Minimally Processed Vegetables in Brazil: An Overview of Marketing, Processing, and Microbiological Aspects
Abstract
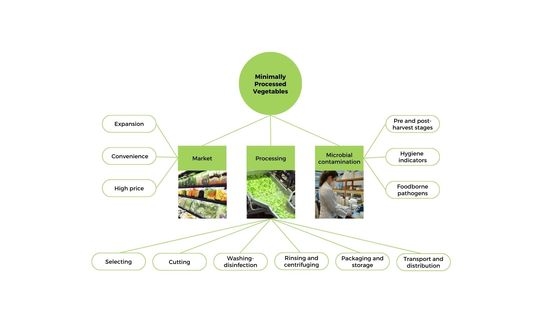
:1. Introduction
2. Market of MPVs in Brazil
3. Processing of MPVs
4. Microbiological Quality and Safety of MPVs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brasil. Ministério da Saúde; Secretaria de Atenção à Saúde; Departamento de Atenção Básica. Guia Alimentar para a População Brasileira, 2nd ed.; Ministério da Saúde: Brasília, Brazil, 2014; p. 156.
- Saini, R.K.; Ko, E.Y.; Keum, Y.S. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Maffei, D.F.; Silveira, M.A.; Silva, M.B.R.D.; Moreira, D.A.; Lourenço, F.R.; Schaffner, D.W.; Franco, B.D.G.M. Consumption data and consumer handling practices of leafy greens in the city of São Paulo, Brazil: Useful information for quantitative microbiological consumer phase risk assessments. Food Prot. Trends 2020, 40, 224–231. [Google Scholar]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Fruit and Vegetables—Your Dietary Essentials. The International Year of Fruits and Vegetables, Background Paper. Rome. 2021. Available online: https://www.fao.org/publications/card/en/c/CB2395EN (accessed on 10 January 2023).
- Alvarenga, A.L.B.; Toledo, J.C.D.; Paulillo, L.F.D.O. Qualidade e segurança de vegetais minimamente processados: Proposta de estruturas de governança entre os agentes da cadeia e os sinais da qualidade. Gest. Prod. 2014, 21, 341–354. [Google Scholar] [CrossRef] [Green Version]
- Vieira, S.L.V.; da Silva, I.C.P. Alimentos minimamente processados: Novo perfil de escolha do consumidor. Arq. MUDI 2017, 21, 26–38. Available online: https://periodicos.uem.br/ojs/index.php/ArqMudi/article/view/37199 (accessed on 10 January 2023). [CrossRef] [Green Version]
- Maldonade, I.R.; Ginani, V.C.; Riquette, R.F.R.; Gurgel-Gonçalves, R.; Mendes, V.S.; Machado, E.R. Good manufacturing practices of minimally processed vegetables reduce contamination with pathogenic microorganisms. Rev. Inst. Med. Trop. São Paulo 2019, 61, e14. [Google Scholar] [CrossRef] [Green Version]
- de Corato, U. Improving the shelf-life and quality of fresh and minimally-processed fruits and vegetables for a modern food industry: A comprehensive critical review from the traditional technologies into the most promising advancements. Crit. Rev. Food Sci. Nutr. 2020, 60, 940–975. [Google Scholar] [CrossRef]
- Santos, T.S.; Campos, F.B.; Padovani, N.F.D.A.; Dias, M.; Mendes, M.A.; Maffei, D.F. Assessment of the microbiological quality and safety of minimally processed vegetables sold in Piracicaba, SP, Brazil. Lett. Appl. Microbiol. 2020, 71, 187–194. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; López-Gámez, G.M.; Domínguez-Avila, J.A.; González-Aguilar, G.A.; Soliva-Fortuny, R.; Ayala-Zavala, J.F. Minimal processing. In Postharvest Technology of Perishable Horticultural Commodities; Woodhead Publishing: Querétaro, Mexico, 2019; pp. 353–374. [Google Scholar] [CrossRef]
- de Corato, U.; Cancellara, F.A. Measures, technologies, and incentives for cleaning the minimally processed fruits and vegetables supply chain in the Italian food industry. J. Clean. Prod. 2019, 237, 117735. [Google Scholar] [CrossRef]
- Perez, R.; Ramos, A.M.; Binoti, M.L.; Sousa, P.H.M.D.; Machado, G.D.M.; Cruz, I.B. Perfil dos consumidores de hortaliças minimamente processadas de Belo Horizonte. Hortic. Bras. 2008, 26, 441–446. [Google Scholar] [CrossRef]
- Finger, J.A.F.F.; Costa, D.A.; Alves, V.F.; Baroni, W.S.G.V.; Malheiros, P.S.; Alves, E.A.; Maffei, D.F.; Pinto, U.M. Minimally Processed Vegetables: Consumer Profile, Consumption Habits, and Perceptions of Microbiological Risk. Food Prot. Trends 2023, 43, 167–178. [Google Scholar] [CrossRef]
- Moretti, C.L. Manual de Processamento Mínimo de Frutas e Hortaliças; Embrapa Hortaliças: Brasília, Brazil, 2007; pp. 25–40. [Google Scholar]
- Sant’Anna, P.B.; Franco, B.D.G.M.; Maffei, D.F. Microbiological safety of ready-to-eat minimally processed vegetables in Brazil: An overview. J. Sci. Food Agric. 2020, 100, 4664–4670. [Google Scholar] [CrossRef]
- Maffei, D.F.; Alvarenga, V.O.; Sant’Ana, A.S.; Franco, B.D.G.M. Assessing the effect of washing practices employed in Brazilian processing plants on the quality of ready-to-eat vegetables. LWT Food Sci. Technol. 2016, 69, 474–481. [Google Scholar] [CrossRef]
- Fröhling, A.; Rademacher, A.; Rumpold, B.; Klocke, M.; Schlüter, O. Screening of microbial communities associated with endive lettuce during postharvest processing on industrial scale. Heliyon 2018, 4, e00671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, S.A.; Shah, M.A.; Mir, M.M.; Dar, B.N.; Greiner, R.; Roohinejad, S. Microbiological contamination of ready-to-eat vegetable salads in developing countries and potential solutions in the supply chain to control microbial pathogens. Food Control. 2018, 85, 235–244. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [Green Version]
- Finger, J.A.F.F.; Maffei, D.F.; Dias, M.; Mendes, M.A.; Pinto, U.M. Microbiological quality and safety of minimally processed parsley (Petroselinum crispum) sold in food markets, southeastern Brazil. J. Appl. Microbiol. 2021, 131, 272–280. [Google Scholar] [CrossRef]
- CDC (Centers for Disease Control and Prevention). Center of Disease Control. Food Safety. Foodborne Outbreaks. 2022. Available online: https://www.cdc.gov/foodsafety/outbreaks/index.html (accessed on 18 May 2023).
- Paoletti, F.; Raffo, A. Fresh-Cut Vegetables Processing: Environmental Sustainability and Food Safety Issues in a Comprehensive Perspective. Front. Sustain. Food Syst. 2022, 5, 681459. [Google Scholar] [CrossRef]
- Nascimento, K.D.O.; Augusta, I.M.; da Rocha Rodrigues, N.; Pires, T.; Batista, E.; Júnior, J.L.B.; Barbosa, M.I.M.J. Alimentos minimamente processados: Uma tendência de mercado. Acta Tecnol. 2014, 9, 48–61. [Google Scholar] [CrossRef]
- Costa, D.A.; Finger, J.A.F.F.; Alves, V.F.; Baroni, W.S.G.V.; Malheiros, P.S.; Alves, E.A.; Maffei, D.F.; Pinto, U.M. Minimally Processed Vegetables: Consumer Profile, Consumption Habits, Perceptions of Microbiological Risk and Labeling. In Anais do 31° Congresso Brasileiro de Microbiologia: Brasil. 2021. Available online: https://sbmicrobiologia.org.br/31cbm-anais/lista_area_01.htm (accessed on 16 May 2023).
- Embrapa (Empresa Brasileira de Pesquisa Agropecuária Agroindústria Tropical). Processamento Mínimo de Frutas E Hortaliças: Tecnologia, Qualidade E Sistemas de Embalagem/Coordenador, Sergio Agostinho Cenci; Embrapa Agroindústria de Alimentos: Rio de Janeiro, Brasil, 2011; pp. 1–144. Available online: https://www.infoteca.cnptia.embrapa.br/bitstream/doc/907934/1/LivroProcessamentoMinimo.pdf (accessed on 5 November 2022).
- Sato, G.S.; Martins, V.A.; Bueno, C.R.F. Análise exploratória do perfil do consumidor de produtos minimamente processados na cidade de São Paulo. Inf. Econômicas 2007, 37, 63–71. [Google Scholar]
- Degiovanni, G.C.; Japur, C.C.; Sanches, A.P.L.M.; Mattos, C.H.P.D.S.; Martins, L.D.S.; Reis, C.V.D.; Vieira, M.N.C.M. Hortaliças in natura ou minimamente processadas em unidades de alimentação e nutrição: Quais aspectos devem ser considerados na sua aquisição? Rev. Nutr. 2010, 23, 813–822. [Google Scholar] [CrossRef]
- Pena, F.L.; Paulo, K.H.; Soragni, L.; Duarte, L.T.; Antunes, A.E.C. Avaliação microbiológica de hortaliças minimamente processadas disponíveis no mercado e servidas em redes de fast-food e em unidades de alimentação e nutrição nas cidades de Limeira e Campinas, São Paulo, Brasil. Rev. Segur. Aliment. Nutr. 2015, 22, 633–643. [Google Scholar] [CrossRef]
- Melo, V.T.P.; Strasburg, V.J. Geração de resíduos na aquisição de vegetais in natura e minimamente processados por serviço de nutrição e dietética de um hospital público. Braz. J. Food Technol. 2020, 23, e2019069. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.; Siddiqui, M.W.; Rahman, M.S. Minimally processed foods: Overview. In Minimally Processed Foods: Technologies for Safety, Quality, and Convenience; Siddiqui, M., Rahman, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; p. 306. [Google Scholar] [CrossRef]
- Castro-Ibáñez, I.; Gil, M.I.; Allende, A. Ready-to-eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT Food Sci. Technol. 2017, 85, 284–292. [Google Scholar] [CrossRef]
- López-Gálvez, F.; Gil, M.I.; Truchado, P.; Selma, M.V.; Allende, A. Cross-contamination of fresh-cut lettuce after a short-term exposure during pre-washing cannot be controlled after subsequent washing with chlorine dioxide or sodium hypochlorite. Food Microbiol. 2010, 27, 199–204. [Google Scholar] [CrossRef]
- Maffei, D.F.; Sant’Ana, A.S.; Franco, B.D.G.M.; Schaffner, D.W. Quantitative assessment of the impact of cross-contamination during the washing step of ready-to-eat leafy greens on the risk of illness caused by Salmonella. Food Res. Int. 2017, 92, 106–112. [Google Scholar] [CrossRef]
- Paulo, S. Secretária do Estado da Saúde. Portaria CVS 5, de 09 de abril de 2013. In Aprova o Regulamento Técnico Sobre Boas Práticas Para Estabelecimentos Comerciais de Alimentos e Para Serviços de Alimentação, e o Roteiro de Inspeção, Anexo; Diário Oficial do Estado de São Paulo: São Paulo, Brazil, 2013; pp. 32–35. [Google Scholar]
- Ferreira, M.R.; Santos, T.S.D.; Maffei, D.F. Assessing Brazilian food establishments’ hygienic handling of leafy vegetables and their microbiological quality. Acta Aliment. 2021, 50, 189–198. [Google Scholar] [CrossRef]
- Lee, W.N.; Huang, C.H.; Zhu, G. Analytical methods for conventional and emerging disinfection by-products in fresh-cut produce. Food Chem. 2019, 291, 30–37. [Google Scholar] [CrossRef]
- Joshi, K.; Mahendran, R.; Alagusundaram, K.; Norton, T.; Tiwari, B.K. Novel disinfectants for fresh produce. Trends Food Sci. Technol. 2013, 34, 54–61. [Google Scholar] [CrossRef]
- Feliziani, E.; Lichter, A.; Smilanick, J.L.; Ippolito, A. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol. Technol. 2016, 122, 53–69. [Google Scholar] [CrossRef]
- Gadelha, J.R.; Allende, A.; López-Gálvez, F.; Fernández, P.; Gil, M.I.; Egea, J.A. Chemical risks associated with ready-to-eat vegetables: Quantitative analysis to estimate formation and/or accumulation of disinfection byproducts during washing. EFSA J. 2019, 17, e170913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.H.; Lee, S.Y. Comparison of the effectiveness of decontaminating strategies for fresh fruits and vegetables and related limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 3189–3208. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.; Giaouris, E.; Simões, M. Alternative disinfection methods to chlorine for use in the fresh-cut industry. Food Res. Int. 2016, 82, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Huang, R.; Chen, H. Application of ultraviolet C technology for surface decontamination of fresh produce. Trends Food Sci. Technol. 2017, 70, 9–19. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z. Recent developments in novel shelf-life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar] [CrossRef] [Green Version]
- Balbinot Filho, C.A.; Borges, C.D. Efeitos da radiação UV-C em alface e maçã minimamente processadas: Uma revisão. Braz. J. Food Technol. 2020, 23, e2018321. [Google Scholar] [CrossRef]
- Zhang, H.; Tikekar, R.V.; Ding, Q.; Gilbert, A.R.; Wimsatt, S.T. Inactivation of foodborne pathogens by the synergistic combinations of food processing technologies and food-grade compounds. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2110–2138. [Google Scholar] [CrossRef]
- Lepaus, B.M.; Rocha, J.S.; de São José, J.F.B. Organic Acids and Hydrogen Peroxide Can Replace Chlorinated Compounds as Sanitizers on Strawberries, Cucumbers and Rocket Leaves. Food Sci. Technol. 2020, 40, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Lippman, B.; Yao, S.; Huang, R.; Chen, H. Evaluation of the Combined Treatment of Ultraviolet Light and Peracetic Acid as an Alternative to Chlorine Washing for Lettuce Decontamination. Int. J. Food Microbiol. 2020, 323, 108590. [Google Scholar] [CrossRef]
- Onwude, D.I.; Chen, G.; Eke-Emezie, N.; Kabutey, A.; Khaled, A.Y.; Sturm, B. Recent advances in reducing food losses in the supply chain of fresh agricultural produce. Processes 2020, 8, 1431. [Google Scholar] [CrossRef]
- Denoya, G.I.; Vaudagna, S.R.; Polenta, G. Effect of high pressure processing and vacuum packaging on the preservation of fresh-cut peaches. LWT Food Sci. Technol. 2015, 62, 801–806. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre-and postharvest preventive measures and intervention strategies to control microbial food safety hazards of fresh leafy vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 453–468. [Google Scholar] [CrossRef]
- Maffei, D.F.; Batalha, E.Y.; Landgraf, M.; Schaffner, D.W.; Franco, B.D.G.M. Microbiology of organic and conventionally grown fresh produce. Braz. J. Microbiol. 2016, 47, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beuchat, L.R. Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables. Microbes Infect. 2002, 4, 413–423. [Google Scholar] [CrossRef]
- Fröder, H.; Martins, C.G.; de Souza, K.L.O.; Landgraf, M.; Franco, B.D.G.M.; Destro, M.T. Minimally processed vegetable salads: Microbial quality evaluation. J. Food Prot. 2007, 70, 1277–1280. [Google Scholar] [CrossRef]
- Silva, S.R.; Verdin, S.E.F.; Pereira, D.C.; Schatkoski, A.M.; Rott, M.B.; Corção, G. Microbiological quality of minimally processed vegetables sold in Porto Alegre, Brazil. Braz. J. Microbiol. 2007, 38, 594–598. [Google Scholar] [CrossRef]
- de Oliveira, M.A.; de Souza, V.M.; Bergamini, A.M.M.; de Martinis, E.C.P. Microbiological quality of ready-to-eat minimally processed vegetables consumed in Brazil. Food Control. 2011, 22, 1400–1403. [Google Scholar] [CrossRef]
- Sant’Ana, A.S.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Prevalence and counts of Salmonella spp. in minimally processed vegetables in São Paulo, Brazil. Food Microbiol. 2011, 28, 1235–1237. [Google Scholar] [CrossRef]
- Maistro, L.C.; Miya, N.T.N.; Sant’Ana, A.S.; Pereira, J.L. Microbiological quality and safety of minimally processed vegetables marketed in Campinas, SP–Brazil, as assessed by traditional and alternative methods. Food Control. 2012, 28, 258–264. [Google Scholar] [CrossRef]
- Sant’Ana, A.S.; Igarashi, M.C.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Prevalence, populations and pheno-and genotypic characteristics of Listeria monocytogenes isolated from ready-to-eat vegetables marketed in São Paulo, Brazil. Int. J. Food Microbiol. 2012, 155, 1–9. [Google Scholar] [CrossRef]
- Vasconcellos, L.; Carvalho, C.T.; Tavares, R.O.; Medeiros, V.M.; Rosas, C.O.; Silva, J.N.; Lopes, S.M.D.R.; Forsythe, S.J.; Brandão, M.L.L. Isolation, molecular and phenotypic characterization of Cronobacter spp. in ready-to-eat salads and foods from Japanese cuisine commercialized in Brazil. Food Res. Int. 2018, 107, 353–359. [Google Scholar] [CrossRef]
- Cruz, M.R.G.D.; Leite, Y.J.B.D.S.; Marques, J.D.L.; Pavelquesi, S.L.S.; Oliveira, L.R.D.A.; Silva, I.C.R.D.; Orsi, D.C. Microbiological quality of minimally processed vegetables commercialized in Brasilia, DF, Brazil. Food Sci. Technol. 2019, 39, 498–503. [Google Scholar] [CrossRef] [Green Version]
- Schuh, V.; Schuh, J.; Fronza, N.; Foralosso, F.B.; Verruck, S.; Vargas Junior, A.; Silveira, S.M.D. Evaluation of the microbiological quality of minimally processed vegetables. Food Sci. Technol. 2020, 40, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Santos, L.S.; Silva, L.V.; Lepaus, B.M.; São José, J.F.B. Microbial quality and labeling of minimally processed fruits and vegetables. Biosci. J. 2021, 37, 1981–3163. [Google Scholar] [CrossRef]
- Szabo, E.A.; Scurrah, K.J.; Burrows, J.M. Survey for psychrotrophic bacterial pathogens in minimally processed lettuce. Lett. Appl. Microbiol. 2000, 30, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Abadias, M.; Usall, J.; Anguera, M.; Solsona, C.; Viñas, I. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int. J. Food Microbiol. 2008, 123, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.H.; Jang, J.H.; Moon, K.D. Microbial evaluation of minimally processed vegetables and sprouts produced in Seoul, Korea. Food Sci. Biotechnol. 2010, 19, 1283–1288. [Google Scholar] [CrossRef]
- Xanthopoulos, V.; Tzanetakis, N.; Litopoulou-Tzanetaki, E. Occurrence and characterization of Aeromonas hydrophila and Yersinia enterocolitica in minimally processed fresh vegetable salads. Food Control. 2010, 21, 393–398. [Google Scholar] [CrossRef]
- Althaus, D.; Hofer, E.; Corti, S.; Julmi, A.; Stephan, R. Bacteriological survey of ready-to-eat lettuce, fresh-cut fruit, and sprouts collected from the Swiss market. J. Food Prot. 2012, 75, 1338–1341. [Google Scholar] [CrossRef]
- Moreno, Y.; Sánchez-Contreras, J.; Montes, R.M.; García-Hernández, J.; Ballesteros, L.; Ferrús, M.A. Detection and enumeration of viable Listeria monocytogenes cells from ready-to-eat and processed vegetable foods by culture and DVC-FISH. Food Control. 2012, 27, 374–379. [Google Scholar] [CrossRef]
- Santos, M.I.; Cavaco, A.; Gouveia, J.; Novais, M.R.; Nogueira, P.J.; Pedroso, L.; Ferreira, M.A.S.S. Evaluation of minimally processed salads commercialized in Portugal. Food Control 2012, 23, 275–281. [Google Scholar] [CrossRef]
- Eckert, C.; Burghoffer, B.; Barbut, F. Contamination of ready-to-eat raw vegetables with Clostridium difficile in France. J. Med. Microbiol. 2013, 62, 1435–1438. [Google Scholar] [CrossRef] [PubMed]
- Kovačević, M.; Burazin, J.; Pavlović, H.; Kopjar, M.; Piližota, V. Prevalence and level of Listeria monocytogenes and other Listeria sp. in ready-to-eat minimally processed and refrigerated vegetables. World J. Microbiol. Biotechnol. 2013, 29, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Jeddi, M.Z.; Yunesian, M.; Gorji, M.E.H.; Noori, N.; Pourmand, M.R.; Khaniki, G.R.J. Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J. Health Popul. Nutr. 2014, 32, 391. [Google Scholar] [PubMed]
- Cerna-Cortes, J.F.; Leon-Montes, N.; Cortes-Cueto, A.L.; Salas-Rangel, L.P.; Helguera-Repetto, A.C.; Lopez-Hernandez, D.; Gonzalez-y-Merchand, J.A. Microbiological quality of ready-to-eat vegetables collected in Mexico City: Occurrence of aerobic-mesophilic bacteria, fecal coliforms, and potentially pathogenic nontuberculous mycobacteria. BioMed Res. Int. 2015, 2015, 789508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurler, Z.; Pamuk, S.; Yildirim, Y.; Ertas, N. The microbiological quality of ready-to-eat salads in Turkey: A focus on Salmonella spp. and Listeria monocytogenes. Int. J. Food Microbiol. 2015, 196, 79–83. [Google Scholar] [CrossRef]
- Nousiainen, L.L.; Joutsen, S.; Lunden, J.; Hänninen, M.L.; Fredriksson-Ahomaa, M. Bacterial quality and safety of packaged fresh leafy vegetables at the retail level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. [Google Scholar] [CrossRef]
- Abaza, A. Bacteriological assessment of some vegetables and ready-to-eat salads in Alexandria Egypt. J. Egypt Public Health Assoc. 2017, 92, 177–187. [Google Scholar] [CrossRef]
- Berthold-Pluta, A.; Garbowska, M.; Stefańska, I.; Pluta, A. Microbiological quality of selected ready-to-eat leaf vegetables, sprouts and non-pasteurized fresh fruit-vegetable juices including the presence of Cronobacter spp. Food Microbiol. 2017, 65, 221–230. [Google Scholar] [CrossRef]
- Bencardino, D.; Vitali, L.A.; Petrelli, D. Microbiological evaluation of ready-to-eat iceberg lettuce during shelf-life and effectiveness of household washing methods. Ital. J. Food Saf. 2018, 7, 6913. [Google Scholar] [CrossRef] [Green Version]
- Hualpa, D.; Toledo, Z.; Meneses, M.A.; Feng, P. Microbiological Quality of Minimally Processed, Ready-to-Eat, Vegetables in Loja, Ecuador. Rev. Politec. 2018, 41, 45–50. [Google Scholar]
- Azimirad, M.; Nadalian, B.; Alavifard, H.; Panirani, S.N.; Bonab, S.M.V.; Azimirad, F.; Gholami, F.; Jabbari, P.; Yadegar, A.; Busani, L.; et al. Microbiological survey and occurrence of bacterial foodborne pathogens in raw and ready-to-eat green leafy vegetables marketed in Tehran, Iran. Int. J. Hyg. Environ. Health 2021, 237, 113824. [Google Scholar] [CrossRef] [PubMed]
- Baraquet, M.L.; Camiletti, O.F.; Moretti, C.I.; Rodríguez, L.E.; Vázquez, C.; Oberto, M.G. Microbiological Status and Quality Traits of Ready-to-Eat Minimally Processed Vegetables Sold in Córdoba, Argentina. J. Food Qual. Hazards Control. 2021, 8, 119–124. [Google Scholar] [CrossRef]
- Łepecka, A.; Zielińska, D.; Szymański, P.; Buras, I.; Kołożyn-Krajewska, D. Assessment of the Microbiological Quality of Ready-to-Eat Salads—Are There Any Reasons for Concern about Public Health? Int. J. Environ. Res. Public Health 2022, 19, 1582. [Google Scholar] [CrossRef]
- WHO (World Health Organization); FAO (Food and Agriculture Organization of the United Nations). Shiga Toxin-Producing Escherichia coli (STEC) and Food: Attribution, Characterization, and Monitoring: Report; World Health Organization: Rome, Italy, 2018. Available online: https://apps.who.int/iris/handle/10665/272871. (accessed on 16 May 2023).
- Miralles, M.M.; Maestre-Carballa, L.; Lluesma-Gomez, M.; Martinez-Garcia, M. High-throughput 16S rRNA sequencing to assess potentially active bacteria and foodborne pathogens: A case example in ready-to-eat food. Foods 2019, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Tatsika, S.; Karamanoli, K.; Karayanni, H.; Genitsaris, S. Metagenomic characterization of bacterial communities on ready-to-eat vegetables and effects of household washing on their diversity and composition. Pathogens 2019, 8, 37. [Google Scholar] [CrossRef] [Green Version]
- Manthou, E.; Coeuret, G.; Chaillou, S.; Nychas, G.J.E. Metagenetic characterization of bacterial communities associated with ready-to-eat leafy vegetables and study of temperature effect on their composition during storage. Food Res. Int. 2022, 158, 111563. [Google Scholar] [CrossRef]
- Jung, Y.; Jang, H.; Matthews, K.R. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. J. Microbial. Biotechnol. 2014, 7, 517–527. [Google Scholar] [CrossRef]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [CrossRef]
- Garner, D.; Kathariou, S. Fresh produce-associated listeriosis outbreaks, sources of concern, teachable moments, and insights. J. Food Prot. 2016, 79, 337–344. [Google Scholar] [CrossRef]
- Elias, S.O.; Decol, L.T.; Tondo, E.C. Foodborne outbreaks in Brazil associated with fruits and vegetables: 2008 through 2014. Food Qual. Saf. 2018, 2, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Ministério da Saúde; Secretaria de Vigilância em Saúde; Sistema de Informação de Agravos de Notificação. Situação Epidemiológica—Doenças Transmitidas Por Alimentos; Banco de dados 2000 a 2021; Ministério da Saúde: Brasília, Brazil, 2022.
- Ministério da Saúde; Agência Nacional de Vigilância Sanitária. Instrução Normativa n 161, de 01 de Julho de 2022; Estabelece os padrões microbiológicos dos alimentos; Ministério da Saúde: Brasília, Brazil, 2022.
- CFS (Centre for Food Safety). Microbiological Guidelines for Food (For Ready-To-Eat Food in General and Specific Food Items). In Food and Environmental Hygiene Department, Hong Kong. 2014. Available online: https://www.cfs.gov.hk/english/food_leg/files/food_leg_Microbiological_Guidelines_for_Food_e.pdf (accessed on 20 December 2022).
- EU (European Union). Commission Regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02005R2073-20200308&rid=1 (accessed on 10 January 2023).
- FDA (Food and Drug Administration). FDA Circular 2013-010-Food and Drug Administration Philippines. 2013. Available online: https://members.wto.org/crnattachments/2021/TBT/PHL/21_3930_00_e.pdf (accessed on 10 January 2023).
| Vegetables | Fresh Vegetables (BRL/100g) | MPVs (BRL/100g) | Price Difference | ||||
|---|---|---|---|---|---|---|---|
| Mean | Minimum | Maximum | Mean | Minimum | Maximum | BRL (%) | |
| Arugula | 1.88 | 0.78 | 0.79 | 8.72 | 5.32 | 18.87 | 6.84 (463.8) |
| Cassava | 0.70 | 0.47 | 0.86 | 1.00 | 1.00 | 1.00 | 0.30 (142.8) |
| Escarole | 0.89 | 0.36 | 1.31 | 7.15 | 4.49 | 9.43 | 6.26 (803.4) |
| Kale | 1.58 | 0.59 | 4.00 | 3.88 | 2.00 | 4.50 | 2.30 (245.6) |
| Lettuce | 1.05 | 0.68 | 2.39 | 6.12 | 2.99 | 11.32 | 5.07 (582.9) |
| Pumpkin | 1.02 | 0.30 | 2.00 | 1.55 | 2.10 | 2.10 | 0.53 (152.0) |
| Spinach | 1.83 | 1.66 | 2.00 | 5.65 | 4.61 | 6.00 | 3.82 (308.7) |
| Watercress | 1.05 | 1.05 | 1.05 | 6.71 | 6.24 | 7.98 | 5.66 (639.0) |
| Microorganisms | Number of Samples | Range Counts | Unit | Reference | |
|---|---|---|---|---|---|
| Total n | Positive n (%) | ||||
| Total psychrotrophic bacteria | 133 | 133 (100) | 1.0–6.0 | Log CFU/g | [54] |
| Enterobacteriaceae | 133 (100) | 1.0 > 6.0 | Log CFU/g | ||
| Total coliforms | 133 (100) | 1.0–>6.0 | Log CFU/g | ||
| Thermotolerant coliforms | 133 (100) | 1.0–>6.0 | Log CFU/g | ||
| Salmonella | 4 (3) | - | - | ||
| Listeria monocytogenes | 181 | 1 (0.6) | - | – | |
| Listeria welshimeri | 1 (0.6) | - | - | ||
| Listeria innocua | 2 (1.1) | - | - | ||
| Total mesophilic bacteria | 56 | 56 (100) | 5.7–8.2 | Log CFU/g | [55] |
| Total psychrotrophic bacteria | 56 (100) | 6.9–8.2 | Log CFU/g | ||
| Thermotolerant coliforms | 56 (100) | <0.5–4.0 | Log MNP/g | ||
| Escherichia coli | 8 (28.6) | <0.5 | Log MNP/g | ||
| Oocysts of Eimeria | 52 | 8 (15.3) | - | - | |
| Total psychrotrophic bacteria | 162 | 157 (96.7) | 7.1–9.4 | Log CFU/g | [56] |
| Total coliforms | 158 (97.5) | - | - | ||
| Thermotolerant coliforms | 107 (66) | - | - | ||
| Escherichia coli | 86 (53.1) | 1.0–6.0 | Log MNP/g | ||
| Listeria | 6 (3.7) | - | - | ||
| Listeria monocytogenes | 2 (1.2) | - | - | ||
| Listeria innocua | 4 (2.4) | - | - | ||
| Salmonella | 2 (1.2) | - | - | ||
| Total coliforms | 512 | 512 (100) | 2.0–>6.0 | Log CFU/g | [57] |
| Escherichia coli | 512 (100) | 2.0–5.0 | Log CFU/g | ||
| Salmonella | 4 (0.8) | 2.4–2.9 | Log CFU/g | ||
| Total mesophilic bacteria | 172 | 172 (100) | 4.0–6.8 | Log CFU/g | [58] |
| Total coliforms | 172 (100) | 1.0–3.7 | Log CFU/g | ||
| Escherichia coli | 10 (17.2) | <1.0–3.5 | Log CFU/g | ||
| Listeria monocytogenes | 3 (1.2) | - | - | ||
| Salmonella | 1 (0.6) | - | - | ||
| Listeria monocytogenes | 512 | 16 (3.1) | 1.0–2.4 | Log CFU/g | [59] |
| Cronobacter | 30 | 13 (43.3) | - | - | [60] |
| Total mesophilic bacteria | 32 | 32 (100) | 4.0–8.0 | Log CFU/g | [61] |
| Total psychrotrophic bacteria | 32 (100) | 4.0–8.0 | Log CFU/g | ||
| Total coliforms | 32 (100) | 1.0–4.0 | Log MPN/g | ||
| Thermotolerant coliforms | 32 (100) | 1.0–4.0 | Log MPN/g | ||
| Escherichia coli | 16 (50) | - | - | ||
| Staphylococcus aureus | 14 (43.8) | 1.0–5.0 | Log CFU/g | ||
| Salmonella | 4 (12.5) | - | - | ||
| Enterobacteriaceae | 100 | 86 (25.9) | 5.2–6.8 | Log MPN/g | [10] |
| Total coliforms | 100 (100) | 2.6–3.0 | Log MPN/g | ||
| Thermotolerant coliforms | 20 (20) | <0.5–3.0 | Log MPN/g | ||
| Escherichia coli | 16 (16) | <0.5–1.9 | Log MPN/g | ||
| Salmonella | 1 (1) | - | - | ||
| Total mesophilic bacteria | 21 | 21 (100) | 2.4–7.4 | Log CFU/g | [62] |
| Total coliforms | 9 (37.5) | 0.5–>3.0 | Log MPN/g | ||
| Thermotolerant coliforms | 1 (4.1) | <0.5–2.3 | Log MPN/g | ||
| Staphylococcus | 21 (100) | <2.0–7.2 | Log CFU/g | ||
| Yeasts and molds | 21 (100) | 2.7–5.7 | Log CFU/g | ||
| Enterobacteriaceae | 100 | 100 (100) | - | CFU/g | [21] |
| Escherichia coli | 3 (3) | - | - | ||
| Listeria innocua | 2 (2) | - | - | ||
| Listeria fleischmannii | 1 (1) | - | - | ||
| Total mesophilic bacteria | 30 | 30 (100) | 4.3–>6.3 | Log CFU/g | [63] |
| Total coliforms | 30 (100) | 4.0–>6.3 | Log CFU/g | ||
| Escherichia coli | 4 (13.3) | 3.0–3.6 | Log CFU/g | ||
| Yeasts and molds | 30 (100) | 3.4–>6.3 | Log CFU/g | ||
| Country | Microorganisms | Number of Samples | Range Counts | Unit | Reference | |
|---|---|---|---|---|---|---|
| Total | Positive | |||||
| n | n (%) | |||||
| Australia | Total psychrotrophic bacteria | 120 | 120 (100) | 3.0–9.0 | Log CFU/g | [64] |
| Aeromonas hydrophila or A. caviae | 66 (55) | - | - | |||
| Aeromonas sobria | 14 (12.7) | - | - | |||
| Listeria monocytogenes | 3 (2.5) | - | - | |||
| Yersinia enterocolitica | 71 (59.2) | - | - | |||
| Spain | Total mesophilic bacteria | 236 | 236 (100) | 4.3–8.9 | Log CFU/g | [65] |
| Total psychrotrophic bacteria | 236 (100) | 4.3–8.9 | Log CFU/g | |||
| Lactic acid bacteria | 236 (100) | <1.0–8.5 | Log CFU/g | |||
| Enterobacteriaceae | 236 (100) | <1.0–8.0 | Log CFU/g | |||
| Escherichia coli | 27 (11.4) | - | - | |||
| Listeria monocytogenes | 2 (0.8) | - | - | |||
| Salmonella | 4 (1.7) | - | - | |||
| Yeasts and molds | 236 (100) | 2.0–7.8 | Log CFU/g | |||
| Korea | Total mesophilic bacteria | 159 | 159 (100) | 4.2–8.9 | Log CFU/g | [66] |
| Total psychrotrophic bacteria | 159 (100) | 3.2–8.5 | Log CFU/g | |||
| Total coliforms | 159 (100) | 2.2–8.2 | Log CFU/g | |||
| Escherichia coli | 7 (4.4) | - | - | |||
| Clostridium perfringens | 6 (3.7) | - | - | |||
| Salmonella | 2 (1.2) | - | - | |||
| Yeasts and molds | 159 (100) | 1.7–7.5 | Log CFU/g | |||
| Greece | Total mesophilic bacteria | 26 | 26 (100) | 5.4–8.6 | Log CFU/g | [67] |
| Escherichia coli | 3 (11.5) | - | - | |||
| Aeromonas | 16 (61.5) | - | - | |||
| Aeromonas hydrophila | 12 (46.1) | - | - | |||
| Yersinia enterocolitica | 2 (7.7) | - | - | |||
| Yeasts and molds | 26 (100) | <3.0 | Log CFU/g | |||
| Switzerland | Total viable count | 142 | 142 (100) | 5.0–>8.0 | Log CFU/g | [68] |
| Cronobacter | 2 (1.4) | - | - | |||
| Escherichia coli (EPEC) | 11 (7.7) | <2.0–3.0 | Log CFU/g | |||
| Escherichia coli (STEC) | 1 (0.7) | <2.0 | Log CFU/g | |||
| Listeria monocytogenes | 5 (3.5) | <2.0 | Log CFU/g | |||
| Spain | Listeria monocytogenes | 191 | 8 (4.2) | <100.0 | CFU/g | [69] |
| Portugal | Total psychrotrophic bacteria | 151 | 151 (100) | 0.7–0.9 | Log CFU/g | [70] |
| Enterobacteriaceae | 151 (100) | 2.0–8.0 | Log CFU/g | |||
| Escherichia coli | 4 (2.6) | <1.0–2.3 | Log CFU/g | |||
| Listeria | 3 (2) | <1.0–2.0 | Log CFU/g | |||
| Listeria innocua | 2 (1.3) | 2.0–2.3 | Log CFU/g | |||
| Listeria monocytogenes | 1 (0.7) | <2.0 | Log CFU/g | |||
| Aeromonas hydrophila | 11 (7.3) | 3.1–5.1 | Log CFU/g | |||
| Bacillus cereus | 66 | 15 (22.7) | <2.0–3.2 | Log CFU/g | ||
| France | Clostridium difficile | 104 | 3 (2.9) | - | - | [71] |
| Croatia | Listeria monocytogenes | 100 | 1 (1) | 1.8 | Log CFU/g | [72] |
| Listeria | 20 (20) | - | - | |||
| Iran | Total mesophilic bacteria | 32 | 32 (100) | 5.3–7.5 | Log CFU/g | [73] |
| Total coliforms | 28 (87.5) | ND *–5.5 | Log CFU/g | |||
| Thermotolerant coliforms | 11 (34.4) | - | - | |||
| Escherichia coli | 3 (9.4) | - | - | |||
| Yeasts and molds | 32 (100) | 5.4–7.6 | Log CFU/g | |||
| Mexico | Total mesophilic bacteria | 100 | 100 (100) | 3.0–6.6 | Log CFU/g | [74] |
| Total coliforms | 96 (100) | <0.5–>3.0 | Log NMP/g | |||
| Thermotolerant coliforms | 32 (32) | <0.5–>3.0 | Log NMP/g | |||
| Nontuberculous mycobacteria | 7 (7) | - | - | |||
| Turkey | Total psychrotrophic bacteria | 261 | 235 (90) | 2.0–> 6.0 | Log CFU/g | [75] |
| Total coliforms | 155 (59.3) | >0.5 | Log NMP/g | |||
| Escherichia coli | 10 (3.8) | >0.5 | Log NMP/g | |||
| Listeria monocytogenes | 15 (5.7) | - | - | |||
| Listeria ivanovi | 14 (5.3) | - | - | |||
| Listeria grayi | 21 (8) | - | - | |||
| Listeria welshimeri | 23 (8.8) | - | - | |||
| Salmonella | 21 (8) | - | - | |||
| Finland | Total mesophilic bacteria | 100 | 100 (100) | 6.2–10.6 | Log CFU/g | [76] |
| Total coliforms | 100 (100) | 4.2–8.3 | Log CFU/g | |||
| Escherichia coli | 15 (15) | - | - | |||
| Escherichia coli (STEC) | 7 (7) | - | - | |||
| Listeria | 4 (4) | - | - | |||
| Listeria monocytogenes | 2 (2) | - | - | |||
| Yersinia | 33 (33) | - | - | |||
| Yersinia enterocolitica | 3 (3) | - | - | |||
| Salmonella | 2 (2) | - | - | |||
| Egypt | Total mesophilic bacteria | 50 | 10 (35.7) | 3.8–9.4 | Log CFU/g | [77] |
| Total coliforms | 33 (66) | - | - | |||
| Thermotolerant coliforms | 33 (66) | - | - | |||
| Escherichia coli | 4 (18.2) | - | - | |||
| Poland | Total mesophilic bacteria | 20 | 20 (100) | 5.6–7.6 | Log CFU/g | [78] |
| Cronobacter | 6 (35) | - | - | |||
| Cronobacter sakazakii | 3 (15) | - | - | |||
| Italy | Total mesophilic bacteria | 78 | 78 (100) | 6.0–9.2 | Log CFU/g | [79] |
| Ecuador | Total mesophilic bacteria | 60 | 60 (100) | 4.5–7.8 | Log CFU/g | [80] |
| Total coliforms | 60 (100) | 0.4–>5.0 | Log MNP/g | |||
| Escherichia coli | 13 (21.7) | <0.8 | Log MNP/g | |||
| Canada | Listeria monocytogenes | 5379 | 13 (0.2) | - | - | [46] |
| Iran | Escherichia coli | 92 | 28 (30.4) | - | - | [81] |
| Clostridium perfringens | 8 (8.7) | - | - | |||
| Bacillus cereus | 10 (10.9) | - | - | |||
| Listeria monocytogenes | 4 (4.3) | - | - | |||
| Staphylococcus aureus | 18 (19.6) | - | - | |||
| Pseudomonas aeruginosa | 4 (4.3) | - | - | |||
| Shigella | 2 (2.2) | - | - | |||
| Salmonella | 3 (3.3) | - | - | |||
| Argentina | Total coliforms | 60 | 60 (100) | 1.3–3.3 | Log MPN/g | [82] |
| Thermotolerant coliforms | 60 (100) | 0.3–1.9 | Log MPN/g | |||
| Escherichia coli | 15 (25) | 3.4–8.4 | Log CFU/g | |||
| Staphylococcus aureus | 3 (5) | - | - | |||
| Poland | Total mesophilic bacteria | 30 | 30 (100) | 2.3–9.3 | Log CFU/g | [83] |
| Enterobacteriaceae | 30 (100) | <1.0–7.4 | Log CFU/g | |||
| Escherichia coli | 30 (100) | <1.0–5.5 | Log CFU/g | |||
| Staphylococcus aureus | 30 (100) | <1.0–3.5 | Log CFU/g | |||
| Lactic acid bacteria | 30 (100) | <1.0–8.4 | Log CFU/g | |||
| Listeria monocytogenes | 10 (33.3) | - | - | |||
| Salmonella | 8 (26.7) | - | - | |||
| Yeasts and molds | 30 (100) | <1.0–7.0 | Log CFU/g | |||
| Etiological Agents | Outbreaks |
Sick
Individuals |
Dead
Individuals | ||
|---|---|---|---|---|---|
| n | % | n | % | n | |
| Not identified | 39 | 25.5 | 703 | 19.6 | 1 |
| Escherichia coli * | 27 | 17.6 | 752 | 21 | 0 |
| Salmonella spp. | 25 | 16.3 | 681 | 19 | 0 |
| Bacillus cereus | 20 | 13.1 | 543 | 15.2 | 0 |
| Staphylococcus aureus | 14 | 9.2 | 515 | 14.4 | 0 |
| Others | 28 | 18.3 | 388 | 10.8 | 1 |
| Total | 153 | 100 | 3582 | 100 | 2 |
| Sites of occurrence | |||||
| Restaurants/bakeries | 31 | 20.3 | 270 | 7.5 | 0 |
| Homes | 31 | 20.3 | 222 | 6.2 | 2 |
| Other institutions (accommodation facilities, workplace) | 29 | 19 | 1271 | 35.5 | 0 |
| Others | 62 | 40.4 | 1819 | 50.8 | 0 |
| Total | 153 | 100 | 3582 | 100 | 2 |
| Source | Criteria | Guidelines | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacillus cereus | Campylobacter spp. | Clostridium perfringens | Escherichia coli | Listeria monocytogenes | Salmonella spp. | Staphylococcus aureus | Vibrio cholerae | Vibrio parahaemolyticus | Yeasts and Molds | ||
| Brazilian Ministry of Health [93] | Satisfactory | N/A | N/A | N/A | 10 | 102 | Abs/25 g | N/A | N/A | N/A | N/A |
| Acceptable | N/A | N/A | N/A | 102 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Centre for Food Safety China [94] | Satisfactory | <103 | Abs/25 g | <10 | Abs/25 g | Abs/25 g | Abs/25 g | <20 | Abs/25 g | <20 | Abs/25 g |
| Acceptable | 103–≤105 | N/A | 10–≤104 | N/A | N/A | N/A | 20–≤104 | N/A | 20–≤103 | N/A | |
| European Union [95] | Satisfactory | N/A | N/A | N/A | 102 | 102 | Abs/25 g | N/A | N/A | N/A | N/A |
| Acceptable | N/A | N/A | N/A | 103 | N/A | N/A | N/A | N/A | N/A | N/A | |
| Food and Drug Administration USA [96] | Satisfactory | N/A | N/A | N/A | <3 * | N/A | Abs/25 g | 10 | N/A | N/A | 102 |
| Acceptable | N/A | N/A | N/A | N/A | Abs/25 g | N/A | N/A | N/A | N/A | 104 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finger, J.A.F.F.; Santos, I.M.; Silva, G.A.; Bernardino, M.C.; Pinto, U.M.; Maffei, D.F. Minimally Processed Vegetables in Brazil: An Overview of Marketing, Processing, and Microbiological Aspects. Foods 2023, 12, 2259. https://doi.org/10.3390/foods12112259
Finger JAFF, Santos IM, Silva GA, Bernardino MC, Pinto UM, Maffei DF. Minimally Processed Vegetables in Brazil: An Overview of Marketing, Processing, and Microbiological Aspects. Foods. 2023; 12(11):2259. https://doi.org/10.3390/foods12112259
Chicago/Turabian StyleFinger, Jéssica A. F. F., Isabela M. Santos, Guilherme A. Silva, Mariana C. Bernardino, Uelinton M. Pinto, and Daniele F. Maffei. 2023. "Minimally Processed Vegetables in Brazil: An Overview of Marketing, Processing, and Microbiological Aspects" Foods 12, no. 11: 2259. https://doi.org/10.3390/foods12112259
APA StyleFinger, J. A. F. F., Santos, I. M., Silva, G. A., Bernardino, M. C., Pinto, U. M., & Maffei, D. F. (2023). Minimally Processed Vegetables in Brazil: An Overview of Marketing, Processing, and Microbiological Aspects. Foods, 12(11), 2259. https://doi.org/10.3390/foods12112259