Technol. 2013, 54, 257–264. [Google Scholar] [CrossRef]
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef]
- Marsol-Vall, A.; Kelanne, N.; Nuutinen, A.; Yang, B.; Laaksonen, O. Influence of enzymatic treatment on the chemical composition of lingonberry (Vaccinium vitis-idaea) juice. Food Chem. 2020, 339, 128052. [Google Scholar] [CrossRef]
- Walker, R.P.; Famiani, F. Organic Acids in Fruits. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 2018; pp. 371–430. [Google Scholar]
- Samkumar, A.; Karppinen, K.; Dhakal, B.; Martinussen, I.; Jaakola, L. Insights into sugar metabolism during bilberry (Vaccinium myrtillus L.) fruit development. Physiol. Plant. 2022, 174, e13657. [Google Scholar] [CrossRef]
- Wang, Y.; Johnson-Cicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in American cranberry (Vaccinium macrocarpon) cultivars using HPLC and APCI-MS/MS. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chem. 2012, 135, 2138–2146. [Google Scholar] [CrossRef]
- Jensen, H.D.; Krogfelt, K.A.; Cornett, C.; Hansen, S.H.; Christensen, S.B. Hydrophilic carboxylic acids and iridoid glycosides in the juice of American and European cranberries (Vaccinium macrocarpon and V. oxycoccos), lingonberries (V. vitis-idaea), and blueberries (V. myrtillus). J. Agric. Food Chem. 2002, 50, 6871–6874. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Raudonis, R.; Motiekaityte, V.; Vainoriene, R.; Burdulis, D.; Viskelis, J.; Raudone, L. Composition of sugars in wild and cultivated lingonberries (Vaccinium vitis-idaea L.). Molecules 2019, 24, 4225. [Google Scholar] [CrossRef]
- Grace, M.H.; Esposito, D.; Dunlap, K.L.; Lila, M.A. Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. J. Agric. Food Chem. 2014, 62, 4007–4017. [Google Scholar] [CrossRef]
- Andersen, Ø.M. Chromatographic Separation of Anthocyanins in Cowberry (Lingonberry) Vaccinium vites-idaea L. J. Food Sci. 1985, 50, 1230–1232. [Google Scholar] [CrossRef]
- Kelanne, N.; Laaksonen, O.; Seppälä, T.; Yang, W.; Tuukkanen, K.; Loponen, J.; Yang, B. Impact of cyclodextrin treatment on composition and sensory properties of lingonberry (Vaccinium vitis-idaea) juice. LWT-Food Sci. Technol. 2019, 113, 108295. [Google Scholar] [CrossRef]
- Ek, S.; Kartimo, H.; Mattila, S.; Tolonen, A. Characterization of phenolic compounds from lingonberry (Vaccinium vitis-idaea). J. Agric. Food Chem. 2006, 54, 9834–9842. [Google Scholar] [CrossRef]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of phenolic compounds from lingonberry (Vaccinium vitis-idaea L.), bilberry (Vaccinium myrtillus L.) and hybrid bilberry (Vaccinium x intermedium Ruthe L.) leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef]
- McGhie, T.K.; Ainge, G.D.; Barnett, L.E.; Cooney, J.M.; Jensen, D.J. Anthocyanin Glycosides from Berry Fruit Are Absorbed and Excreted Unmetabolized by Both Humans and Rats. J. Agric. Food Chem. 2003, 51, 4539–4548. [Google Scholar] [CrossRef]
- Šedbarė, R.; Pašakinskienė, I.; Janulis, V. Changes in the Composition of Biologically Active Compounds during the Ripening Period in Fruit of Different Large Cranberry (Vaccinium macrocarpon Aiton) Cultivars Grown in the Lithuanian Collection. Plants 2023, 12, 202. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimia, R.; Westerlund-Wikstrom, B.; Leppanen, T.; Welling, J.; Moilanen, E.; Heinonen, M. Lingonberry (Vaccinium vitis-idaea) and European cranberry (Vaccinium microcarpon) proanthocyanidins: Isolation, identification, and bioactivities. J. Agric. Food Chem. 2011, 59, 3373–3384. [Google Scholar] [CrossRef]
- Anjou, K.; Von Sydow, E. Aroma of cranberries juice of Vaccinium vitis-idaea L. Acta Chem. Scand. 1969, 23, 109–114. [Google Scholar] [CrossRef]
- Anjou, K.; Von Sydow, E. Aroma cranberries. I. Vaccinium vitis-idaea L. Acta Chem. Scand. 1967, 21, 945–952. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the Key Aroma Volatile Compounds in Cranberry (Vaccinium macrocarpon Ait.) Using Gas Chromatography–Olfactometry (GC-O) and Odor Activity Value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef]
- Gilbert, J.L.; Schwieterman, M.L.; Colquhoun, T.A.; Clark, D.G.; Olmstead, J.W. Potential for Increasing Southern Highbush Blueberry Flavor Acceptance by Breeding for Major Volatile Components. HortScience 2013, 48, 835–843. [Google Scholar] [CrossRef]
- Dashbaldan, S.; Becker, R.; Pączkowski, C.; Szakiel, A. Various Patterns of Composition and Accumulation of Steroids and Triterpenoids in Cuticular Waxes from Screened Ericaceae and Caprifoliaceae Berries during Fruit Development. Molecules 2019, 24, 3826. [Google Scholar] [CrossRef]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef]
- Klavins, L.; Mezulis, M.; Nikolajeva, V.; Klavins, M. Composition, sun protective and antimicrobial activity of lipophilic bilberry (Vaccinium myrtillus L.) and lingonberry (Vaccinium vitis-idaea L.) extract fractions. LWT 2021, 138, 110784. [Google Scholar] [CrossRef]
- Vilkickyte, G.; Motiekaityte, V.; Vainoriene, R.; Raudone, L. Promising cultivars and intraspecific taxa of lingonberries (Vaccinium vitis-idaea L.): Profiling of phenolics and triterpenoids. J. Food Compost. Anal. 2022, 114, 104796. [Google Scholar] [CrossRef]
- Vyas, P.; Curran, N.H.; Igamberdiev, A.U.; Debnath, S.C. Antioxidant properties of lingonberry (Vaccinium vitis-idaea L.) leaves within a set of wild clones and cultivars. Can. J. Plant Sci. 2015, 95, 663–669. [Google Scholar] [CrossRef]


| Harvest Date | Fruit Color | Weight (g/berry) | Dry Weight (%) | Temp Mean (°C) | Max (°C) | Min (°C) | Rain (mm) | |
|---|---|---|---|---|---|---|---|---|
| 23 July 2020 | Unripe green |  | 0.21 ± 0.05 | 15.5 ± 0.2 | 13.2 | 19.4 | 5.0 | 0 |
| 24 July 2020 | Half red |  | 0.23 ± 0.08 | 15.3 ± 1.1 | 14.2 | 20.4 | 7.8 | 0 |
| 8 August 2020 | Ripening fully red |  | 0.28 ± 0.10 | 15.6 ± 1.2 | 19.0 | 25.2 | 12.4 | 0.1 |
| 27 August 2020 | Fully ripe scarlet red |  | 0.28 ± 0.05 | 15.0 ± 1.5 | 11.8 | 19.9 | 4.2 | 0 |
| 28 September 2020 | Late season scarlet red |  | 0.27 ± 0.02 | 15.1 ± 1.2 | 9.7 | 15.4 | 2.5 | 0.1 |
| Green | Half Red | Red | Fully Ripe | Late Season | |
|---|---|---|---|---|---|
| Sucrose | 0.23 ± 0.01 | 0.37 ± 0.07 | 0.38 ± 0.07 | 0.34 ± 0.06 | 0.31 ± 0.02 |
| Fructose | 1.1 ± 0.3 d | 1.9 ± 0.3 c | 2.7 ± 0.5 b | 3.2 ± 0.1 ab | 3.5 ± 0.4 a |
| Glucose | 1.3 ± 0.2 b | 2.2 ± 0.2 b | 3.0 ± 0.5 a | 3.3 ± 0.4 a | 3.4 ± 0.1 a |
| Total sugars | 2.7 ± 0.5 c | 4.4 ± 0.4 b | 6.2 ± 1.0 a | 6.9 ± 0.4 a | 7.2 ± 0.4 a |
| Citric acids | 2.1 ± 0.4 | 2.2 ± 0.3 | 2.1 ± 0.3 | 2.1 ± 0.6 | 1.7 ± 0.4 |
| Quinic acid | 2.7 ± 0.5 a | 1.9 ± 0.1 b | 1.5 ± 0.2 bc | 1.1 ± 0.2 cd | 0.9 ± 0.1 d |
| Malic acid | 155 ± 39 a | 114 ± 54 ab | 93 ± 24 abc | 79 ± 25 bc | 48 ± 17 c |
| Shikimic acid | 3.0 ± 0.7 | 2.4 ± 0.3 | 2.3 ± 0.5 | 2.3 ± 1.0 | 2.3 ± 0.9 |
| Total organic acids | 4.9 ± 0.4 a | 4.2 ± 40.3 ab | 3.8 ± 0.1 bc | 3.2 ± 0.7 cd | 2.7 ± 0.5 d |
| Sugar: acid ratio | 0.6 ± 0.2 b | 1.0 ± 0.1 b | 1.6 ± 0.3 b | 2.2 ± 0.4 ab | 2.7 ± 0.3 a |
| Green | Half Red | Red | Fully Ripe | Late Season | |
|---|---|---|---|---|---|
| Cyanidin-3-O-galactoside | 0.4 ± 0.1 b | 17.8 ± 1.3 b | 57.9 ± 4.0 a | 65.4 ± 11.0 a | 79.3 ± 17.5 a |
| Cyanidin-3-O-glucoside | 0.0 ± 0.0 c | 0.6 ± 0.1 c | 3.1 ± 0.5 b | 4.5 ± 1.0 ab | 5.6 ± 1.5 a |
| Cyanidin-3-O-arabinoside | 0.0 ± 0.0 c | 0.9 ± 0.0 c | 6.6 ± 0.2 b | 10.0 ± 0.7 ab | 13.0 ± 3.4 a |
| Cyanidin-3-O-pentoside | 0.0 ± 0.0 c | 0.1 ± 0.0 c | 0.6 ± 0.1 b | 0.9 ± 0.1 ab | 1.2 ± 0.4 a |
| Cyanidin-3-O-(acetyl)glucoside | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Total anthocyanins | 0.4 ± 0.1 c | 19.4 ± 0.7 c | 68.3 ± 2.3 b | 80.9 ± 7.0 ab | 98.5 ± 12.6 a |
| Quercetin-3-O-galactoside | 10.4 ± 2.3 a | 10.5 ± 4.1 a | 7.6 ± 2.8 ab | 5.5 ± 0.6 b | 4.6 ± 2.0 b |
| Quercetin-3-O-glucoside | 2.4 ± 0.2 a | 2.3 ± 0.4 a | 1.6 ± 0.3 ab | 1.2 ± 0.1 b | 1.2 ± 0.3 b |
| Quercetin-3-O-xyloside | 1.7 ± 0.2 | 1.6 ± 0.3 | 1.4 ± 0.1 | 1.3 ± 0.2 | 1.2 ± 0.2 |
| Quercetin-3-O-arabinoside | 10.4 ± 0.8 a | 10.8 ± 0.6 a | 9.5 ± 1.3 ab | 7.8 ± 0.5 bc | 6.6 ± 0.8 c |
| Quercetin-3-O-arabinofuranoside | 1.0 ± 0.1 a | 0.8 ± 0.1 b | 0.6 ± 0.1 c | 0.5 ± 0.1 d | 0.4 ± 0.1 d |
| Quercetin-3-O-rhamnoside | 6.3 ± 4.5 | 6.3 ± 4.8 | 5.2 ± 3.6 | 3.8 ± 2.6 | 2.4 ± 1.7 |
| Quercetin-(HMG)-pentoside | 0.4 ± 0.1 a | 0.4 ± 0.1 ab | 0.3 ± 0.0 abc | 0.3 ± 0.0 bc | 0.2 ± 0.0 c |
| Quercetin-3-O-(HMG)-pentoside 2 b | 0.0 ± 0.0 b | 0.1 ± 0.1 ab | 0.2 ± 0.1 a | 0.0 ± 0.1 b | 0.0 ± 0.0 b |
| Kaempferol-3-O-rhamnoside | 0.4 ± 0.2 a | 0.3 ± 0.2 ab | 0.3 ± 0.1 ab | 0.2 ± 0.1 ab | 0.2 ± 0.0 b |
| Quercetin-3-O-(HMG)-rhamnoside b | 8.2 ± 7.8 | 7.6 ± 5.8 | 5.7 ± 4.9 | 4.6 ± 4.3 | 2.8 ± 2.6 |
| Kaempferol-3-O-(HMG)-rhamnoside b | 0.0 ± 0.0 b | 0.1 ± 0.0 b | 0.4 ± 0.1 ab | 0.7 ± 0.2 b | 0.5 ± 0.1 b |
| Total flavonols | 41.2 ± 7.7 a | 40.8 ± 7.3 a | 32.8 ± 5.3 ab | 25.8 ± 4.2 b | 20.1 ± 1.2 b |
| Ferulic acid-hexoside 1 | 1.2 ± 0.8 c | 2.4 ± 0.4 ab | 2.8 ± 0.6 a | 1.5 ± 0.2 bc | 1.3 ± 0.2 c |
| Ferulic acid-hexoside 2 | 31.4 ± 11.2 a | 30 ± 19.2 a | 3.6 ± 1.5 b | 0.8 ± 0.1 b | 0.7 ± 0.1 b |
| Coumaroyl iridoid | 5.7 ± 2.2 a | 2.9 ± 1.4 ab | 1.6 ± 0.8 b | 1.1 ± 0.8 b | 1.0 ± 0.8 b |
| Caffeic acid hexoside 1 | 2.1 ± 0.3 a | 1.9 ± 0.5 ab | 1.1 ± 0.1 bc | 0.8 ± 0.2 c | 0.7 ± 0.1 c |
| Caffeic acid hexoside 2 | 3.1 ± 0.3 a | 2.9 ± 1.6 a | 1.0 ± 0.3 b | 0.5 ± 0.1 b | 0.3 ± 0.0 b |
| p-Coumaric acid hexoside | 5.7 ± 0.5 | 4.7 ± 1.1 | 5.3 ± 0.1 | 4.2 ± 0.3 | 5.7 ± 1.6 |
| Chlorogenic acid | 2.6 ± 0.6 | 3.8 ± 4.4 | 1.8 ± 1.0 | 1.3 ± 0.1 | 1.7 ± 0.7 |
| Sinapic acid hexoside | 0.1 ± 0.0 a | 0.1 ± 0.0 ab | 0.1 ± 0.0 ab | 0.1 ± 0.0 b | 0.1 ± 0.0 b |
| Total cinnamic acid derivatives | 51.9 ± 6.4 a | 48.8 ± 14.1 a | 17.4 ± 1.7 b | 10.3 ± 0.7 b | 11.3 ± 1.1 b |
| Proanthocyanidin dimer A | 103.8 ± 41.7 a | 78.8 ± 34.6 ab | 46.8 ± 28.3 b | 34.1 ± 13.6 b | 27.2 ± 4.3 b |
| Proanthocyanidin dimer B | 30.8 ± 7.4 a | 26.7 ± 8.3 ab | 17.8 ± 3.5 bc | 14.1 ± 3.5 c | 14.2 ± 4.7 c |
| Catechin | 71.4 ± 24.6 a | 54.4 ± 20.3 ab | 36.7 ± 10.7 bc | 26.1 ± 7.9 c | 31.4 ± 11.4 c |
| Total Flavan-3-ols | 206.0 ± 67.8 a | 159.8 ± 61 ab | 101.4 ± 33.4 bc | 74.3 ± 10.7 c | 72.8 ± 14.6 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amundsen, M.; Hykkerud, A.L.; Kelanne, N.; Tuominen, S.; Schmidt, G.; Laaksonen, O.; Yang, B.; Martinussen, I.; Jaakola, L.; Aaby, K. Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages. Foods 2023, 12, 2154. https://doi.org/10.3390/foods12112154
Amundsen M, Hykkerud AL, Kelanne N, Tuominen S, Schmidt G, Laaksonen O, Yang B, Martinussen I, Jaakola L, Aaby K. Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages. Foods. 2023; 12(11):2154. https://doi.org/10.3390/foods12112154
Chicago/Turabian StyleAmundsen, Mathias, Anne Linn Hykkerud, Niina Kelanne, Sanni Tuominen, Gesine Schmidt, Oskar Laaksonen, Baoru Yang, Inger Martinussen, Laura Jaakola, and Kjersti Aaby. 2023. "Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages" Foods 12, no. 11: 2154. https://doi.org/10.3390/foods12112154
APA StyleAmundsen, M., Hykkerud, A. L., Kelanne, N., Tuominen, S., Schmidt, G., Laaksonen, O., Yang, B., Martinussen, I., Jaakola, L., & Aaby, K. (2023). Composition of Sugars, Organic Acids, Phenolic Compounds, and Volatile Organic Compounds in Lingonberries (Vaccinium vitis-idaea L.) at Five Ripening Stages. Foods, 12(11), 2154. https://doi.org/10.3390/foods12112154