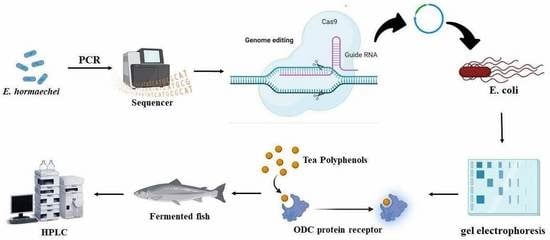
Characterization and Mechanism of Tea Polyphenols Inhibiting Biogenic Amine Accumulation in Marinated Spanish Mackerel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Total DNA Extraction
2.3. ODC Sequence and Cloning
2.4. ODC Bioinformatics Analysis
2.5. ODC Prokaryotic Expression and Purification
2.6. Evaluation of ODC Protein Function
2.7. Screening of Targeted Inhibitors of Putrescine
2.8. Analysis of the Mechanism of ODC Protein Binding to the Ornithine Molecule
2.9. Statistical Analysis
3. Results
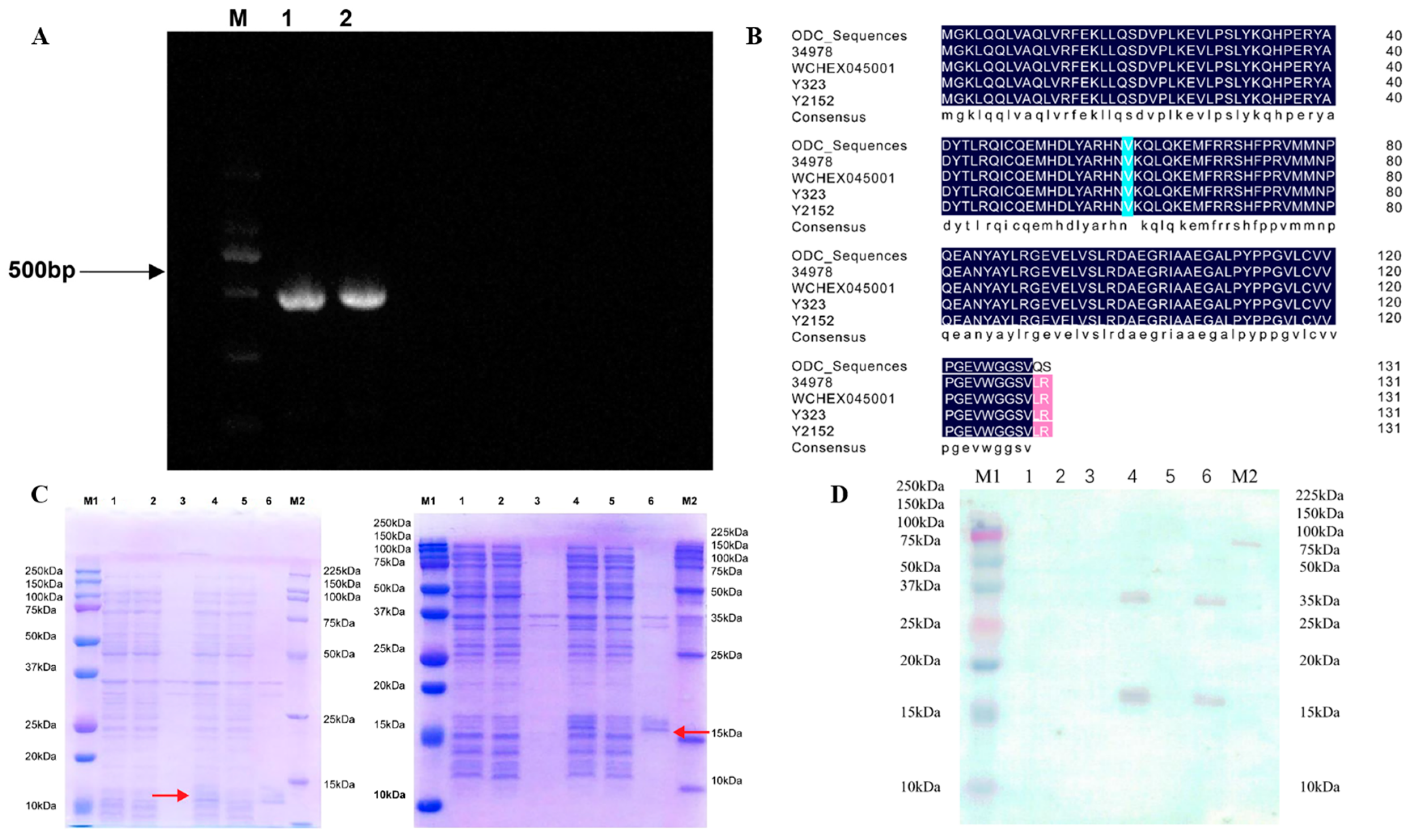
3.1. Cloning ODCs and Bioinformatics Evaluation
3.2. ODC Expression and Protein Purification
3.3. ODC Protein Functional Verification
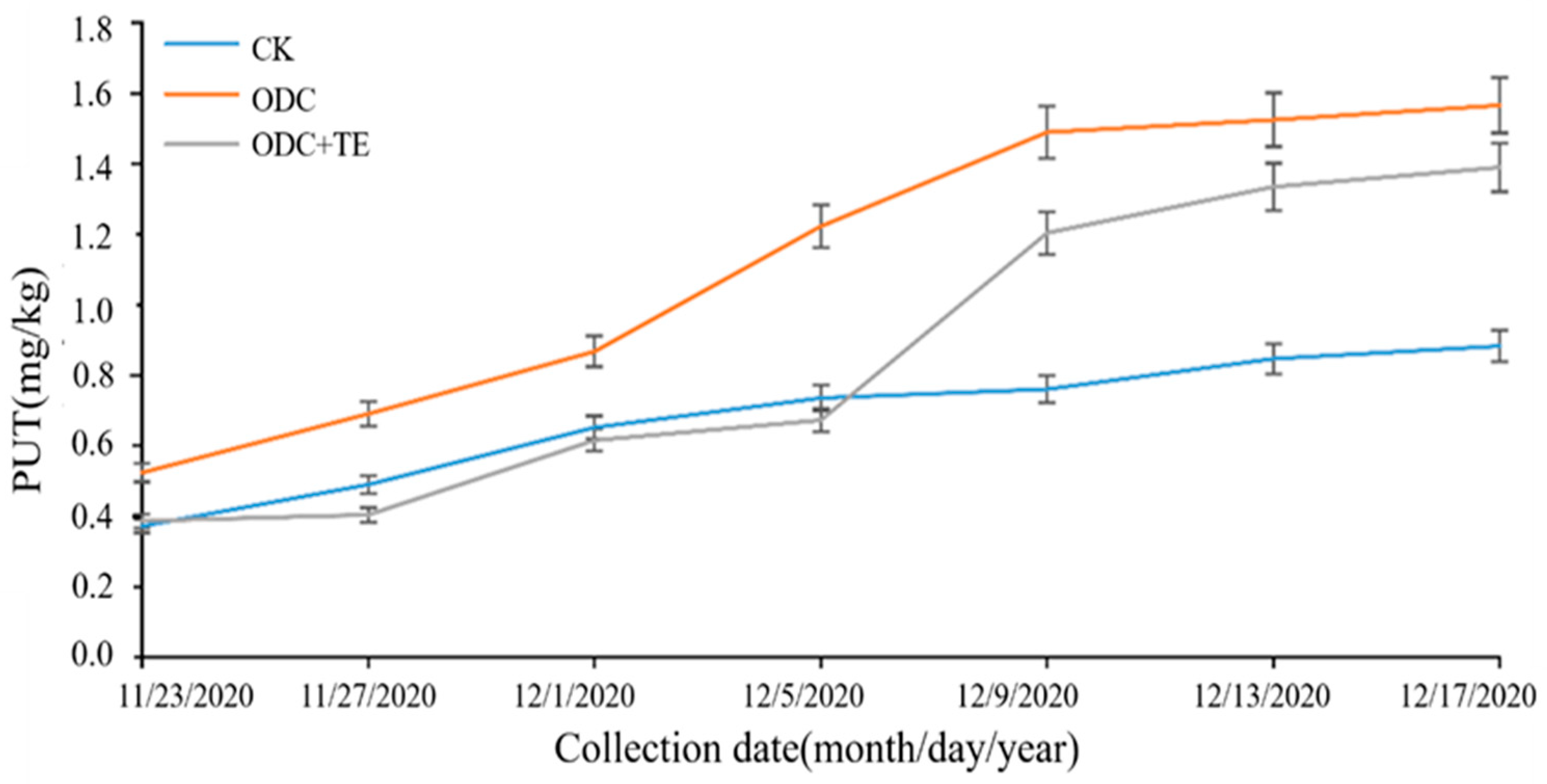
3.4. Screening of Targeted Inhibitors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schirone, M.; Visciano, P.; Conte, F.; Paparella, A. Formation of biogenic amines in the cheese production chain: Favouring and hindering factors. Int. Dairy J. 2022, 133, 105420. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Lee, J.H.; Mah, J.-H. Occurrence and Reduction of Biogenic Amines in Kimchi and Korean Fermented Seafood Products. Foods 2019, 8, 547. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Update on Biogenic Amines in Fermented and Non-Fermented Beverages. Foods 2022, 11, 353. [Google Scholar] [CrossRef]
- Schirone, M.; Esposito, L.; D’onofrio, F.; Visciano, P.; Martuscelli, M.; Mastrocola, D.; Paparella, A. Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives. Foods 2022, 11, 788. [Google Scholar] [CrossRef]
- Bao, X.; Wang, F.; Yang, R.; Zhang, Y.; Fu, L.; Wang, Y. Ornithine Decarboxylation System of Shewanella baltica Regulates Putrescine Production and Acid Resistance. J. Food Prot. 2021, 84, 303–309. [Google Scholar] [CrossRef]
- Rathod, N.B.; Phadke, G.G.; Tabanelli, G.; Mane, A.; Ranveer, R.C.; Pagarkar, A.; Ozogul, F. Recent advances in bio-preservatives impacts of lactic acid bacteria and their metabolites on aquatic food products. Food Biosci. 2021, 44, 101440. [Google Scholar] [CrossRef]
- Moniente, M.; Garcia-Gonzalo, D.; Ontanon, I.; Pagan, R.; Botello-Morte, L. Histamine accumulation in dairy products: Microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1481–1523. [Google Scholar] [CrossRef]
- Carballo, D.E.; Mateo, J.; Andrés, S.; Giráldez, F.J.; Quinto, E.J.; Khanjari, A.; Operta, S.; Caro, I. Microbial Growth and Biogenic Amine Production in a Balkan-Style Fresh Sausage during Refrigerated Storage under a CO2-Containing Anaerobic Atmosphere: Effect of the Addition of Zataria multiflora Essential Oil and Hops Extract. Antibiotics 2019, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S. Polyamine metabolism and transport in gut microbes. Biosci. Biotechnol. Biochem. 2022, 86, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Yang, Q.; Wan, W.; Zhu, Q.; Zeng, X. Physicochemical properties and adaptability of amine-producing Enterobacteriaceae isolated from traditional Chinese fermented fish (Suan yu). Food Chem. 2022, 369, 130885. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-K.; Mah, J.-H.; Hwang, H.-J. Biogenic amine formation and bacterial contribution in fish, squid and shellfish. Food Chem. 2009, 116, 87–95. [Google Scholar] [CrossRef]
- Madrigal Pulido, J.; Padilla Guerrero, I.; Magana Martinez, I.d.J.; Cacho Valadez, B.; Torres Guzman, J.C.; Salazar Solis, E.; Gutierrez Corona, J.F.; Schrank, A.; Jimenez Bremont, F.; Gonzalez Hernandez, A. Isolation, characterization and ex-pression analysis of the ornithine decarboxylase gene (ODC1) of the entomopathogenic fungus, Metarhizium anisopliae. Microbiol. Res. 2011, 166, 494–507. [Google Scholar] [CrossRef] [PubMed]
- Gerdt, J.P.; McInnis, C.E.; Schell, T.L.; Blackwell, H.E. Unraveling the contributions of hydrogen-bonding inter-actions to the activity of native and non-native ligands in the quorum-sensing receptor LasR. Org. Biomol. Chem. 2015, 13, 1453–1462. [Google Scholar] [CrossRef]
- Ding, T.; Li, T.; Li, J. Identification of natural product compounds as quorum sensing inhibitors in Pseudomonas fluorescens P07 through virtual screening. Bioorganic Med. Chem. 2018, 26, 4088–4099. [Google Scholar] [CrossRef]
- Gerhardtová, I.; Sokol, J.; Maliarová, M.; Martinka, N.; Jankech, T. Determination of Biogenic Amines in Food and Beverage Samples. Chem. Listy 2022, 116, 528–535. [Google Scholar] [CrossRef]
- Buňková, L.; Adamcová, G.; Hudcová, K.; Velichová, H.; Pachlová, V.; Lorencová, E.; Buňka, F. Monitoring of biogenic amines in cheeses manufactured at small-scale farms and in fermented dairy products in the Czech Republic. Food Chem. 2013, 141, 548–551. [Google Scholar] [CrossRef]
- Munoz-Esparza, N.C.; Luz Latorre-Moratalla, M.; Comas-Baste, O.; Toro-Funes, N.; Teresa Veciana-Nogues, M.; Carmen Vidal-Carou, M. Polyamines in Food. Front. Nutr. 2019, 6, 108–119. [Google Scholar] [CrossRef]
- Onal, A. A review: Current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007, 103, 1475–1486. [Google Scholar] [CrossRef]
- Xia, X.; Kong, B.; Liu, Q.; Liu, J. Physicochemical change and protein oxidation in porcine longissimus dorsi as influenced by different freeze–thaw cycles. Meat Sci. 2009, 83, 239–245. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- Omer, A.K.; Mohammed, R.R.; Ameen, P.S.M.; Abas, Z.A.; Ekici, K. Presence of Biogenic Amines in Food and Their Public Health Implications: A Review. J. Food Prot. 2021, 84, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Sentellas, S.; Núñez, O.; Saurina, J. Recent Advances in the Determination of Biogenic Amines in Food Samples by (U)HPLC. J. Agric. Food Chem. 2016, 64, 7667–7678. [Google Scholar] [CrossRef]
- Gardini, F.; Ozogul, Y.; Suzzi, G.; Tabanelli, G.; Ozogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Xu, X.; Zhou, G.; Zhao, G.; Li, C.; Zhang, Y.; Chen, L.; Qi, J. Irradiated Chinese Rugao ham: Changes in volatile N-nitrosamine, biogenic amine and residual nitrite during ripening and post-ripening. Meat Sci. 2009, 81, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Heimer, Y.; Mizrahi, Y.; Bachrach, U. Ornithine decarboxylase activity in rapidly proliferating plant cells. FEBS Lett. 1979, 104, 146–148. [Google Scholar] [CrossRef]
- Kosma, I.; Badeka, A. Determination of six underivatized biogenic amines by LC-MS/MS and study of biogenic amine production during trout (Salmo trutta) storage in ice. Food Addit. Contam. A 2021, 38, 476–487. [Google Scholar] [CrossRef]
- Smith, T.A. Amines in food. Food Chem. 1980, 6, 169–200. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef]
- Martuscelli, M.; Serio, A.; Capezio, O.; Mastrocola, D. Safety, Quality and Analytical Authentication of ḥalāl Meat Products, with Particular Emphasis on Salami: A Review. Foods. 2020, 9, 1111. [Google Scholar] [CrossRef]
- Latorre-Moratalla, M.; Veciana-Nogués, T.; Bover-Cid, S.; Garriga, M.; Aymerich, T.; Zanardi, E.; Ianieri, A.; Fraqueza, M.; Patarata, L.; Drosinos, E.; et al. Biogenic amines in traditional fermented sausages produced in selected European countries. Food Chem. 2008, 107, 912–921. [Google Scholar] [CrossRef]
- Mohamed, R.; Livia, S.-S.; Hassan, S.; Soher, E.-S.; Ahmed-Adel, E.B. Changes in free amino acids and biogenic amines of Egyptian salted-fermented fish (Feseekh) during ripening and storage. Food Chem. 2009, 115, 635–638. [Google Scholar]
- Andrade, S.C.S.; Mársico, E.T.; Godoy, R.L.O.; Franco, R.M.; Junior, C.A.C. Chemical quality indices for freshness evaluation of fish. J. Food Stud. 2014, 3, 71–87. [Google Scholar] [CrossRef]
- Mohan, C.O.; Ravishankara, C.N.; Gopal, T.K.S.; Kumar, K.A.; Lalitha, K.V. Biogenic amines formation in seer fish (Scomberomorus commerson) steaks packed with O2 scavenger during chilled storage. Food Res. Int. 2009, 42, 411–416. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Bo, Y.; Han, L.; He, J.; Lanczycki, C.J.; Lu, S.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; et al. CDD/SPARCLE: Functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017, 45, D200–D203. [Google Scholar] [CrossRef] [PubMed]
- Wendakoon, C.N.; Sakaguchi, M. Inhibition of amino acid decarboxylase activity of Enterobacter aerogenes by active components in spices. J. Food Prot. 1995, 58, 280–283. [Google Scholar] [CrossRef]
- Gupta, N.; Zhang, H.; Liu, P. Chronic difluoromethylornithine treatment impairs spatial learning and memory in rats. Pharmacol. Biochem. Behav. 2012, 100, 464–473. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Y.; Li, X.; Bi, J.; Zhang, G.; Hao, H.; Hou, H. Impacts of salt-tolerant Staphylococcus nepalensis 5-5 on bacterial composition and biogenic amines accumulation in fish sauce fermentation. Int. J. Food Microbiol. 2022, 361, 109464. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, Q.; Zhang, B.; Zhang, W.; Wang, W. Insights into the Biogenic Amine Metabolic Landscape during Industrial Semidry Chinese Rice Wine Fermentation. J. Agric. Food Chem. 2016, 64, 7385–7393. [Google Scholar] [CrossRef]
- Zaman, M.Z.; Bakar, F.A.; Selamat, J.; Bakar, J.; Ang, S.S.; Chong, C.Y. Degradation of histamine by the halotolerant Staphylococcus carnosus FS19 isolate obtained from fish sauce. Food Control 2014, 40, 58–63. [Google Scholar] [CrossRef]
- Pištěková, H.; Jančová, P.; Berčíková, L.; Buňka, F.; Sokolová, I.; Šopík, T.; Maršálková, K.; Amaral, O.M.R.P.d.; Buňková, L. Application of qPCR for multicopper oxidase gene (MCO) in biogenic amines degradation by Lactobacillus casei. Food Microbiol. 2020, 91, 103550. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Choi, Y.-J.; Hwang, I.M.; Hong, S.W.; Lee, M.-A. Relationship between chemical characteristics and bacterial community of a Korean salted-fermented anchovy sauce, Myeolchi-Aekjeot. LWT 2016, 73, 251–258. [Google Scholar] [CrossRef]
- Lee, Y.-C.; Kung, H.-F.; Huang, C.-Y.; Huang, T.-C.; Tsai, Y.-H. Reduction of histamine and biogenic amines during salted fish fermentation by Bacillus polymyxa as a starter culture. J. Food Drug Anal. 2016, 24, 157–163. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, C.; Du, S.; Liu, C.; Zheng, F.; Wang, J.; Li, Q. Reduction of biogenic amines formation during soybean paste fermentation by using Staphylococcus carnosus M43 and Pediococcus acidilactici M28 as starter culture. LWT 2020, 133, 109917. [Google Scholar] [CrossRef]
- Smirnova, O.A.; Isaguliants, M.G.; Hyvonen, M.T.; Keinanen, T.A.; Tunitskaya, V.L.; Vepsalainen, J.; Alhonen, L.; Kochetkov, S.N.; Ivanov, A.V. Chemically induced oxidative stress increases polyamine levels by activating the transcription of ornithine decarboxylase and spermidine/spermine-N1-acetyltransferase in human hepatoma HUH7 cells. Biochimie 2012, 94, 1876–1883. [Google Scholar] [CrossRef]
- Jeon, A.R.; Lee, J.H.; Mah, J.-H. Biogenic amine formation and bacterial contribution in Cheonggukjang, a Korean traditional fermented soybean food. LWT 2018, 92, 282–289. [Google Scholar] [CrossRef]
- Mobin, L.; Haq, M.A.; Ali, R.; Naz, S.; Saeed, S.G. Antibacterial and antioxidant potential of the phenolic extract and its fractions isolated from Allium ascalonicum (onion) peel. Nat. Prod. Res. 2022, 36, 3163–3167. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Lee, Y.R. Systematic study on active compounds as antibacterial and antibiofilm agent in aging onions. J. Food Drug Anal. 2018, 26, 518–528. [Google Scholar] [CrossRef]


| Name of Primers | Sequences of Primers (5′ → 3′) |
|---|---|
| ODC-F | GCACGGAACCGCCCCAGACT |
| ODC-R | GCCACCATCCTCGCCAAC |
| ODC-IF-F | GACGGAGCTCGAATTATGGGTAAACTGCAGCAGCTG |
| ODC-IF-R | ACAATTCCCCTCTAGTTAAACAGAACCACCCCAAACTTC |
| M13-M4 | GTTTTCCCAGTCACGAC |
| M13-RV | CAGGAAACAGCTATGAC |
| T7 | TAATACGACTCACTATAGGG |
| T7 terminator | GCTAGTTATTGCTCAGCGG |
| Name | CAS | Structural Formula |
|---|---|---|
| Sodium 1-carboxylatoethyl stearate | 18200-72-1 | 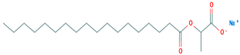 |
| D-glucitol | 50-70-4 | 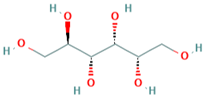 |
| Tea polyphenol | 84650-60-2 | 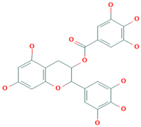 |
| Poly(L-lysine) hydrobromide | 25988-63-0 |  |
| Gallic acid | 149-91-7 |  |
| 5-propyl-2-thiouracil | 2954-52-1 | 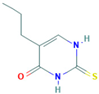 |
| Chlorogenic acid | 327-97-9 | 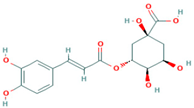 |
| Name | CAS | Binding Energy (kcal mol−1) |
|---|---|---|
| Sodium 1-carboxylatoethyl Stearate | 18200-72-1 | −5.1 |
| D-glucitol | 50-70-4 | −4.5 |
| Tea polyphenol | 84650-60-2 | −7.2 |
| Poly(L-lysine) hydrobromide | 25988-63-0 | −4.8 |
| Gallic acid | 149-91-7 | −5.1 |
| 5-propyl-2-thiouracil | 2954-52-1 | −4.3 |
| Chlorogenic acid | 327-97-9 | −6.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Chang, J.; Zhou, J.; Shi, Y.; Chen, H.; Han, L.; Tu, M.; Li, T. Characterization and Mechanism of Tea Polyphenols Inhibiting Biogenic Amine Accumulation in Marinated Spanish Mackerel. Foods 2023, 12, 2347. https://doi.org/10.3390/foods12122347
Xu Z, Chang J, Zhou J, Shi Y, Chen H, Han L, Tu M, Li T. Characterization and Mechanism of Tea Polyphenols Inhibiting Biogenic Amine Accumulation in Marinated Spanish Mackerel. Foods. 2023; 12(12):2347. https://doi.org/10.3390/foods12122347
Chicago/Turabian StyleXu, Zhe, Jiale Chang, Jiamin Zhou, Yixin Shi, Hui Chen, Lingyu Han, Maolin Tu, and Tingting Li. 2023. "Characterization and Mechanism of Tea Polyphenols Inhibiting Biogenic Amine Accumulation in Marinated Spanish Mackerel" Foods 12, no. 12: 2347. https://doi.org/10.3390/foods12122347
APA StyleXu, Z., Chang, J., Zhou, J., Shi, Y., Chen, H., Han, L., Tu, M., & Li, T. (2023). Characterization and Mechanism of Tea Polyphenols Inhibiting Biogenic Amine Accumulation in Marinated Spanish Mackerel. Foods, 12(12), 2347. https://doi.org/10.3390/foods12122347