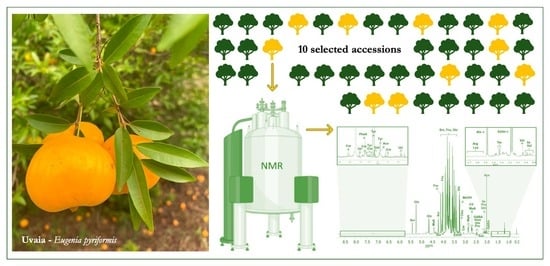
Quality Attributes and Metabolic Profiles of Uvaia (Eugenia pyriformis), a Native Brazilian Atlantic Forest Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Physicochemical Characterization and Accession Selection
Plant Material
2.2. Metabolite Identification and Quantification in Selected Uvaia Accessions
2.2.1. Sample Preparation and Metabolite Extraction
2.2.2. Metabolomics Analyses
2.2.3. Statistical Analyses
3. Results
3.1. Physicochemical Uvaia Characterization and Accession Selection
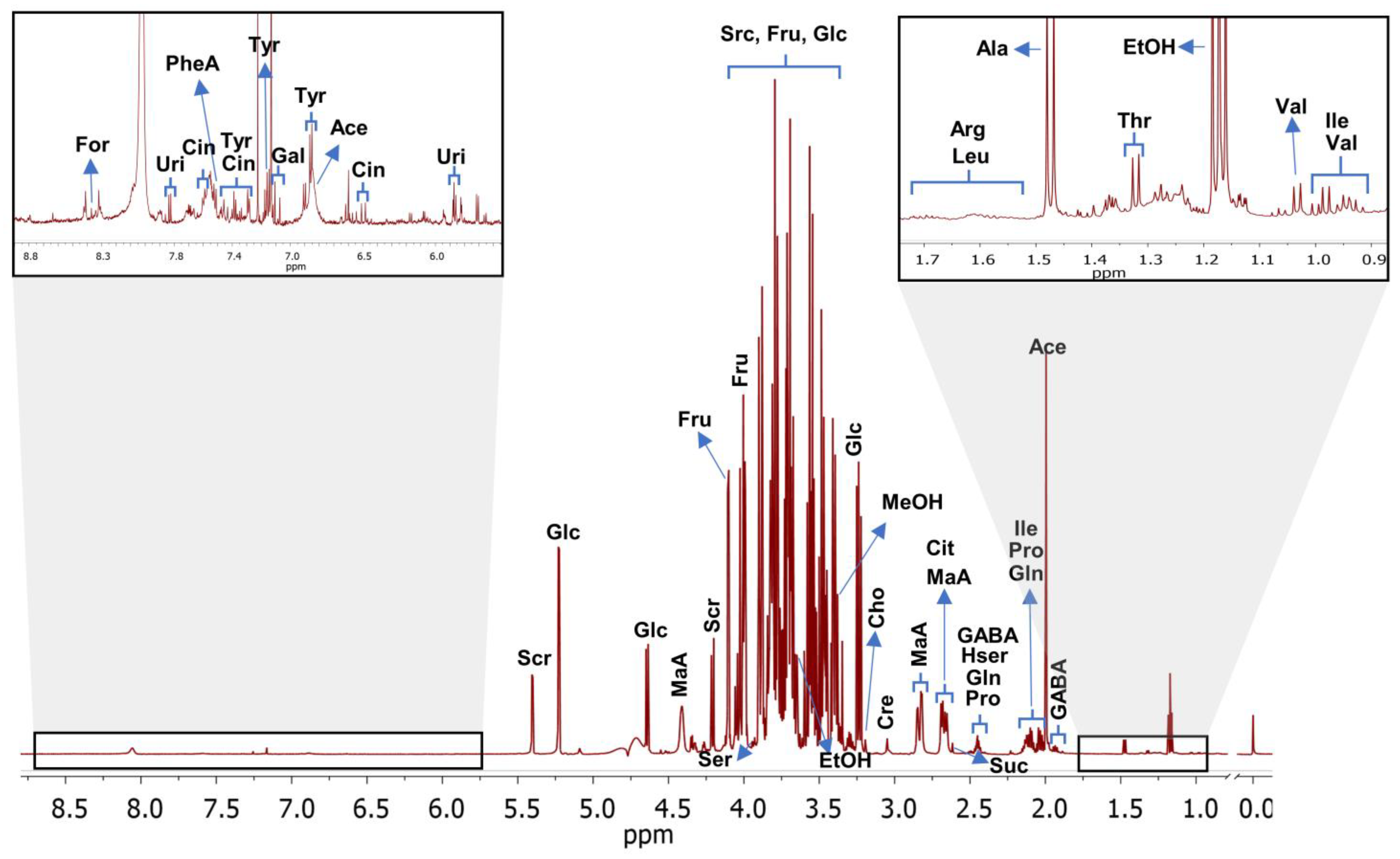
3.2. Metabolite Identification and Quantification in Selected Uvaia Accessions
3.3. Selected Uvaia Accession Classification
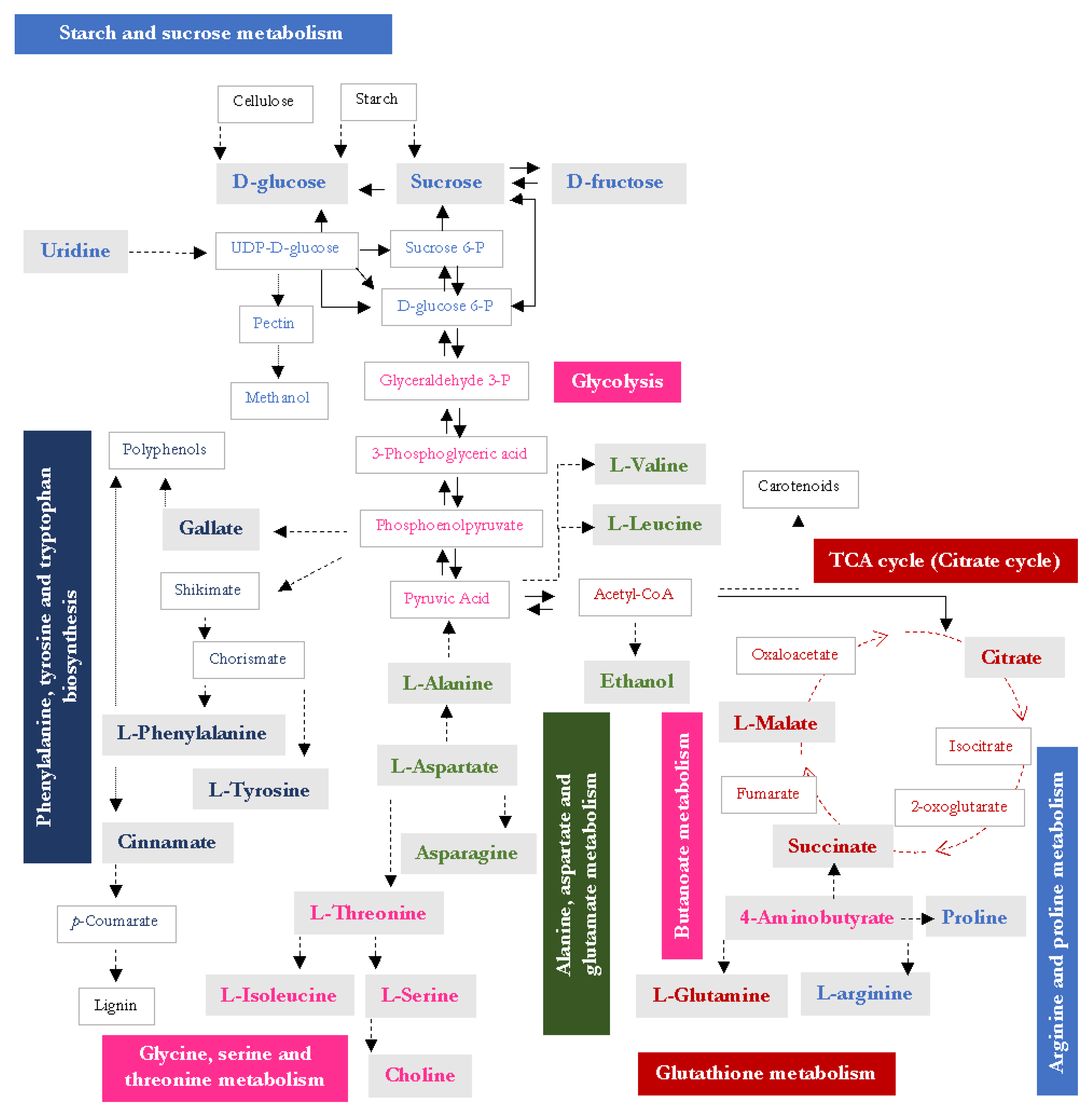
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Spricigo, P.C.; Correia, B.S.B.; Borba, K.R.; Taver, I.B.; Machado, G.D.O.; Wilhelms, R.Z.; Queiroz, L.H.K.; Jacomino, A.P.; Colnago, L.A. Classical food quality attributes and the metabolic profile of cambuci, a native brazilian atlantic rainforest fruit. Molecules 2021, 26, 3613. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; de Alencar, S.M.; Jacomino, A.P. Volatile Compounds Determined by SPME-GC, Bioactive Compounds, In Vitro Antioxidant Capacity and Physicochemical Characteristics of Four Native Fruits from South America. Plant Foods Hum. Nutr. 2019, 74, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Jacomino, A.P.; da Silva, A.P.G.; de Freitas, T.P.; de Paula Morais, V.S. Uvaia—Eugenia pyriformis Cambess. In Exotic Fruits; Elsevier: Amsterdam, The Netherlands, 2018; pp. 435–438. [Google Scholar]
- da Silva, A.P.G.; Spricigo, P.C.; Purgatto, E.; de Alencar, S.M.; Sartori, S.F.; Jacomino, A.P. Chemical composition, nutritional value and bioactive compounds in six uvaia accessions. Food Chem. 2019, 294, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Simpson, M.G. 8—Diversity and Classification of Flowering Plants: Eudicots; Simpson, M.G.B.T.-P.S., Second, E., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 275–448. ISBN 978-0-12-374380-0. [Google Scholar]
- Taver, I.B.; Spricigo, P.C.; Neto, H.B.; de Alencar, S.M.; Massarioli, A.P.; Jacomino, A.P. Bioactive Compounds and In Vitro Antioxidant Capacity of Cambuci and Uvaia: An Extensive Description of Little-Known Fruits from the Myrtaceae Family with High Consumption Potential. Foods 2022, 11, 2612. [Google Scholar] [CrossRef]
- Freitas, T.P.; Spricigo, P.C.; Purgatto, E.; Jacomino, A.P. Aroma and soluble solid contents of the uvaia—A native Atlantic rainforest fruit—Are negatively affected by early harvest. J. Food Biochem. 2019, 43, e12881. [Google Scholar] [CrossRef]
- Azevedo, N.; Silva, D.; Rodrigues, E.; Mercadante, A.Z.; Vera De Rosso, V. Phenolic Compounds and Carotenoids from Four Fruits Native from the Brazilian Atlantic Forest. J. Agric. Food Chem. 2014, 62, 5072–5084. [Google Scholar] [CrossRef]
- Cho, K.S.; Lim, Y.-R.; Lee, K.; Lee, J.; Lee, J.H.; Lee, I.-S. Terpenes from Forests and Human Health. Toxicol. Res. 2017, 33, 97–106. [Google Scholar] [CrossRef]
- Rufino, M.; Fernandes, F.; Alves, R.; De Brito, E. Free radical-scavenging behaviour of some north-east Brazilian fruits in a DPPH system. Food Chem. 2009, 114, 693–695. [Google Scholar] [CrossRef]
- Wanderley, B.R.D.S.M.; Haas, I.C.D.S.; Biluca, F.C.; Brugnerotto, P.; Gomes, T.M.; Aquino, A.C.M.D.S.; Costa, A.C.O.; Burin, V.M.; Amboni, R.D.D.M.C.; Fritzen-Freire, C.B. Phenolic profiling, organic acids and sugars composition of feijoa (Acca sellowiana (O. Berg) Burret) and uvaia (Eugenia pyriformis Cambess) from the southern Brazilian highlands. Cienc. Rural 2022, 52, 1–7. [Google Scholar] [CrossRef]
- Pereira, M.C.; Steffens, R.S.; Hertz, P.F.; de Rios, A.O.; Vizzotto, M.; Flo, S.H. Characterization and Antioxidant Potential of Brazilian Fruits from the Myrtaceae Family. J. Agric. Food Chem. 2012, 60, 3061–3067. [Google Scholar] [CrossRef]
- Sobolev, A.P.; Mannina, L.; Proietti, N.; Carradori, S.; Daglia, M.; Giusti, A.M.; Antiochia, R.; Capitani, D. Untargeted NMR-based methodology in the study of fruit metabolites. Molecules 2015, 20, 4088–4108. [Google Scholar] [CrossRef] [PubMed]
- Ayvaz, H.; Santos, A.M.; Moyseenko, J.; Kleinhenz, M.; Rodriguez-saona, L.E. Application of a Portable Infrared Instrument for Simultaneous Analysis of Sugars, Asparagine and Glutamine Levels in Raw Potato Tubers. Plant Foods Hum. Nutr. 2015, 70, 215–220. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Ma, J.; Wang, H.; Li, F.; Qin, D.; Wu, J.; Zhu, G. NMR-based global metabolomics approach to decipher the metabolic effects of three plant growth regulators on strawberry maturation. Food Chem. 2018, 269, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Santucci, C.; Tenori, L.; Luchinat, C. NMR fingerprinting as a tool to evaluate post-harvest time-related changes of peaches, tomatoes and plums. Food Res. Int. 2015, 75, 106–114. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2023. [Google Scholar]
- Carvalho, C.R.L.; Mantovani, D.M.B.; Carvalho, P.R.N.; Moraes, R.M.M. Análises Químicas de Alimentos; ITAL: Campinas, Brazil, 1990. [Google Scholar]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Sganzerla, W.G.; Beling, P.C.; Ferreira, A.L.A.; Azevedo, M.S.; Ferrareze, J.P.; Komatsu, R.A.; Nunes, M.R.; de Lima Veeck, A.P. Geographical discrimination of uvaia (Eugenia pyriformis Cambess) by principal component analysis. J. Sci. Food Agric. 2019, 99, 6778–6787. [Google Scholar] [CrossRef]
- de Oliveira, C.S.; Carlos, E.F.; Vieira, L.G.E.; Lião, L.M.; Alcantara, G.B. HR-MAS NMR metabolomics of ‘Swingle’ citrumelo rootstock genetically modified to overproduce proline. Magn. Reson. Chem. 2014, 52, 422–429. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Nehring, P.; Della Betta, F.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Nutritional and bioactive potential of Myrtaceae fruits during ripening. Food Chem. 2018, 239, 649–656. [Google Scholar] [CrossRef]
- da Silva, A.P.G.; Sganzerla, W.G.; Jacomino, A.P.; da Silva, E.P.; Xiao, J.; Simal-Gandara, J. Chemical composition, bioactive compounds, and perspectives for the industrial formulation of health products from uvaia (Eugenia pyriformis Cambess–Myrtaceae): A comprehensive review. J. Food Compos. Anal. 2022, 109, 104500. [Google Scholar] [CrossRef]
- Saltveit, M.E. Chapter 4—Respiratory Metabolism; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 73–91. ISBN 978-0-12-813278-4. [Google Scholar]
- Freitas, T.P.; Taver, I.B.; Spricigo, P.C.; Do Amaral, L.B.; Purgatto, E.; Jacomino, A.P. Volatile compounds and physicochemical quality of four jabuticabas (plinia sp.). Molecules 2020, 25, 4543. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Sheshukova, E.V.; Komarova, T.V. Methanol in Plant Life. Front. Plant Sci. 2018, 9, 1623. [Google Scholar] [CrossRef] [PubMed]
- Possner, D.; Zimmer, T.; Kürbel, P.; Dietrich, H. Methanol contents of fruit juices and smoothies in comparison to fruits and a simple method for the determination thereof. Dtsch. Leb. 2014, 110, 65–69. [Google Scholar]
- World Health Organization. World Health Organization Methanol Health and Safety Guide; World Health Organization: Geneva, Switzerland, 1997; Volume 105, pp. 1–31. [Google Scholar]
- Hou, C.-Y.; Lin, Y.-S.; Tai Wang, Y.; Jiang, C.-M.; Wu, M.-C. Effect of storage conditions on methanol content of fruit and vegetable juices. J. Food Compos. Anal. 2008, 21, 410–415. [Google Scholar] [CrossRef]
- Li, S.; Yuan, X.; Xu, Y.; Li, Z.; Feng, Z.; Yue, X.; Paoletti, E. Biogenic volatile organic compound emissions from leaves and fruits of apple and peach trees during fruit development. J. Environ. Sci. 2021, 108, 152–163. [Google Scholar] [CrossRef]
- Decker, D.; Kleczkowski, L.A. UDP-sugar producing pyrophosphorylases: Distinct and essential enzymes with overlapping substrate specificities, providing de novo precursors for glycosylation reactions. Front. Plant Sci. 2019, 9, 1822. [Google Scholar] [CrossRef]
- Søltoft-Jensen, J.; Hansen, F. 15-New Chemical and Biochemical Hurdles; Sun, D.-W., Ed.; Academic Press: London, UK, 2005; pp. 387–416. ISBN 978-0-12-676757-5. [Google Scholar]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus Species: Correlation with Apple Domestication. Metabolites 2018, 8, 31–74. [Google Scholar] [CrossRef]
- Li, W.; Ruan, C.J.; da Silva, J.A.T.; Guo, H.; Zhao, C.E. NMR metabolomics of berry quality in sea buckthorn (Hippophae L.). Mol. Breed. 2013, 31, 57–67. [Google Scholar] [CrossRef]
- Nakamura, K.; Nara, K.; Noguchi, T.; Ohshiro, T.; Koga, H. Contents of GAMMA.-Aminobutyric Acid (GABA) in Potatoes and Processed Potato Products. J. Japanese Soc. Food Sci. Technol. 2006, 53, 514–517. [Google Scholar] [CrossRef]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health effects, sources, utilization and safety of tannins: A critical review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- He, H.-F. Recognition of Gallotannins and the Physiological Activities: From Chemical View. Front. Nutr. 2022, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shuab, R.; Lone, R.; Koul, K.K. Cinnamate and cinnamate derivatives in plants. Acta Physiol. Plant. 2016, 38, 64. [Google Scholar] [CrossRef]
- Pérez-Alonso, M.-M.; Ortiz-García, P.; Moya-Cuevas, J.; Lehmann, T.; Sánchez-Parra, B.; Björk, R.G.; Karim, S.; Amirjani, M.R.; Aronsson, H.; Wilkinson, M.D.; et al. Endogenous indole-3-acetamide levels contribute to the crosstalk between auxin and abscisic acid, and trigger plant stress responses in Arabidopsis. J. Exp. Bot. 2021, 72, 459–475. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Meyers, L.D.; Hellwig, J.P.; Otten, J.J. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006; ISBN 0309157420. [Google Scholar]
- Hou, Y.; Wu, G. Nutritionally Essential Amino Acids. Adv. Nutr. 2018, 9, 849–851. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; He, W.; Hu, S.; Wu, G. Composition of polyamines and amino acids in plant-source foods for human consumption. Amino Acids 2019, 51, 1153–1165. [Google Scholar] [CrossRef]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L. An amino-acid taste receptor. Nature 2002, 5124, 199–202. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant Volatiles: Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- De Oliveira, C.R.; Carneiro, R.L.; Ferreira, A.G. Tracking the degradation of fresh orange juice and discrimination of orange varieties: An example of NMR in coordination with chemometrics analyses. Food Chem. 2014, 164, 446–453. [Google Scholar] [CrossRef]
- Beckles, D.M.; Roessner, U. 5-Plant Metabolomics: Applications and Opportunities for Agricultural Biotechnology; Altman, A., Hasegawa, Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 67–81. ISBN 978-0-12-381466-1. [Google Scholar]



| H | D | FW | S | Y | TSS | TA | |
|---|---|---|---|---|---|---|---|
| UV016 | 16.21 | 20.83 | 4.80 | 1.26 | 74.13 | 10.08 | 1.69 |
| UV022 | 24.83 | 30.22 | 13.85 | 4.26 | 70.91 | 8.12 | 1.48 |
| UV027 | 17.96 | 21.11 | 5.41 | 1.19 | 78.30 | 12.73 | 2.08 |
| UV028 | 22.08 | 26.18 | 8.91 | 1.34 | 85.07 | 15.30 | 1.39 |
| UV029 | 26.38 | 33.82 | 14.18 | 3.46 | 75.94 | 7.52 | 1.25 |
| UV030 | 20.67 | 25.09 | 12.88 | 1.24 | 89.95 | 13.92 | 1.00 |
| UV036 | 19.20 | 19.51 | 4.29 | 1.26 | 70.79 | 8.73 | 1.10 |
| UV037 | 24.11 | 28.03 | 10.28 | 2.48 | 75.92 | 8.40 | 1.06 |
| UV039 | 20.30 | 25.51 | 6.83 | 1.10 | 84.04 | 6.57 | 0.95 |
| UV040 | 24.99 | 30.36 | 11.64 | 1.73 | 85.58 | 12.07 | 1.29 |
| UV041 | 23.10 | 25.80 | 9.30 | 2.89 | 69.24 | 8.42 | 1.36 |
| UV042 | 19.92 | 24.05 | 6.51 | 1.15 | 82.53 | 9.10 | 1.22 |
| UV043 | 20.92 | 25.50 | 6.65 | 1.48 | 64.87 | 8.72 | 1.10 |
| UV046 | 21.94 | 23.69 | 7.44 | 2.26 | 70.72 | 10.02 | 2.02 |
| UV047 | 23.82 | 27.04 | 9.92 | 1.56 | 84.06 | 12.30 | 1.11 |
| UV048 | 18.59 | 21.65 | 5.55 | 1.40 | 74.60 | 10.27 | 2.31 |
| UV049 | 26.16 | 30.21 | 13.14 | 3.18 | 76.35 | 8.95 | 2.26 |
| UV050 | 20.49 | 23.67 | 6.54 | 2.15 | 65.95 | 8.62 | 1.47 |
| UV052 | 25.56 | 31.99 | 12.92 | 2.42 | 81.39 | 7.28 | 0.83 |
| UV056 | 21.50 | 24.65 | 7.75 | 1.50 | 80.76 | 9.12 | 1.47 |
| UV057 | 20.19 | 26.24 | 7.36 | 1.14 | 84.70 | 8.13 | 1.18 |
| UV058 | 24.97 | 28.03 | 12.46 | 3.45 | 73.51 | 7.58 | 1.27 |
| UV059 | 20.09 | 24.90 | 7.46 | 1.53 | 79.65 | 8.98 | 1.38 |
| UV060 | 20.00 | 23.19 | 6.81 | 1.15 | 83.10 | 11.38 | 2.07 |
| UV062 | 28.17 | 32.03 | 26.23 | 3.96 | 85.60 | 10.43 | 1.69 |
| UV067 | 19.81 | 23.88 | 7.61 | 1.06 | 86.20 | 10.20 | 1.49 |
| UV068 | 32.62 | 41.17 | 27.90 | 5.47 | 80.47 | 10.20 | 1.03 |
| UV069 | 21.39 | 24.00 | 8.08 | 2.58 | 69.27 | 10.45 | 3.48 |
| UV072 | 19.10 | 24.97 | 6.59 | 1.82 | 72.99 | 5.98 | 1.77 |
| UV073 | 30.09 | 34.94 | 18.19 | 3.02 | 83.45 | 10.53 | 3.46 |
| UV075 | 26.74 | 31.25 | 13.63 | 4.05 | 70.33 | 10.38 | 3.13 |
| UV076 | 20.06 | 24.59 | 6.79 | 1.91 | 72.49 | 8.77 | 3.58 |
| UV088 | 24.05 | 28.82 | 11.12 | 4.24 | 61.95 | 5.87 | 3.17 |
| UV089 | 21.40 | 26.93 | 7.40 | 1.54 | 79.37 | 9.50 | 3.08 |
| UV112 | 22.11 | 26.91 | 9.22 | 1.60 | 82.65 | 8.92 | 2.85 |
| UV114 | 20.03 | 24.39 | 6.64 | 0.84 | 87.59 | 10.17 | 3.16 |
| UV116 | 22.79 | 29.55 | 13.81 | 2.72 | 80.37 | 9.55 | 3.01 |
| UV120 | 20.50 | 24.19 | 7.45 | 1.47 | 80.79 | 9.05 | 2.87 |
| UV122 | 22.42 | 24.91 | 9.37 | 2.08 | 77.09 | 9.78 | 1.65 |
| UV125 | 21.40 | 24.24 | 6.85 | 1.26 | 81.58 | 10.28 | 3.70 |
| UV146 | 25.62 | 28.14 | 10.49 | 1.81 | 82.59 | 11.17 | 3.13 |
| Accession | Sucrose | Glucose | Fructose | Ethanol | Methanol | Uridine | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 302.6 | b | 1800.9 | b | 1643.1 | b | 23.5 | b | 10.8 | b | 1.27 | ab |
| 2 | 567.1 | b | 2832.3 | ab | 2832.1 | ab | 65.7 | a | 24.0 | a | 1.40 | ab |
| 3 | 303.2 | b | 1285.6 | b | 1258.5 | b | 23.8 | b | 10.7 | b | 0.93 | b |
| 4 | 1999.5 | a | 5203.6 | a | 4279.8 | a | 51.6 | ab | 10.1 | b | 2.12 | a |
| 5 | 405.0 | b | 2022.3 | b | 1819.6 | b | 18.3 | b | 9.9 | b | 1.59 | ab |
| 6 | 193.9 | b | 1739.4 | b | 1660.7 | b | 28.1 | b | 14.1 | ab | 1.17 | ab |
| 7 | 569.8 | b | 1970.3 | b | 1752.4 | b | 22.4 | b | 13.5 | ab | 1.36 | ab |
| 8 | 600.9 | b | 2044.9 | b | 1639.2 | b | 21.2 | b | 6.6 | b | 1.44 | ab |
| 9 | 253.1 | b | 1045.0 | b | 1178.0 | b | 25.7 | b | 11.8 | b | 0.81 | b |
| 10 | 360.3 | b | 1398.8 | b | 1294.0 | b | 20.0 | b | 13.4 | b | 0.62 | b |
| Mean | 555.5 | 2134.3 | 1935.7 | 30.0 | 12.5 | 1.27 | ||||||
| CV (%) | 85.28 | 50.22 | 44.78 | 49.30 | 36.92 | 30.26 | ||||||
| Accession | Citrate | Malate | Gallate | Succinate | 4-Aminobutyrate | Cinnamate | Formate | Acetamide | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 49.41 | a | 241.95 | b | 0.52 | a | 0.038 | ab | 1.392 | b | 0.79 | b | 0.113 | b | 107.44 | abc |
| 2 | 33.22 | b | 312.14 | b | 0.47 | a | 0.065 | ab | 0.520 | b | 0.58 | b | 0.120 | b | 88.11 | abc |
| 3 | 23.97 | b | 250.54 | b | 0.63 | a | 0.041 | ab | 1.379 | b | 0.68 | b | 0.062 | b | 97.35 | abc |
| 4 | 65.27 | ab | 737.97 | a | 0.66 | a | 0.079 | a | 0.887 | b | 0.72 | b | 0.205 | a | 109.36 | abc |
| 5 | 36.68 | ab | 299.35 | b | 0.50 | a | 0.061 | ab | 2.251 | ab | 0.48 | b | 0.075 | b | 138.87 | a |
| 6 | 21.61 | b | 196.79 | b | 0.58 | a | 0.032 | ab | 1.090 | b | 0.39 | b | 0.111 | b | 72.53 | bc |
| 7 | 33.11 | b | 296.04 | b | 0.37 | a | 0.040 | ab | 1.922 | ab | 0.31 | b | 0.130 | b | 120.057 | abc |
| 8 | 41.38 | ab | 369.61 | b | 0.36 | a | 0.063 | ab | 3.611 | a | 1.43 | a | 0.166 | a | 106.37 | abc |
| 9 | 27.03 | b | 141.53 | b | 0.47 | a | 0.034 | ab | 1.437 | b | 0.85 | ab | 0.060 | b | 76.39 | bc |
| 10 | 21.54 | b | 318.96 | b | 0.43 | a | 0.019 | b | 0.999 | b | 0.55 | b | 0.071 | b | 56.92 | c |
| Mean | 35.32 | 316.49 | 0.50 | 0.05 | 1.55 | 0.68 | 0.10 | 97.34 | ||||||||
| CV (%) | 36.29 | 47.91 | 19.83 | 35.68 | 53.90 | 46.84 | 38.60 | 23.20 | ||||||||
| Accession | Ala | Arg | Asp | Cho | Gln | Ile | Leu | Phe | Pro | Ser | Thr | Tyr | Val | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8.32 | abc | 2.57 | ab | 4.56 | ab | 1.53 | b | 37.36 | ab | 0.64 | ab | 0.82 | abc | 0.86 | b | 1.89 | a | 3.84 | a | 2.44 | ab | 2.00 | b | 1.50 | ab |
| 2 | 10.90 | a | 3.37 | a | 6.46 | ab | 3.34 | a | 10.62 | b | 0.88 | a | 0.97 | a | 0.91 | b | 1.95 | a | 4.61 | a | 2.57 | ab | 2.96 | b | 1.34 | ab |
| 3 | 7.88 | abc | 0.97 | b | 6.90 | ab | 1.60 | b | 36.47 | ab | 0.49 | b | 0.53 | bc | 0.83 | b | 1.07 | ab | 3.11 | a | 2.59 | ab | 1.80 | b | 1.13 | ab |
| 4 | 8.44 | abc | 2.26 | ab | 8.42 | a | 1.06 | b | 14.06 | b | 0.38 | b | 0.49 | bc | 0.62 | b | 1.30 | ab | 4.14 | a | 2.23 | ab | 5.47 | a | 0.73 | b |
| 5 | 8.95 | abc | 1.96 | ab | 5.29 | ab | 1.88 | ab | 51.12 | ab | 0.62 | ab | 0.75 | abc | 1.00 | b | 1.28 | ab | 3.82 | a | 2.99 | ab | 1.51 | b | 1.73 | a |
| 6 | 10.30 | ab | 2.30 | ab | 3.96 | b | 2.22 | ab | 36.15 | ab | 0.88 | a | 0.85 | ab | 0.63 | b | 1.73 | ab | 4.28 | a | 2.80 | ab | 2.89 | b | 1.52 | ab |
| 7 | 9.14 | abc | 1.56 | ab | 5.10 | ab | 1.69 | b | 45.24 | a | 0.55 | ab | 0.58 | bc | 0.95 | b | 1.00 | ab | 4.87 | a | 3.01 | a | 1.56 | b | 1.35 | ab |
| 8 | 6.46 | abc | 0.88 | b | 8.48 | a | 1.60 | b | 78.65 | ab | 0.76 | ab | 0.96 | a | 2.23 | a | 1.06 | ab | 3.37 | a | 3.09 | a | 3.16 | ab | 1.64 | a |
| 9 | 5.48 | bc | 0.97 | b | 3.26 | b | 1.38 | b | 34.91 | b | 0.47 | b | 0.62 | abc | 1.12 | b | 1.05 | ab | 2.42 | a | 2.00 | ab | 1.44 | b | 1.04 | ab |
| 10 | 4.17 | c | 1.01 | b | 3.35 | b | 0.89 | b | 16.31 | b | 0.38 | b | 0.53 | bc | 0.47 | b | 0.60 | b | 2.41 | a | 1.30 | b | 1.12 | b | 0.72 | b |
| Mean | 8.00 | 1.79 | 5.58 | 1.72 | 36.09 | 0.60 | 0.71 | 0.96 | 1.30 | 3.69 | 2.50 | 2.39 | 1.27 | |||||||||||||
| CV (%) | 26.21 | 47.42 | 34.50 | 39.77 | 55.80 | 30.80 | 25.78 | 50.62 | 33.70 | 23.80 | 21.96 | 54.23 | 28.05 | |||||||||||||
| Accession | Sugar | Rel. Sugar | Organic Acids | Rel. Organic Acids | Am. Acids | Sum | S/OA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3746.60 | b | 34.33 | b | 294.21 | b | 107.44 | abc | 69.59 | b | 4252.17 | 12.73 |
| 2 | 6231.56 | ab | 89.64 | a | 347.11 | b | 88.11 | abc | 52.28 | b | 6808.71 | 17.95 |
| 3 | 2847.35 | b | 34.46 | b | 277.31 | b | 97.35 | abc | 66.30 | b | 3322.78 | 10.27 |
| 4 | 11482.88 | a | 61.75 | ab | 805.79 | a | 109.36 | abc | 51.70 | b | 12511.48 | 14.25 |
| 5 | 4246.93 | b | 28.22 | b | 339.39 | b | 138.88 | a | 84.51 | ab | 4837.92 | 12.51 |
| 6 | 3593.97 | b | 42.17 | b | 220.60 | b | 72.53 | bc | 71.69 | b | 4000.97 | 16.29 |
| 7 | 4292.48 | b | 35.88 | b | 331.92 | b | 120.06 | abc | 77.97 | b | 4858.31 | 12.93 |
| 8 | 4284.93 | b | 27.80 | b | 416.52 | b | 106.37 | abc | 113.78 | a | 4949.40 | 10.29 |
| 9 | 2476.08 | b | 37.44 | b | 171.40 | b | 76.39 | bc | 56.96 | b | 2818.27 | 14.45 |
| 10 | 3053.01 | b | 33.34 | b | 342.56 | b | 56.92 | c | 33.89 | c | 3519.73 | 8.91 |
| Mean | 4625.58 | 42.50 | 354.68 | 97.34 | 67.87 | 5187.97 | 13.06 | |||||
| CV% | 56.77 | 45.04 | 48.79 | 25.03 | 32.15 | 54.03 | 21.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spricigo, P.C.; Almeida, L.S.; Ribeiro, G.H.; Correia, B.S.B.; Taver, I.B.; Jacomino, A.P.; Colnago, L.A. Quality Attributes and Metabolic Profiles of Uvaia (Eugenia pyriformis), a Native Brazilian Atlantic Forest Fruit. Foods 2023, 12, 1881. https://doi.org/10.3390/foods12091881
Spricigo PC, Almeida LS, Ribeiro GH, Correia BSB, Taver IB, Jacomino AP, Colnago LA. Quality Attributes and Metabolic Profiles of Uvaia (Eugenia pyriformis), a Native Brazilian Atlantic Forest Fruit. Foods. 2023; 12(9):1881. https://doi.org/10.3390/foods12091881
Chicago/Turabian StyleSpricigo, Poliana Cristina, Luísa Souza Almeida, Gabriel Henrique Ribeiro, Banny Silva Barbosa Correia, Isabela Barroso Taver, Angelo Pedro Jacomino, and Luiz Alberto Colnago. 2023. "Quality Attributes and Metabolic Profiles of Uvaia (Eugenia pyriformis), a Native Brazilian Atlantic Forest Fruit" Foods 12, no. 9: 1881. https://doi.org/10.3390/foods12091881