Enrichment of Lactoferrin and Immunoglobulin G from Acid Whey by Cross-Flow Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Acid Whey Production and Clarification
2.1.1. Filtration—Calculations and Characteristic Numbers
2.1.2. Filtration—Plant Design and Membrane Selection
- Koch K131 (MWCO: 10 kDa, area: 6.7 m2, spacer: 31 mil, Koch Separation Solutions, Wilmington, MA, USA)
- Trisep UB50 (nominal pore size: 30 nm, area: 5.4 m2, spacer: 46 mil, Microdyn-Nadir GmbH, Wiesbaden, Germany)
- Nadir MV020 (nominal pore size: 200 nm, area: 5.4 m2, spacer: 46 mil, Microdyn-Nadir GmbH, Wiesbaden, Germany)
2.1.3. Filtration—Parameter Setting and Optimization of Transmembrane Pressure
2.1.4. Filtration—Enrichment of Biofunctional Proteins
2.2. Analytical Methods
2.3. Evaluation of the Antimicrobial Activity
2.4. Data Processing and Calculations
3. Results and Discussion
3.1. Membrane Selection
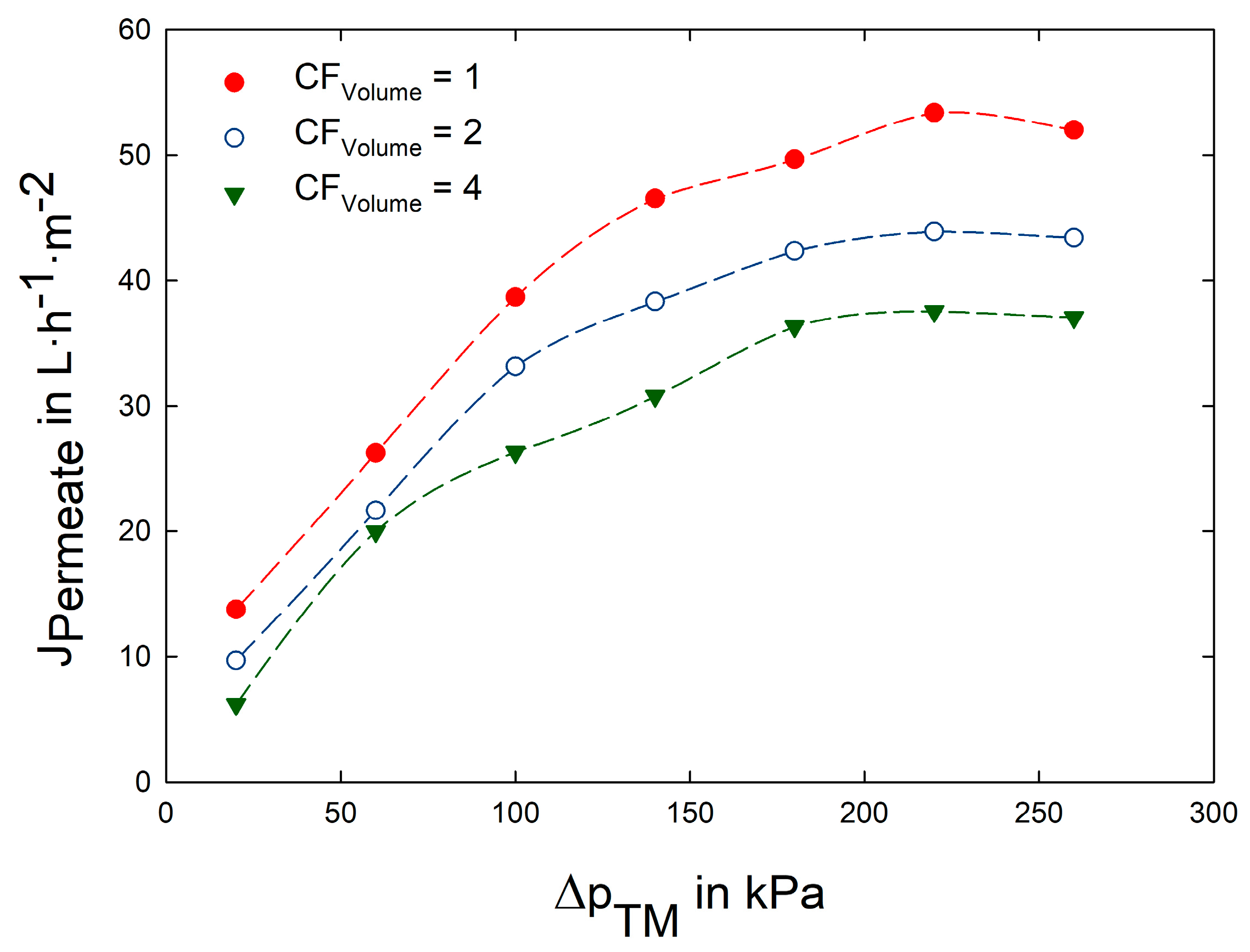
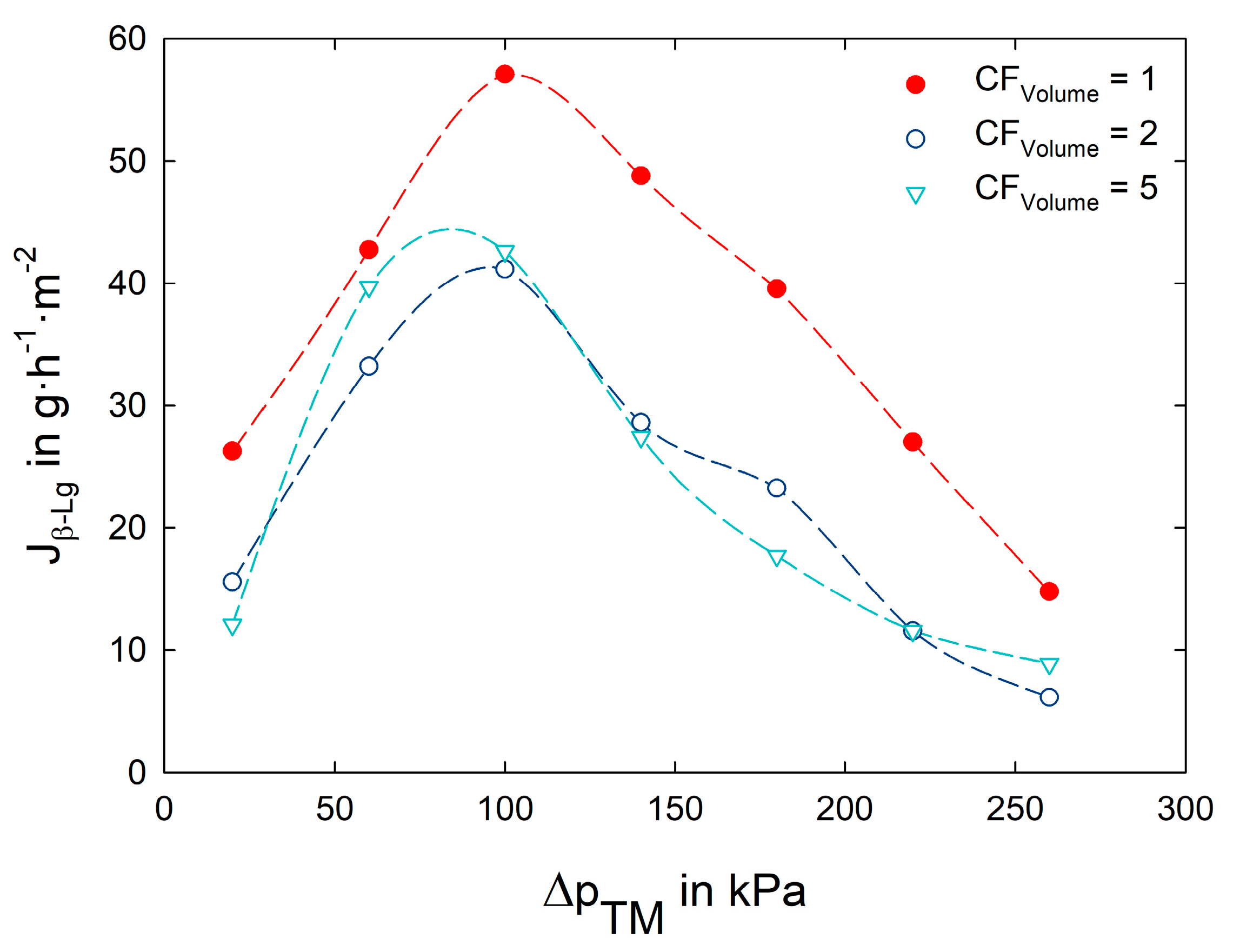
3.2. Impact of Transmembrane Pressure on Flux and Protein Transmission
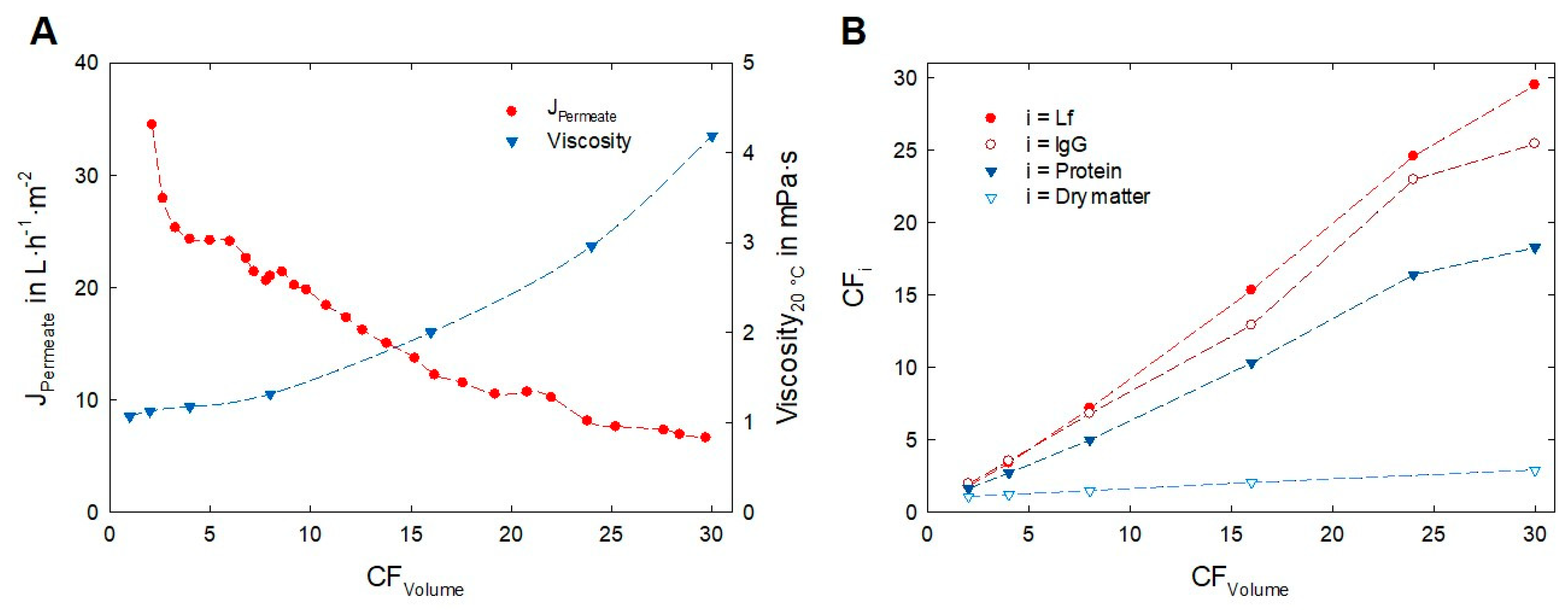
3.3. Enrichment of Lactoferrin and Immunoglobulin G
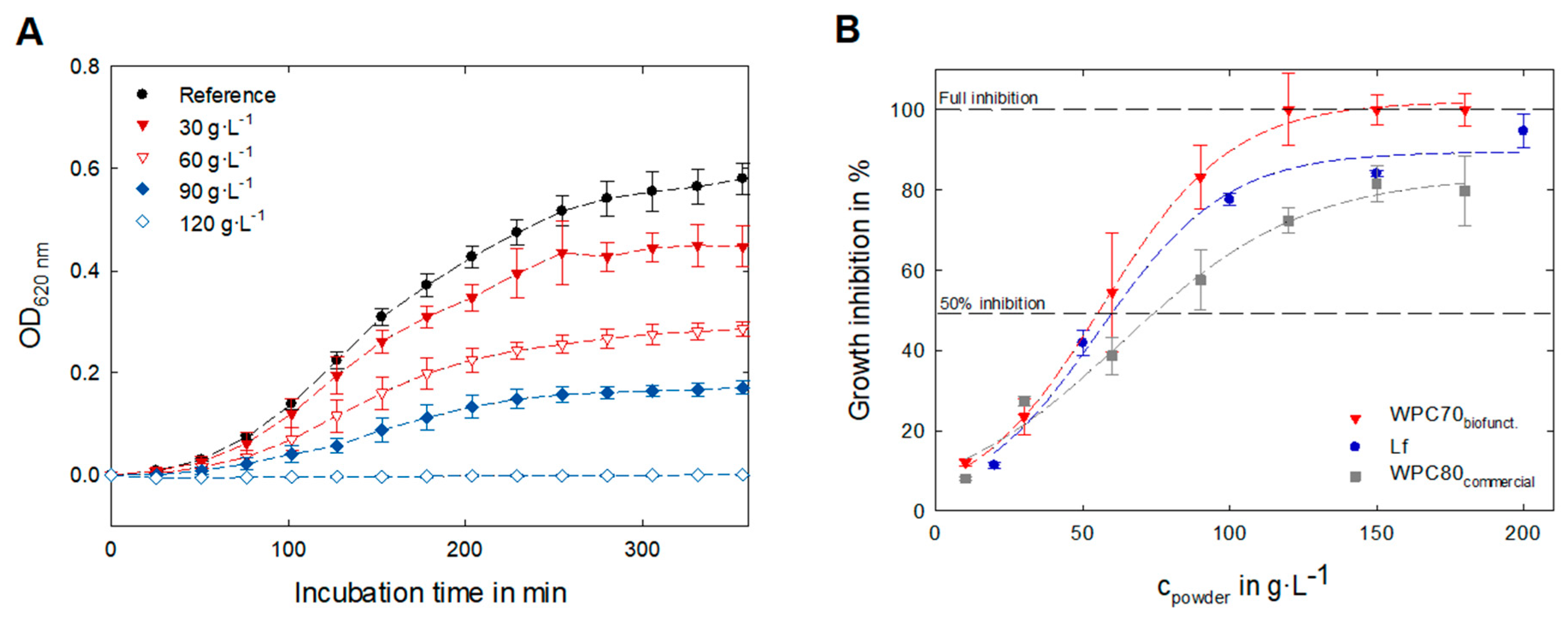
3.4. Evaluation of Biofunctional Properties—Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Domingos, J.M.; Puccio, S.; Martinez, G.A.; Amaral, N.; Reis, M.A.; Bandini, S.; Fava, F.; Bertin, L. Cheese whey integrated valorisation: Production, concentration and exploitation of carboxylic acids for the production of polyhydroxyalkanoates by a fed-batch culture. Chem. Eng. J. 2018, 336, 47–53. [Google Scholar] [CrossRef]
- Rocha-Mendoza, D.; Kosmerl, E.; Krentz, A.; Zhang, L.; Badiger, S.; Miyagusuku-Cruzado, G.; Mayta-Apaza, A.; Giusti, M.; Jiménez-Flores, R.; García-Cano, I. Invited review: Acid whey trends and health benefits. J. Dairy Sci. 2021, 104, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Chandrapala, J.; Duke, M.C.; Gray, S.R.; Weeks, M.; Palmer, M.; Vasiljevic, T. Strategies for maximizing removal of lactic acid from acid whey—Addressing the un-processability issue. Sep. Purif. Technol. 2017, 172, 489–497. [Google Scholar] [CrossRef]
- Hahn, R.; Schulz, P.M.; Schaupp, C.; Jungbauer, A. Bovine whey fractionation based on cation-exchange chromatography. J. Chromatogr. A 1998, 795, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Hirata, Y.; Mukai, F.; Ohtagaki, S. Comparison of Lactoferrin Content in Colostrum Between Different Cattle Breeds. J. Dairy Sci. 1990, 73, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Bylund, G. Dairy Processing Handbook; Revision 1; Tetra Pak® Processing Systems AB: Lund, Sweden, 2015. [Google Scholar]
- Zhang, L. Variations in the chemical composition of human milk. In Human Milk Biochemistry and Infant Formula Manufacturing Technology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 55–111. [Google Scholar]
- Lönnerdal, B.; Jiang, R.; Du, X. Bovine lactoferrin can be taken up by the human intestinal lactoferrin receptor and exert bioactivities. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Tommaselli, G.A.; Morra, I.; Massaro, M.; Formisano, C.; Di Carlo, C. Efficacy and tolerability of oral bovine lactoferrin compared to ferrous sulfate in pregnant women with iron deficiency anemia: A prospective controlled randomized study. Acta Obstet. Gynecol. Scand. 2009, 88, 1031–1035. [Google Scholar] [CrossRef]
- Paesano, R.; Pacifici, E.; Benedetti, S.; Berlutti, F.; Frioni, A.; Polimeni, A.; Valenti, P. Safety and efficacy of lactoferrin versus ferrous sulphate in curing iron deficiency and iron deficiency anaemia in hereditary thrombophilia pregnant women: An interventional study. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Rosa, L.; Lepanto, M.S.; Cutone, A.; Siciliano, R.A.; Paesano, R.; Costi, R.; Musci, G.; Valenti, P. Influence of oral administration mode on the efficacy of commercial bovine Lactoferrin against iron and inflammatory homeostasis disorders. Biometals 2020, 33, 159–168. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; He, J.; Zhu, W. Antibacterial properties of lactoferrin: A bibliometric analysis from 2000 to early 2022. Front. Microbiol. 2022, 13, 947102. [Google Scholar] [CrossRef]
- Fernandes, K.E.; Weeks, K.; Carter, D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020, 64, e02284-19. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Campione, E.; Lanna, C.; Cosio, T.; Rosa, L.; Conte, M.P.; Iacovelli, F.; Romeo, A.; Falconi, M.; Del Vecchio, C.; Franchin, E.; et al. Lactoferrin as Antiviral Treatment in COVID-19 Management: Preliminary Evidence. Int. J. Environ. Res. Public Health 2021, 18, 10985. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, H.; Oda, H.; Yamauchi, K.; Abe, F. Lactoferrin for prevention of common viral infections. J. Infect. Chemother. 2014, 20, 666–671. [Google Scholar] [CrossRef]
- Cornish, J.; Callon, K.E.; Naot, D.; Palmano, K.P.; Banovic, T.; Bava, U.; Watson, M.; Lin, J.; Tong, P.C.; Chen, Q.; et al. Lactoferrin is a potent regulator of bone cell activity and increases bone formation in vivo. Endocrinology 2004, 145, 4366–4374. [Google Scholar] [CrossRef] [PubMed]
- Trybek, G.; Jedliński, M.; Jaroń, A.; Preuss, O.; Mazur, M.; Grzywacz, A. Impact of lactoferrin on bone regenerative processes and its possible implementation in oral surgery—A systematic review of novel studies with metanalysis and metaregression. BMC Oral Health 2020, 20, 232. [Google Scholar] [CrossRef]
- ElSayed, H.L.; Abdelsayed, M.G.; Emara, I.S. Effect of Lactoferrin Supplementation on Appetite and Weight Loss in Obese School Age Children. QJM Int. J. Med. 2021, 114 (Suppl. S1), hcab113-029. [Google Scholar] [CrossRef]
- Sun, J.; Ren, F.; Xiong, L.; Zhao, L.; Guo, H. Bovine lactoferrin suppresses high-fat diet induced obesity and modulates gut microbiota in C57BL/6J mice. J. Funct. Foods 2016, 22, 189–200. [Google Scholar] [CrossRef]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Actor, J.K.; Hwang, S.-A.; Kruzel, M.L. Lactoferrin as a natural immune modulator. Curr. Pharm. Des. 2009, 15, 1956–1973. [Google Scholar] [CrossRef]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Król, J.; Brodziak, A. Antioxidant Activity of Milk and Dairy Products. Animals 2022, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Krolitzki, E.; Schwaminger, S.P.; Pagel, M.; Ostertag, F.; Hinrichs, J.; Berensmeier, S. Current practices with commercial scale bovine lactoferrin production and alternative approaches. Int. Dairy J. 2022, 126, 105263. [Google Scholar] [CrossRef]
- Fortune Business Insights. Bovine Lactoferrin Markez Size, Share & COVID-19 Impact Analysis: By Product (Spray-Dried and Freeze-Dried), By Applcation (Infant Formula, Pharmaceuticals, Dietary Supplements, Food, and Others), and Regional Forecast, 2022–2029. 2021. Available online: https://www.fortunebusinessinsights.com/industry-reports/bovine-lactoferrin-market-101656 (accessed on 15 May 2023).
- EC No 258/97 2012; Authorising the Placing on the Market of Bovine Lactoferrin as a Novel Food Ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council (FrieslandCampina). European Union: Brussels, Belgium, 2012.
- Jańczuk, A.; Brodziak, A.; Czernecki, T.; Król, J. Lactoferrin-The Health-Promoting Properties and Contemporary Application with Genetic Aspects. Foods 2022, 12, 70. [Google Scholar] [CrossRef]
- Stelwagen, K.; Carpenter, E.; Haigh, B.; Hodgkinson, A.; Wheeler, T.T. Immune components of bovine colostrum and milk. J. Anim. Sci. 2009, 87 (Suppl. S13), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.K.; Mahalanabis, D.; Ashraf, H.; Unicomb, L.; Eeckels, R.; Tzipori, S. Hyperimmune cow colostrum reduces diarrhoea due to rotavirus: A double-blind, controlled clinical trial. Acta Paediatr. 1995, 84, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.A.; Casswall, T.H.; Mahalanabis, D.; Alam, N.H.; Albert, M.J.; Brüssow, H.; Fuchs, G.J.; Hammerström, L. Successful treatment of rotavirus diarrhea in children with immunoglobulin from immunized bovine colostrum. Pediatr. Infect. Dis. J. 1998, 17, 1149–1154. [Google Scholar] [CrossRef]
- Serrano, G.; Kochergina, I.; Albors, A.; Diaz, E.; Oroval, M.; Hueso, G.; Serrano, J.M. Liposomal Lactoferrin as Potential Preventative and Cure for COVID-19. IJRHS 2020, 8, 8–15. [Google Scholar] [CrossRef]
- Bacchin, P.; Aimar, P.; Field, R. Critical and sustainable fluxes: Theory, experiments and applications. J. Membr. Sci. 2006, 281, 42–69. [Google Scholar] [CrossRef]
- Steinhauer, T.; Lonfat, J.; Hager, I.; Gebhardt, R.; Kulozik, U. Effect of pH, transmembrane pressure and whey proteins on the properties of casein micelle deposit layers. J. Membr. Sci. 2015, 493, 452–459. [Google Scholar] [CrossRef]
- IDF 185:2002; Milk and Milk Products—Determination of Nitrogen Content—Routine Method Using Combustion According to the Dumas Principle. ISO: Geneva, Switzerland, 2002.
- ISO 5534:2004; Käse und Schmelzkäse—Bestimmung der Gesamttrockenmasse (Referenzverfahren). Beuth Verlag GmbH: Berlin, Germany, 2004.
- Ostertag, F.; Schmidt, C.M.; Berensmeier, S.; Hinrichs, J. Development and validation of an RP-HPLC DAD method for the simultaneous quantification of minor and major whey proteins. Food Chem. 2021, 342, 128176. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, M.; Kulozik, U. Milk protein fractionation by spiral-wound microfiltration membranes in diafiltration mode—Influence of feed protein concentration and composition on the filtration performance. Int. Dairy J. 2020, 102, 104606. [Google Scholar] [CrossRef]
- Konrad, G.; Kleinschmidt, T.; Faber, W. Ultrafiltration flux of acid whey obtained by lactic acid fermentation. Int. Dairy J. 2012, 22, 73–77. [Google Scholar] [CrossRef]
- Morison, K.R.; Mackay, F.M. Viscosity of lactose and whey protein solutions. Int. J. Food Prop. 2001, 4, 441–454. [Google Scholar] [CrossRef]
- Mercadante, D.; Melton, L.D.; Norris, G.E.; Loo, T.S.; Williams, M.A.; Dobson, R.C.; Jameson, G.B. Bovine β-lactoglobulin is dimeric under imitative physiological conditions: Dissociation equilibrium and rate constants over the pH range of 2.5–7.5. Biophys. J. 2012, 103, 303–312. [Google Scholar] [CrossRef] [PubMed]
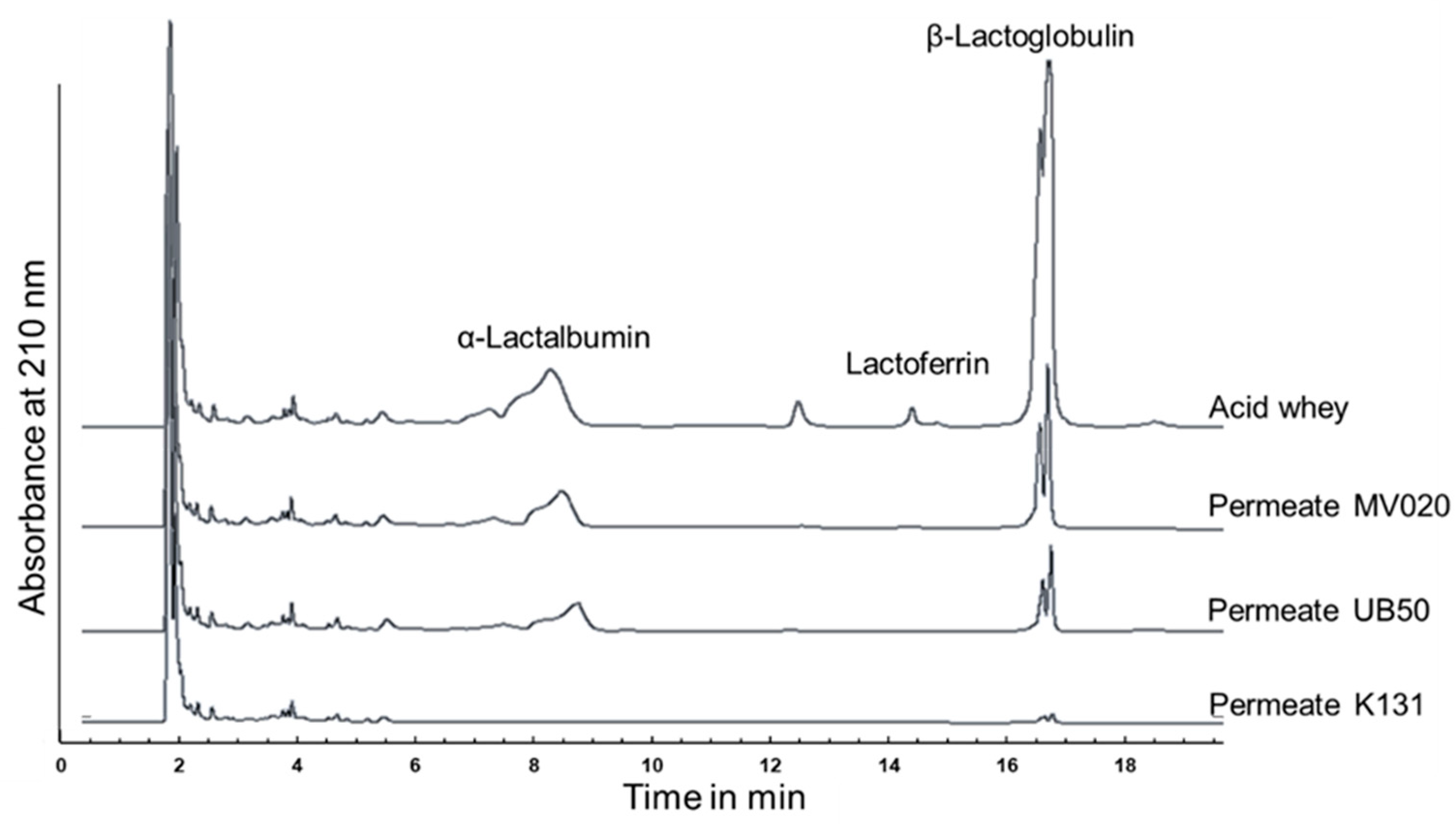
| Membrane | RetLf in % | TrProtein in % | JPermeate in L·m−2·h−1 |
|---|---|---|---|
| MV020 | >95% | 17 ± 4 | 38.6 ± 0.9 |
| UB50 | >95% | 12 ± 2 | 39.6 ± 0.8 |
| K131 | >95% | <5 | 14.1 ± 0.6 |
| Acid Whey | WPC70biofunct. | CFVolume/i | ||
|---|---|---|---|---|
| Volume | in L | 800 | 26 | ×30.8 |
| Viscosity20°C | in mPa·s | 1.063 ± 0.003 | 4.19 ± 0.06 | |
| Dry matter | in g·L−1 | 62.7 ± 0.7 | 181.7 ± 0.8 | ×2.90 |
| Protein | in g·L−1 | 7.0 ± 0.4 | 127.2 ± 0.7 | ×18.2 |
| Lf | in g·L−1 | 0.17 ± 0.01 | 5.1 ± 0.1 | ×29.5 |
| IgG | in g·L−1 | 0.24 ± 0.03 | 6.13 ± 0.44 | ×25.3 |
| α-La | in g·L−1 | 0.89 ± 0.05 | 13.10 ± 0.23 | ×14.8 |
| ß-Lg | in g·L−1 | 3.71 ± 0.18 | 99.8 ± 1.4 | ×26.9 |
| IC50 | ||
|---|---|---|
| cpowder in g∙L−1 | cLf in g∙L−1 | |
| WPC70biofunct. | 55.6 | 1.5 |
| Lf | 60.7 | 58.9 |
| WPC80commercial | 75.5 | ~0.6 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostertag, F.; Hinrichs, J. Enrichment of Lactoferrin and Immunoglobulin G from Acid Whey by Cross-Flow Filtration. Foods 2023, 12, 2163. https://doi.org/10.3390/foods12112163
Ostertag F, Hinrichs J. Enrichment of Lactoferrin and Immunoglobulin G from Acid Whey by Cross-Flow Filtration. Foods. 2023; 12(11):2163. https://doi.org/10.3390/foods12112163
Chicago/Turabian StyleOstertag, Fabian, and Jörg Hinrichs. 2023. "Enrichment of Lactoferrin and Immunoglobulin G from Acid Whey by Cross-Flow Filtration" Foods 12, no. 11: 2163. https://doi.org/10.3390/foods12112163
APA StyleOstertag, F., & Hinrichs, J. (2023). Enrichment of Lactoferrin and Immunoglobulin G from Acid Whey by Cross-Flow Filtration. Foods, 12(11), 2163. https://doi.org/10.3390/foods12112163








