A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
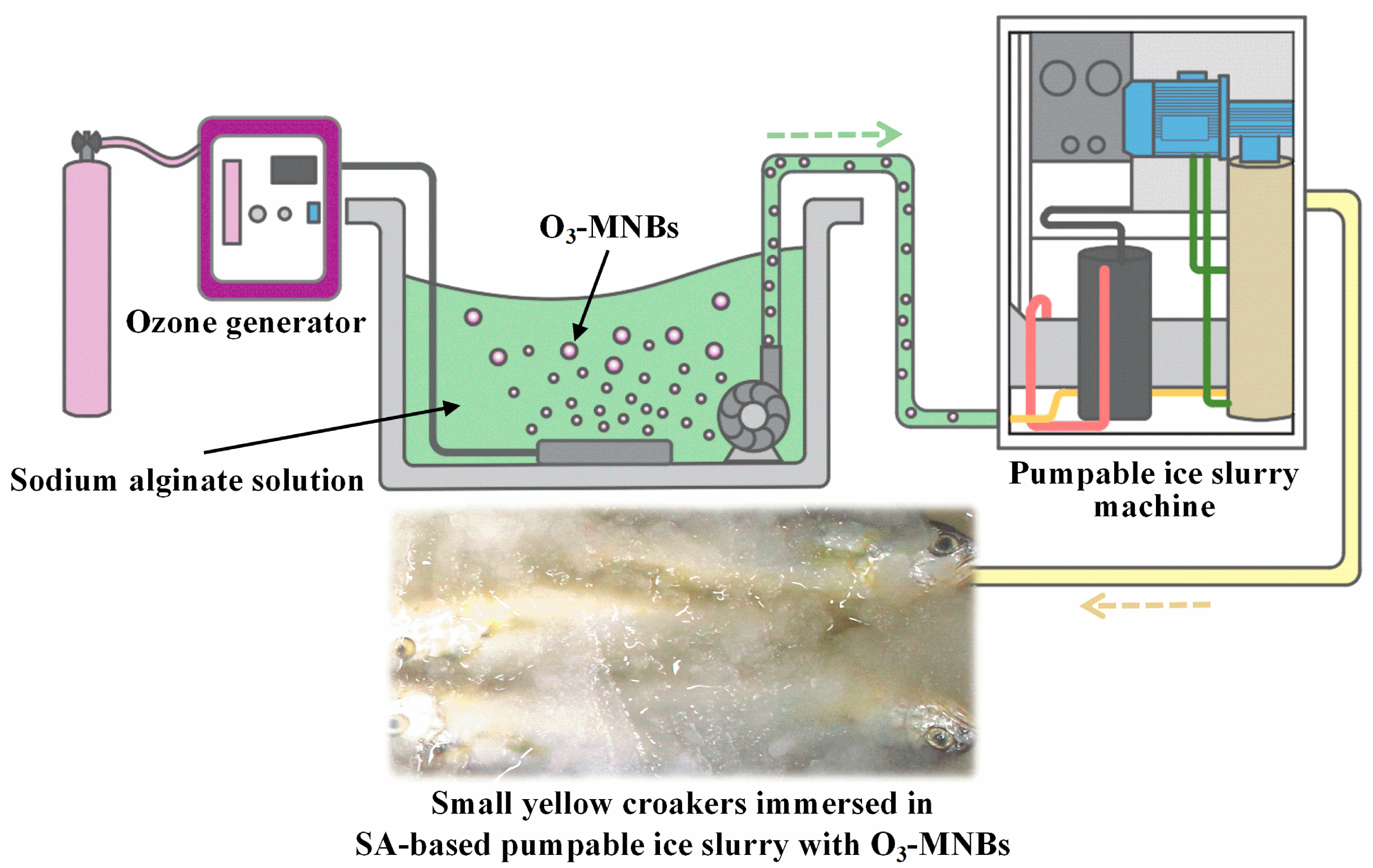
2.2. Preparation of Novel PIS
2.3. Characterization of PIS Production
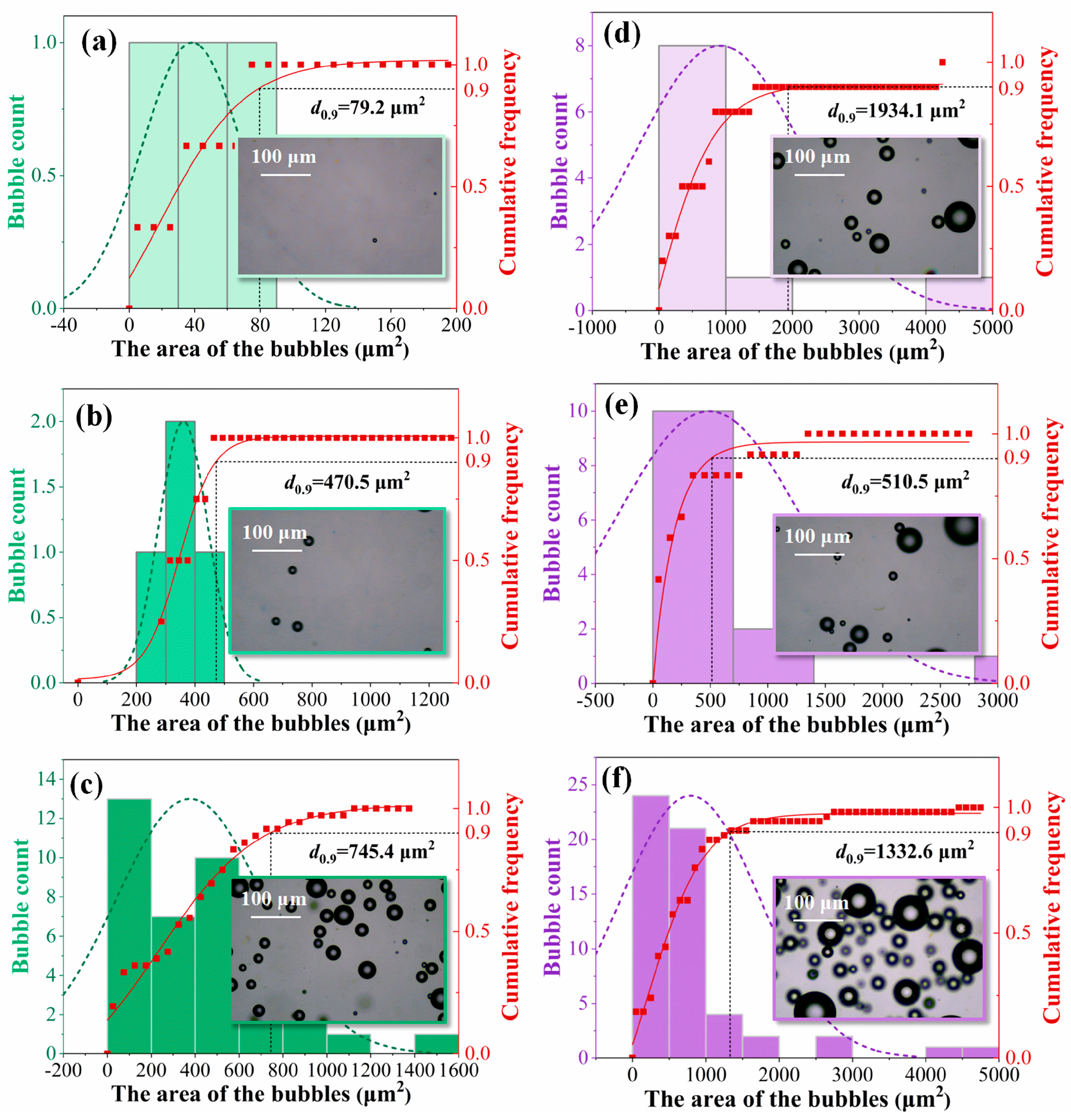
2.3.1. Microscopic Analysis
2.3.2. Freezing Characteristics
2.4. Preservation of Small Yellow Croaker
2.4.1. Preservation Rate of O3 in PIS
2.4.2. Freshness Indicators of Fish Samples
Measurement of Total Plate Counts
Measurement of pH
Measurement of Total Volatile Basic Nitrogen Content
Measurement of Thiobarbituric Acid Content
2.5. Data Analysis
3. Results and Discussion
3.1. Bubble Distribution
3.2. Freezing Characteristics of SA and SA-O3 Solutions
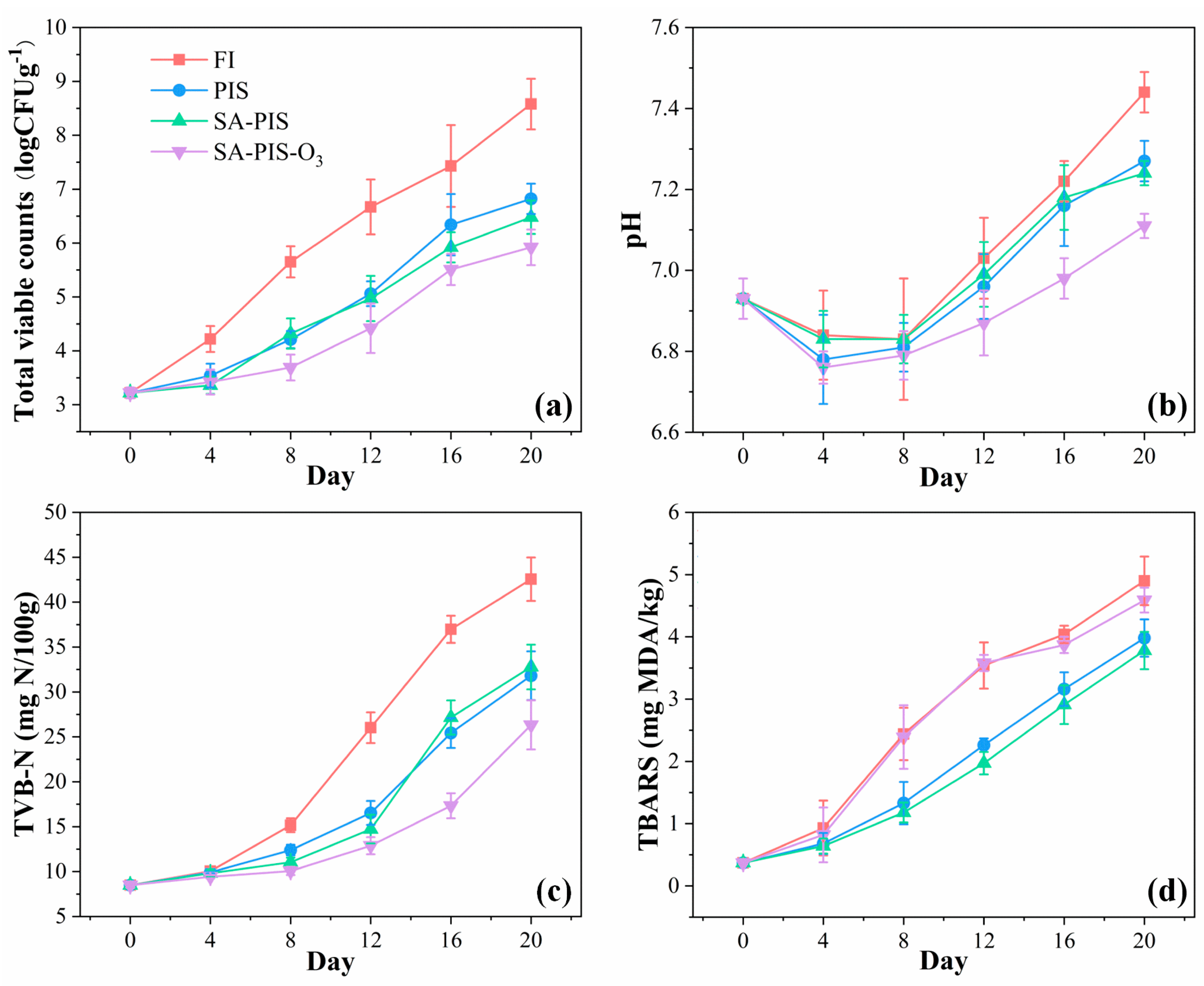
3.3. Preservation of Small Yellow Croaker
3.3.1. Rate of O3 Preservation
3.3.2. Freshness Indicators
Microbiological Analysis
pH
TVB-N
TBA Reactive Substances
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esua, O.J.; Sun, D.-W.; Cheng, J.-H.; Wang, H.; Chen, C. Hybridising plasma functionalized water and ultrasound pretreatment for enzymatic protein hydrolysis of Larimichthys polyactis: Parametric screening and optimization. Food Chem. 2022, 385, 132677. [Google Scholar]
- Choi, M.-J.; Kim, D.-H. Assessment and management of small yellow croaker (Larimichthys polyactis) stocks in South Korea. Sustainability 2020, 12, 8257. [Google Scholar] [CrossRef]
- Karim, N.U.; Nasir, N.; Arifin, B.; Ismail, M. Effect of salt and ozonized-slurry ice on the quality indices of tiger grouper (Epinephelus fuscoguttatus). J. Sustain. Sci. Manag. 2015, 10, 97–102. [Google Scholar]
- Aubourg, S.P.; Losada, V.; Gallardo, J.M.; José, M.; Miranda, J.M.; Velázquez, J.B. On-board quality preservation of megrim (Lepidorhombus whiffiagonis) by a novel ozonised-slurry ice system. Eur. Food Res. Technol. 2006, 223, 232–237. [Google Scholar] [CrossRef][Green Version]
- Lin, H.M.; Deng, S.G.; Huang, S.B.; Guo, H. Effects of precooling with slurry ice on the quality and microstructure of anglerfish (Lophius americanus) liver. J. Food Process Eng. 2016, 39, 3–10. [Google Scholar] [CrossRef]
- FAO. Fisheries and Aquaculture. In Global Production by Production Source Quantity (1950–2020); FAO, Food and Agriculture Organization, Fisheries and Aquaculture Department: Roma, Italy, 2022. [Google Scholar]
- Hozumi, T.; Saito, A.; Okawa, S.; Matsumura, T. Effect of bubble nuclei on freezing of supercooled water. Int. J. Refrig. 2002, 25, 243–249. [Google Scholar] [CrossRef]
- Zhang, X.; Inada, T.; Tezuka, A. Ultrasonic-induced nucleation of ice in water containing air bubbles. Ultrason. Sonochem. 2003, 10, 71–76. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Delgado, A.; Sun, D.-W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011, 44, 2915–2921. [Google Scholar] [CrossRef]
- Fu, X.; Belwal, T.; Cravotto, G.; Luo, Z. Sono-physical and sono-chemical effects of ultrasound: Primary applications in extraction and freezing operations and influence on food components. Ultrason. Sonochem. 2020, 60, 104726. [Google Scholar] [CrossRef]
- Cheng, L.; Sun, D.-W.; Zhu, Z.; Zhang, Z. Emerging techniques for assisting and accelerating food freezing processes: A review of recent research progresses. Crit. Rev. Food Sci. Nutr. 2017, 57, 769–781. [Google Scholar]
- Zhu, Z.; Sun, D.-W.; Zhang, Z.; Li, Y.; Cheng, L. Effects of micro-nano bubbles on the nucleation and crystal growth of sucrose and maltodextrin solutions during ultrasound-assisted freezing process. LWT-Food Sci. Technol. 2018, 92, 404–411. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Tung, V.P.; Truong, T.; Bansal, N.; Bhandari, B. Impact of in-situ CO2 nano-bubbles generation on freezing parameters of selected liquid foods. Food Biophys. 2020, 15, 97–112. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Z.; Zhu, Z.; Sun, D.-W. Effects of nano-bubbles and constant/variable-frequency ultrasound-assisted freezing on freezing behaviour of viscous food model systems. J. Food Eng. 2021, 292, 110284. [Google Scholar] [CrossRef]
- Zhang, R.; Cheng, Z.; Ding, F.; Hua, L.; Fang, Y.; Han, Z.; Shi, J.; Zou, X.; Xiao, J. Improvements in chitosan-based slurry ice production and its application in precooling and storage of Pampus argenteus. Food Chem. 2022, 393, 133266. [Google Scholar] [CrossRef] [PubMed]
- Okpala, C.O.R. Investigation of quality attributes of ice-stored Pacific white shrimp (Litopenaeus vannamei) as affected by sequential minimal ozone treatment. LWT-Food Sci. Technol. 2014, 57, 538–547. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Sunoj, S.; Manikantan, M.R.; Kothakota, A.; Hebbar, K.B. Application and kinetics of ozone in food preservation. Ozone-Sci. Eng. 2017, 39, 115–126. [Google Scholar] [CrossRef]
- Campos, C.A.; Rodríguez, Ó.; Losada, V.; Aubourg, S.P.; Barros-Velásquez, J. Evaluation of an ozone-slurry ice combined refrigeration system for the storage of farmed turbot (Psetta maxima). Food Chem. 2006, 97, 223–230. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Huang, J.; Deng, S.; Huang, Y. Combining ozone and slurry ice to maximize shelf-life and quality of bighead croaker (Collichthys niveatus). J. Food Sci. Technol. 2016, 53, 3651–3660. [Google Scholar] [CrossRef][Green Version]
- Bono, G.; Vitale, S.; Okpala, C.O.; Ferrantelli, V.; Noto, A.D.; Costa, A.; Di, B.C.; Monaco, D.L. Effects of different ozonized slurry-ice treatments and superchilling storage (−1 °C) on microbial spoilage of two important pelagic fish species. Food Sci. Nutr. 2017, 5, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lan, W.; Shen, J.; Xu, Z.; Xie, J. Combining ozone and slurry ice treatment to prolong the shelf-life and quality of large yellow croaker (Pseudosciaena crocea). LWT-Food Sci. Technol. 2022, 154, 112615. [Google Scholar] [CrossRef]
- Pati1, S.; Sarkar, T.; Sheikh, H.I.; Bharadwaj, K.K.; Mohapatra, P.K.; Chatterji, A.; Dash, B.P.; Edinur, H.A.; Nelson, B.R. γ-irradiated chitosan from carcinoscorpius rotundicauda (latreille, 1802) improves the shelf life of refrigerated aquatic products. Front. Mar. Sci. 2021, 8, 664961. [Google Scholar] [CrossRef]
- Watson, R.A.; Nowara, G.B.; Hartmann, K.; Green, B.S.; Tracey, S.R.; Carter, C.G. Marine foods sourced from farther as their use of global ocean primary production increases. Nat. Commun. 2015, 6, 7365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yates, R.S.; Stenstrom, M.K. Gravimetric sampling procedure for aqueous ozone concentrations. Water Res. 2000, 34, 1413–1416. [Google Scholar] [CrossRef]
- Quan, T.H.; Benjakul, S.; Hozzein, W.N. Quality and storage stability of fish tofu as affected by duck albumen hydrolysate-epigalocatechin gallate conjugate. LWT-Food Sci. Technol. 2020, 120, 108927. [Google Scholar] [CrossRef]
- Wen, H.; Ou, C.; Tang, H.; Sang, S.; Du, Y.; Chen, J. Development, Characterization and Application of a three-layer intelligent ph-sensing indicator based on Bromocresol Green (BCG) for monitoring fish freshness. J. Ocean. Univ. China 2023, 22, 565–575. [Google Scholar] [CrossRef]
- Yuan, P.; Deng, S.; Hatab, S.; Ning, Y.; Huo, J. Comparative evaluation of the quality changes in squid (Ommastrephes bartrami) during flake and slurry ice storage. Emir. J. Food Agric. 2017, 29, 339–345. [Google Scholar] [CrossRef][Green Version]
- Bensid, A.; Ucar, Y.; Bendeddouche, B.; Oezogul, F. Effect of the icing with thyme, oregano and clove extracts on quality parameters of gutted and beheaded anchovy (Engraulis encrasicholus) during chilled storage. Food Chem. 2014, 145, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Flores, E.E.; Rodrigues, R.C.; Hertz, P.F.; Norea, C. Effect of deacetylation degree of chitosan on rheological properties and physical chemical characteristics of genipin-crosslinked chitosan beads. Food Hydrocoll. 2020, 106, 105876. [Google Scholar] [CrossRef]
- Xu, B.; Azam, R.; Wang, B.; Zhang, M.; Bhandari, B. Effect of infused CO2 in a model solid food on the ice nucleation during ultrasound-assisted immersion freezing. Int. J. Refrig. 2019, 108, 53–59. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.; Maraqa, M.A.; Hamouda, M.A.; Hassan, A.A.; Ali, J.; Jung, J. A critical review of the recent developments in micro-nano bubbles applications for domestic and industrial wastewater treatment. Alex Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Gezgin, Z.; Lee, T.-C.; Huang, Q. AFM imaging of extracellular ice nucleators. J. Food Sci. 2021, 85, 3355–3362. [Google Scholar] [CrossRef]
- Wei, P.; Huang, C.; Lee, K.W. Nucleation of bubbles on a solidification front—Experiment and analysis. Metall. Mater. Trans. B 2003, 34, 321–332. [Google Scholar] [CrossRef]
- Hu, F.; Sun, D.W.; Gao, W.; Zhang, Z.; Zeng, X.; Han, Z. Effects of pre-existing bubbles on ice nucleation and crystallization during ultrasound-assisted freezing of water and sucrose solution. Innov. Food Sci. Emerg. 2013, 20, 161–166. [Google Scholar] [CrossRef]
- Daghooghi-Mobarakeh, H.; Subramanian, V.; Phelan, P.E. Experimental study of water freezing process improvement using ultrasound. Appl. Therm. Eng. 2022, 202, 1359–4311. [Google Scholar] [CrossRef]
- Chow, R.; Blindt, R.; Chivers, R.; Povey, M. The sonocrystallisation of ice in sucrose solutions: Primary and secondary nucleation. Ultrasonics 2003, 41, 595–604. [Google Scholar] [CrossRef]
- Wang, C.; Wu, J.; Wang, H.; Zhang, Z. Classical nucleation theory of ice nucleation: Second-order corrections to thermodynamic parameters. J. Chem. Phys. 2021, 154, 234503. [Google Scholar] [CrossRef]
- Matsumoto, K.; Zhang, S.; Sekine, K.; Kubota, H. Investigation on influence of dimensions of ice containing ozone micro-bubbles on characteristics of ozone concentration. Int. J. Refrig. 2016, 66, 41–49. [Google Scholar] [CrossRef]
- Pastoriza, L.; Bernardez, M.; Sampedro, G.; Cabo, M.L.; Herrera, J. The use of water and ice with bactericide to prevent onboard and onshore spoilage of refrigerated megrim (Lepidorhombus whiffiagonis). Food Chem. 2008, 110, 31–38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghaly, A.E.; Brooks, M.S.; Dave, D.; Budge, S. Fish spoilage mechanisms and preservation techniques: Review. Am. J. Appl. Sci. 2010, 7, 859–877. [Google Scholar] [CrossRef][Green Version]
- International Commission on Microbiological Specifications for Foods (ICMSF). Microorganisms in Foods 6: Microbial Ecology of Food Commodities, Blackie Academic & Professional; International Commission on Microbiological Specifications for Foods (ICMSF): Baltimore, MD, USA, 1998. [Google Scholar]
- Pandiselvam, R.; Subhashinib, S.; Banuu Priyac, E.P.; Kothakotad, A.; Ramesha, S.V.; Shahi, S. Ozone based food preservation: A promising green technology for enhanced food safety. Ozone-Sci. Eng. 2019, 41, 17–34. [Google Scholar] [CrossRef]
- Huang, H.; Sun, W.; Xiong, G.; Shi, L.; Jiao, C.; Wu, W.; Li, X.; Qiao, Y.; Liao, L.; Ding, A.; et al. Effects of HVEF treatment on microbial communities and physicochemical properties of catfish fillets during chilled storage. LWT-Food Sci. Technol. 2020, 131, 109667. [Google Scholar] [CrossRef]
- Campos, C.A.; Rodríguez, Ó.; Losada, V.; Aubourg, S.P.; Barros-Velázquez, J. Effects of storage in ozonised slurry ice on the sensory and microbial quality of sardine (Sardina pilchardus). Int. J. Food Microbiol. 2005, 103, 121–130. [Google Scholar] [CrossRef][Green Version]
- Okpala, C.; Choo, W.S.; Dykes, G.A. Quality and shelf life assessment of pacific white shrimp (Llitopenaeus vannamei) freshly harvested and stored on ice. LWT-Food Sci Technol. 2014, 55, 110–116. [Google Scholar] [CrossRef]
- Binsi, P.K.; Nayak, N.; Sarkar, P.C.; Sahu, U.; Ninan, G.; Ravishankar, C.N. Comparative evaluation of gumarabic coating and vacuum packaging on chilled storage characteristics of indian mackerel (Rastrelliger kanagurta). J. Food Sci. Technol. 2016, 53, 1889–1898. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, L.; Lan, W.; Pu, T.; Zhou, Y.; Xie, J. Combining slightly acidic electrolyzed water and slurry ice to prolong the shelf-life of mackerel (Pneumatophorus japonicus). J. Food Process. Preserv. 2021, 45, 100706. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, W.; Liu, J.; Xie, J. Effects of precooling with slurry ice on the freshness of farmed perch (Lateolabrax japonicus) during logistics process. J. Aquat. Food Prod. Technol. 2021, 30, 162–175. [Google Scholar] [CrossRef]
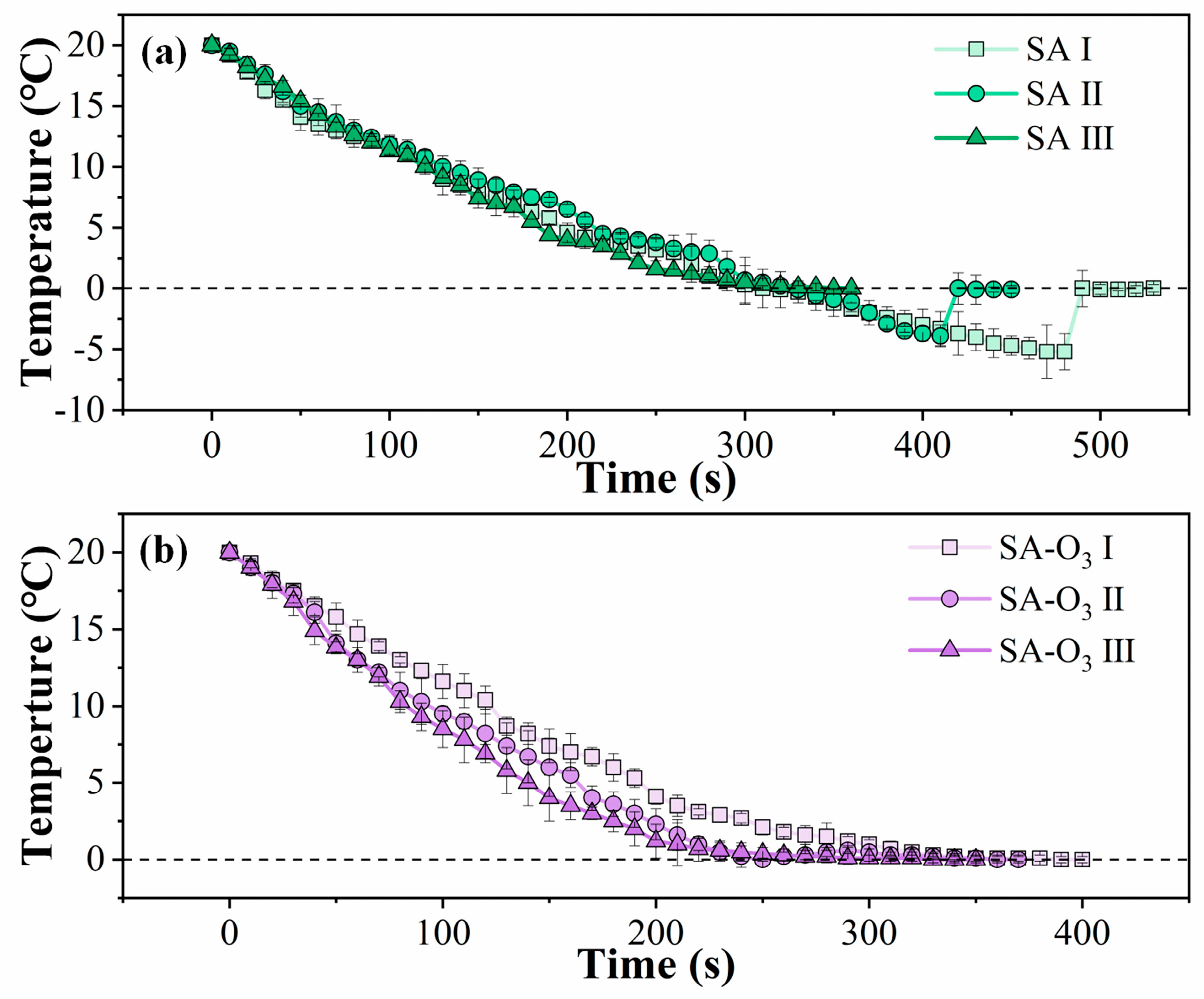

| Solution | Nucleation Temperature (°C) | Supercooling Degree (°C) | Total Freezing Time (s) |
|---|---|---|---|
| SA Ⅰ | −5.2 ± 1.4 c | 5.2 ± 1.4 | 473.3 ± 20.8 a |
| SA Ⅱ | −3.9 ± 0.9 b | 3.9 ± 0.9 | 406.7 ± 5.8 b |
| SA Ⅲ | 0.0 ± 0.3 a | / | 343.3 ± 5.8 cd |
| SA-O3 Ⅰ | 0.0 ± 0.2 a | / | 396.7 ± 11.5 b |
| SA-O3 Ⅱ | 0.0 ± 0.2 a | / | 363.3 ± 5.8 c |
| SA-O3 Ⅲ | 0.0 ± 0.2 a | / | 316.7 ± 5.8 d |
| Ice Type | Fish Sample | Storage Time | Freshness Indicators | Reference | |||
|---|---|---|---|---|---|---|---|
| TVC (log CFU/g) | pH | TVB-N (mg/100 g) | TBARS (mg MDA/kg) | ||||
| FI | squid | Day 15 | 6.61 | - | 40 | - | [27] |
| mackerel | Day 21 | >7.0 | 7.2 | 31.18 | 3.8 | [47] | |
| large yellow croaker | Day 21 | 8.7 | 7.42 | 25.82 | 3.5 | [21] | |
| PIS | farmed perch | Day 20 | 5.60 | - | 32.5 | - | [48] |
| squid | Day 15 | 4.74 | - | 13.26 | - | [27] | |
| PIS combined with slightly acidic electrolyzed water | mackerel | Day 24 | 5.8 | 6.58 | 7.5 | 2.51 | [47] |
| PIS combined with chitosan | silver pomfret | Day 20 | 5.28 | 6.76 | 24.78 | 4.28 | [15] |
| PIS combined with O3 | sardine | Day 22 | - | 6.07 | 32.22 | - | [18] |
| large yellow croaker | Day 21 | 6.85 | 7.28 | 7.09 | 3.10 | [21] | |
| small yellow croaker | Day 20 | 5.92 | 7.11 | 26.32 | 4.59 | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Cheng, Z.; Liang, Y.; Hu, X.; Shen, T.; Li, Y.; Han, Z.; Zhang, X.; Zou, X. A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis. Foods 2023, 12, 2206. https://doi.org/10.3390/foods12112206
Zhang R, Cheng Z, Liang Y, Hu X, Shen T, Li Y, Han Z, Zhang X, Zou X. A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis. Foods. 2023; 12(11):2206. https://doi.org/10.3390/foods12112206
Chicago/Turabian StyleZhang, Roujia, Zhiming Cheng, Yuting Liang, Xuetao Hu, Tingting Shen, Yanxiao Li, Zhi Han, Xinai Zhang, and Xiaobo Zou. 2023. "A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis" Foods 12, no. 11: 2206. https://doi.org/10.3390/foods12112206
APA StyleZhang, R., Cheng, Z., Liang, Y., Hu, X., Shen, T., Li, Y., Han, Z., Zhang, X., & Zou, X. (2023). A Novel Strategy for Accelerating Pumpable Ice Slurry Production with Ozone Micro–Nano Bubbles and Extending the Shelf Life of Larimichthys polyactis. Foods, 12(11), 2206. https://doi.org/10.3390/foods12112206







