Functional, Physical, and Volatile Characterization of Chitosan/Starch Food Films Functionalized with Mango Leaf Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Raw Materials
2.2. Supercritical Extraction of Mango Leaf Extract (MLE)
2.3. Development of Bioactive Films
2.4. Antioxidant Capacity of the MLE and Bioactive Films
2.5. Antimicrobial Capacity of the MLE and the Bioactive Films
2.6. Optical Properties of the Films
2.7. Solubility and Water Vapor Permeability (WVP) of the Films
2.8. Scanning Electron Microscopy
2.9. Study of the Migration of Volatile Organic Compounds (VOCs) from the Films
2.10. Preservation of Raspberry Using Bioactive Films
2.11. Statistical Analysis
3. Results and Discussion
3.1. MLE Production by Enhanced Solvent Extraction
3.2. Manufacturing of Biodegradable Active Films
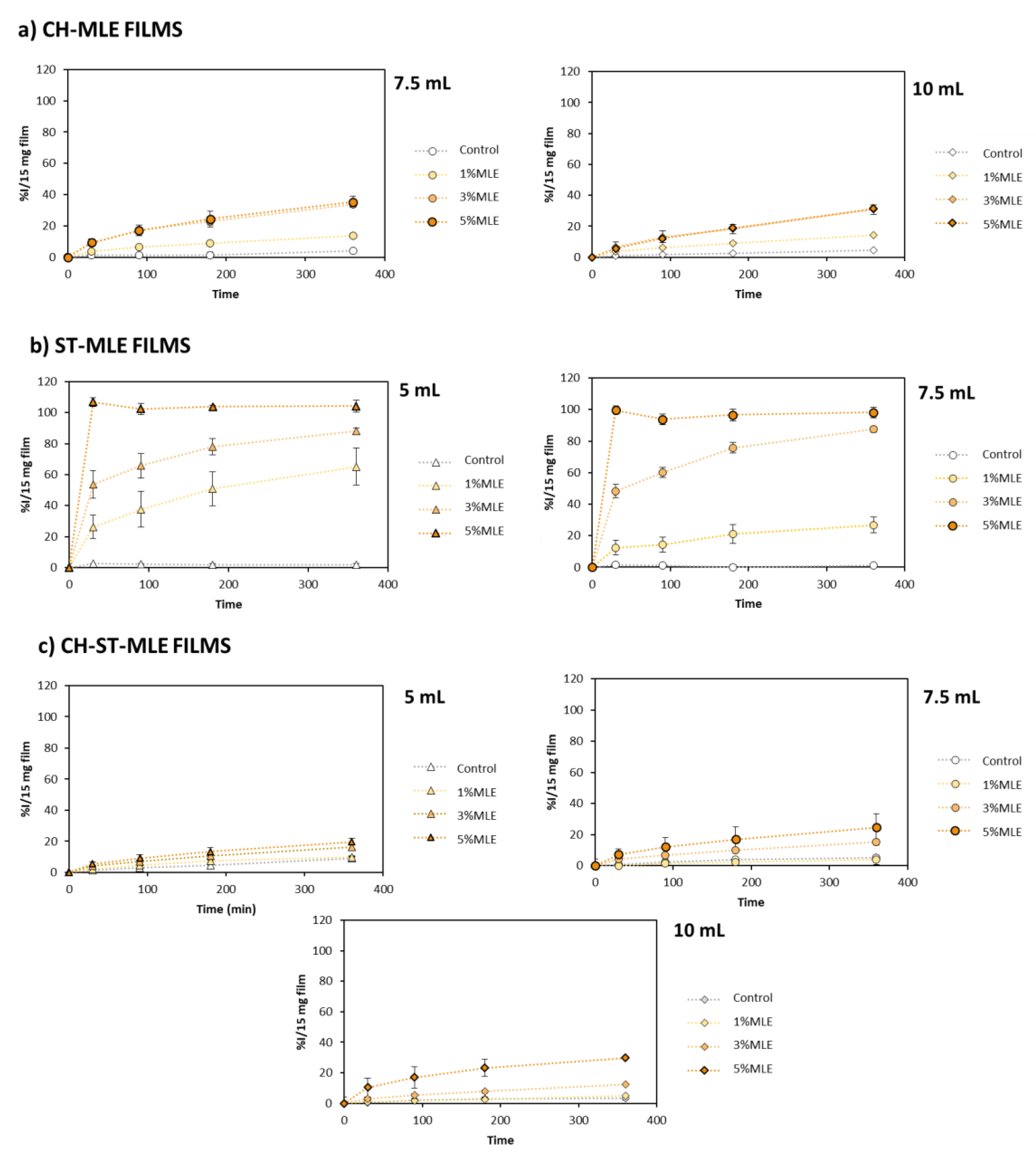
3.3. Bioactive Properties of MLE Films
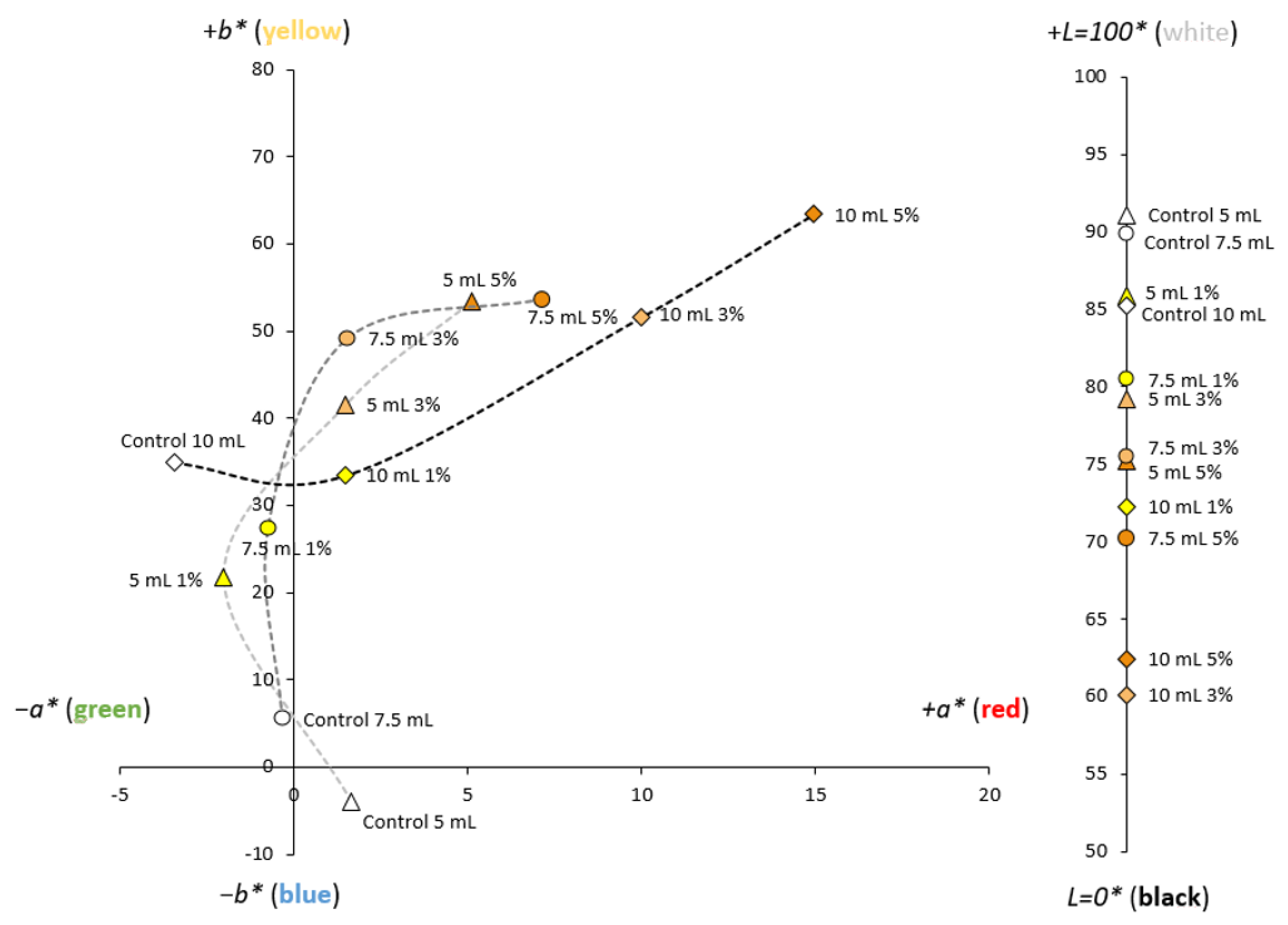
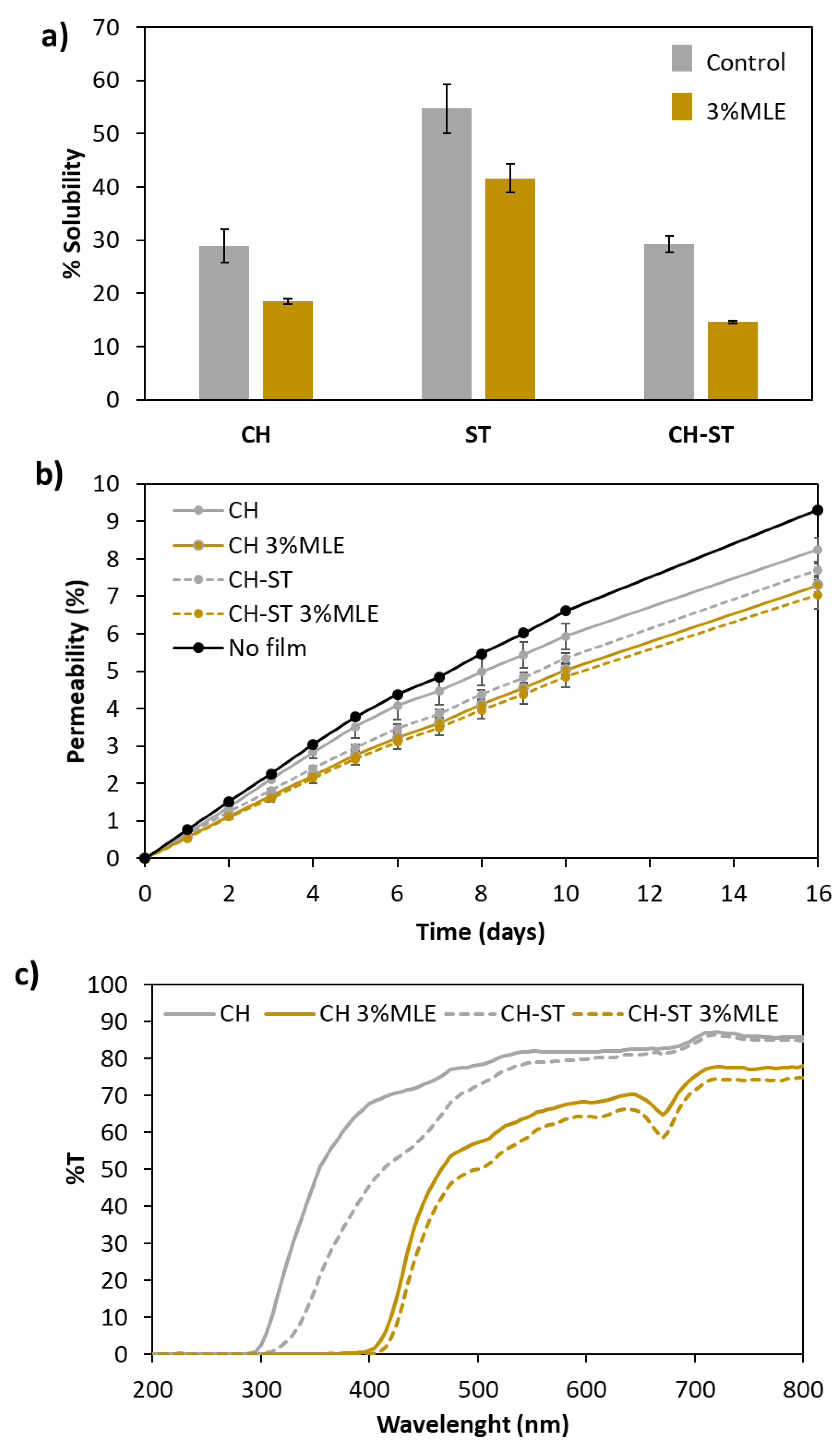
3.4. Physical Properties
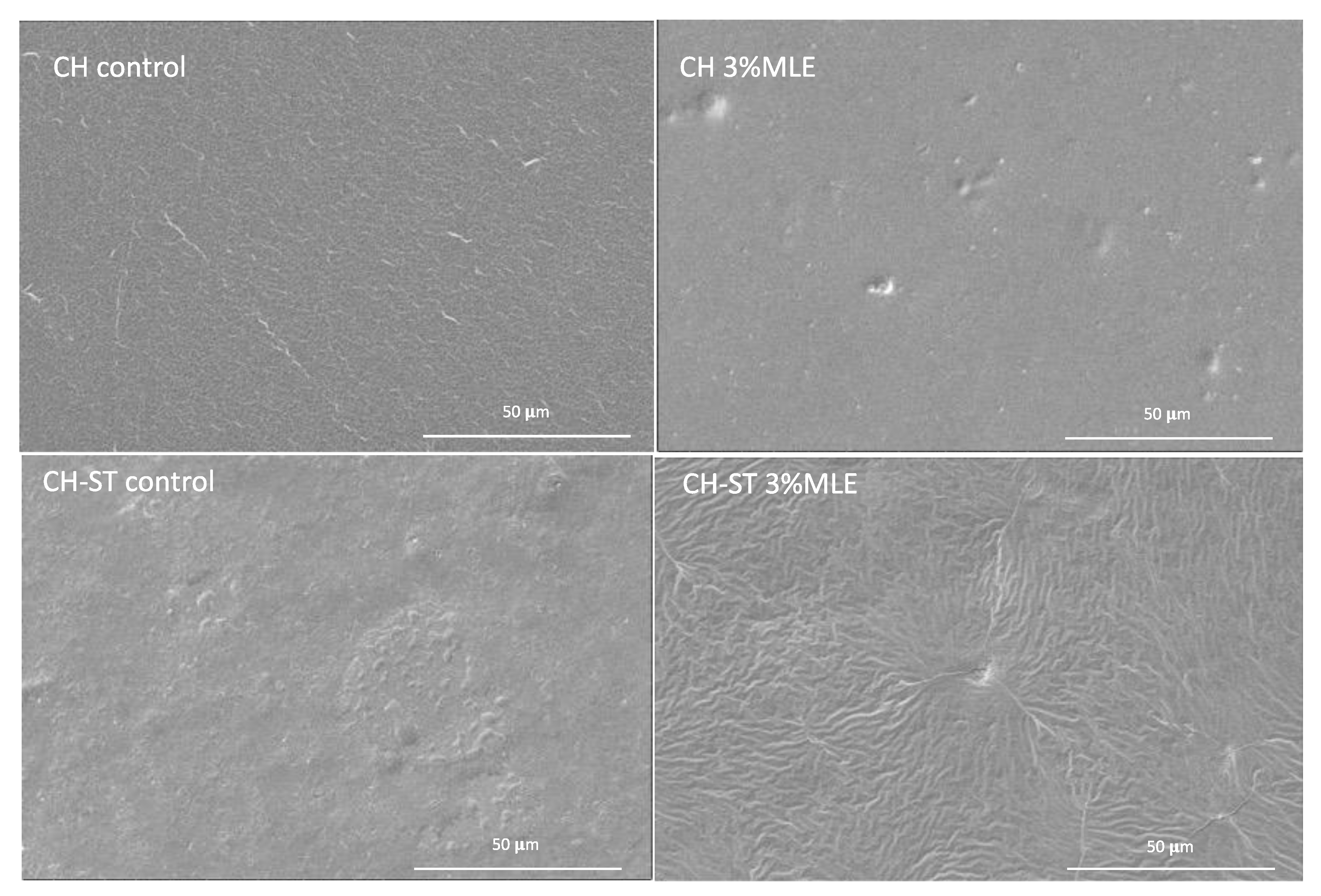
3.5. Scanning Electron Microscopy (SEM)
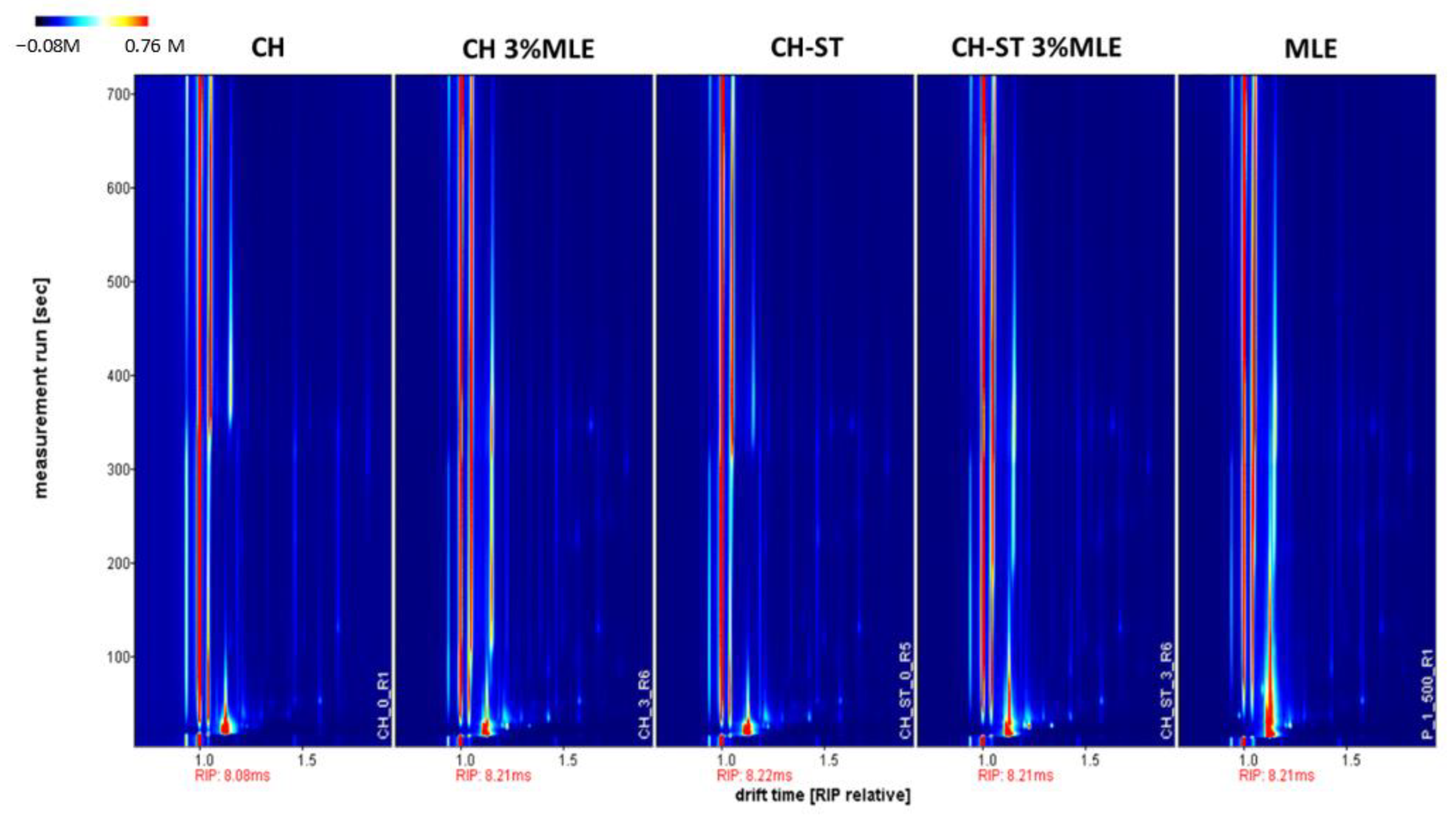
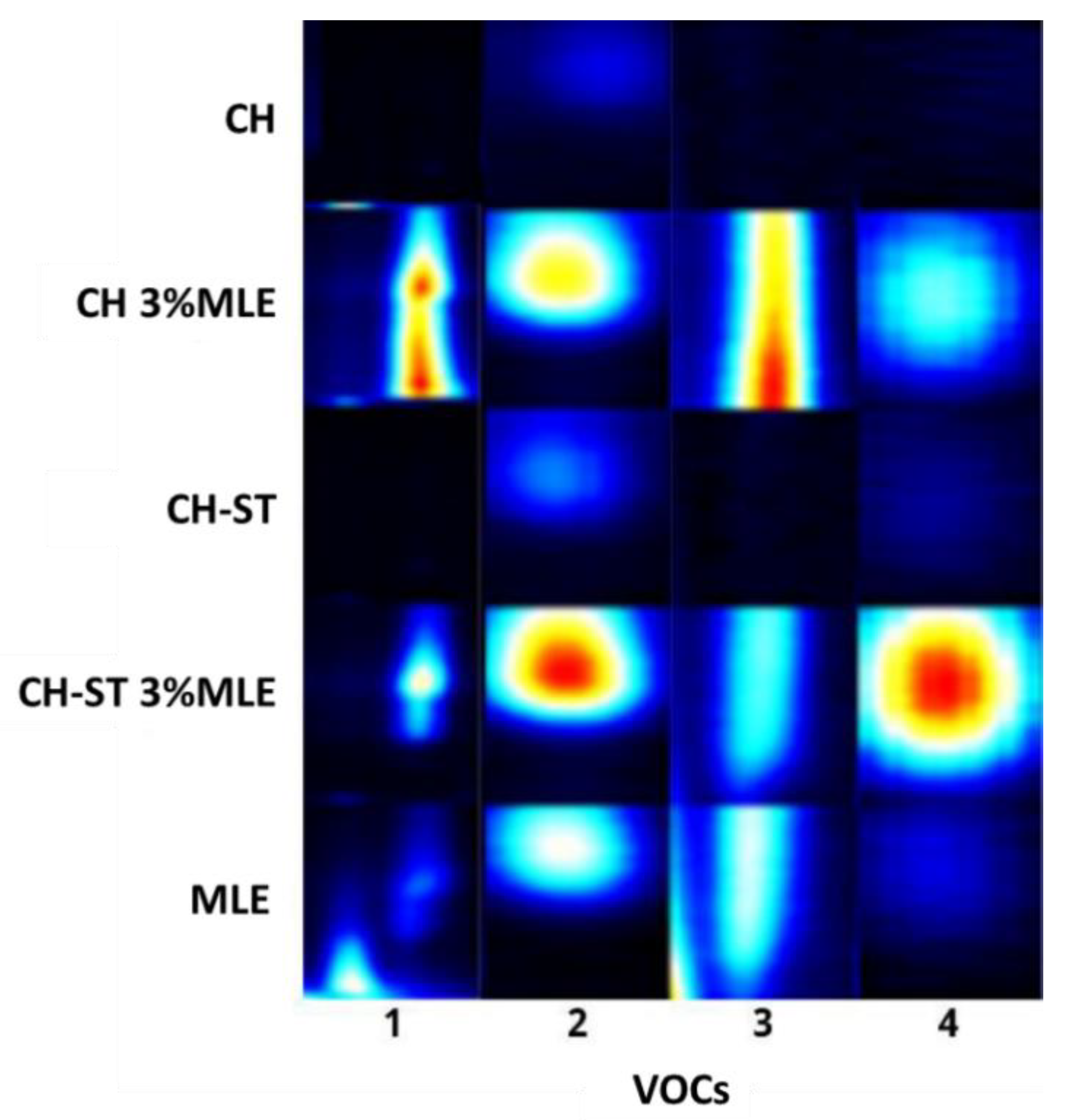
3.6. VOCs Migration
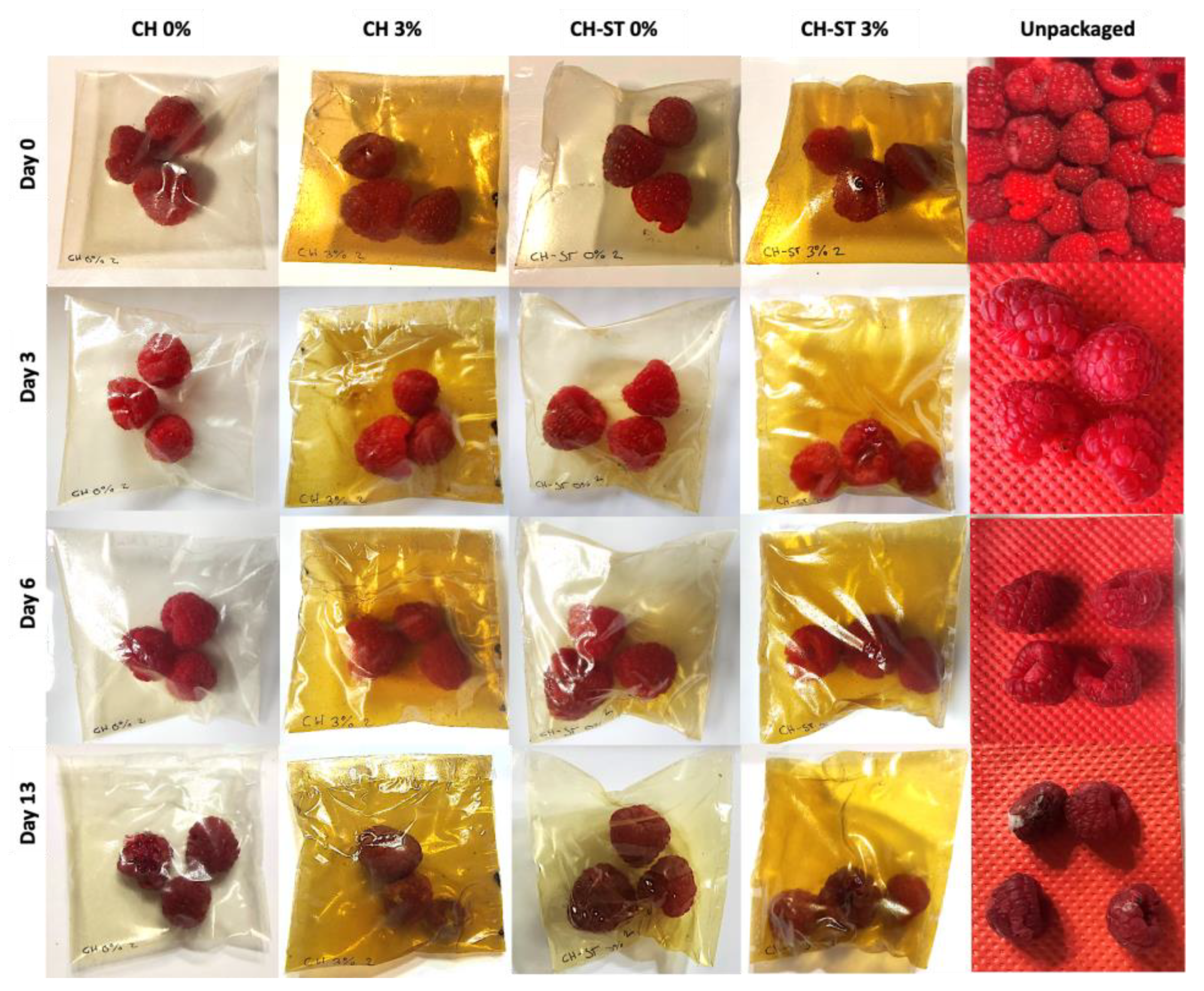
3.7. Raspberry Preservation over Storage Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amit, S.K.; Uddin, M.M.; Rahman, R.; Islam, S.M.R.; Khan, M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017, 6, 51. [Google Scholar] [CrossRef]
- Kong, F.; Singh, R.P. 2—chemical deterioration and physical instability of foods and beverages. In The Stability and Shelf Life of Food, 2nd ed.; Subramaniam, P., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 43–76. [Google Scholar]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of biodegradable polymers in food packaging industry: A comprehensive review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Karimi Sani, I.; Masoudpour-Behabadi, M.; Alizadeh Sani, M.; Motalebinejad, H.; Juma, A.S.M.; Asdagh, A.; Eghbaljoo, H.; Khodaei, S.M.; Rhim, J.-W.; Mohammadi, F. Value-added utilization of fruit and vegetable processing by-products for the manufacture of biodegradable food packaging films. Food Chem. 2023, 405, 134964. [Google Scholar] [CrossRef]
- Azman, N.H.; Khairul, W.M.; Sarbon, N.M. A comprehensive review on biocompatible film sensor containing natural extract: Active/intelligent food packaging. Food Control 2022, 141, 109189. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; HernándezFernández, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bharimalla, A.; Mahapatra, A.; Dhakane-Lad, J.; Arputraj, A.; Kmar, M.; Raja, A.S.M.; Kambli, N. Effect of polymer blending on mechanical and barrier properties of starch-polyvinyl alcohol based biodegradable composite films. Food Biosci. 2021, 44, 101352. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Wu, L.; Lv, S.; Wei, D.; Zhang, S.; Zhang, S.; Li, Z.; Liu, L.; He, T. Structure and properties of starch/chitosan food packaging film containing ultra-low dosage go with barrier and antibacterial. Food Hydrocoll. 2023, 137, 108329. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, L.; Yu, J.; Farag, M.A.; Shao, P. Intelligent starch/chitosan-based film incorporated by anthocyanin-encapsulated amylopectin nanoparticles with high stability for food freshness monitoring. Food Control 2023, 151, 109798. [Google Scholar] [CrossRef]
- Hernández, M.S.; Ludueña, L.N.; Flores, S.K. Citric acid, chitosan and oregano essential oil impact on physical and antimicrobial properties of cassava starch films. Carbohydr. Polym. Technol. Appl. 2023, 5, 100307. [Google Scholar] [CrossRef]
- Hamodin, A.G.; Elgammal, W.E.; Eid, A.M.; Ibrahim, A.G. Synthesis, characterization, and biological evaluation of new chitosan derivative bearing diphenyl pyrazole moiety. Int. J. Biol. Macromol. 2023, 243, 125180. [Google Scholar] [CrossRef] [PubMed]
- Hafsa, J.; Smach, M.A.; Ben Khedher, M.R.; Charfeddine, B.; Limem, K.; Majdoub, H.; Rouatbi, S. Physical, antioxidant and antimicrobial properties of chitosan films containing eucalyptus globulus essential oil. LWT-Food Sci. Technol. 2016, 68, 356–364. [Google Scholar] [CrossRef]
- Sedlaříková, J.; Janalíková, M.; Rudolf, O.; Pavlačková, J.; Egner, P.; Peer, P.; Varaďová, V.; Krejčí, J. Chitosan/thyme oil systems as affected by stabilizing agent: Physical and antimicrobial properties. Coatings 2019, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Maisanaba, S.; Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Puerto, M.; Prieto, A.I.; Jos, A.; Cameán, A.M. New advances in active packaging incorporated with essential oils or their main components for food preservation. Food Rev. Int. 2017, 33, 447–515. [Google Scholar] [CrossRef]
- Luz, R.F.; Ferreira, R.D.R.; Silva, C.N.S.; Miranda, B.M.; Piccoli, R.H.; Silva, M.S.; Paula, L.C.; Leles, M.I.G.; Fernandes, K.F.; Cruz, M.V.; et al. Development of a halochromic, antimicrobial, and antioxidant starch-based film containing phenolic extract from jaboticaba peel. Foods 2023, 12, 653. [Google Scholar] [CrossRef]
- Madureira, J.; Melgar, B.; Alves, V.D.; Moldão-Martins, M.; Margaça, F.M.A.; Santos-Buelga, C.; Barros, L.; Cabo Verde, S. Effect of olive pomace extract application and packaging material on the preservation of fresh-cut royal gala apples. Foods 2023, 12, 1926. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.D.; Canabarro, N.I.; Boeira, C.P.; Melo, P.T.; Aouada, M.R.; da Rosa, C.S. Design of biodegradable films using pecan nut cake extracts for food packing. Foods 2023, 12, 1405. [Google Scholar] [CrossRef]
- Guo, Q.; Yuan, Y.; He, M.; Zhang, X.; Li, L.; Zhang, Y.; Li, B. Development of a multifunctional food packaging for meat products by incorporating carboxylated cellulose nanocrystal and beetroot extract into sodium alginate films. Food Chem. 2023, 415, 135799. [Google Scholar] [CrossRef]
- Song, Z.; Wei, J.; Cao, Y.; Yu, Q.; Han, L. Development and characterization of tapioca starch/pectin composite films incorporated with broccoli leaf polyphenols and the improvement of quality during the chilled mutton storage. Food Chem. 2023, 418, 135958. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; He, B.; Yong, Y.; Zhu, J. Composite films of sodium alginate and konjac glucomannan incorporated with tea polyphenols for food preservation. Int. J. Biol. Macromol. 2023, 242, 124732. [Google Scholar] [CrossRef]
- Oliver-Simancas, R.; Labrador-Fernández, L.; Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Alañón, M.E. Comprehensive research on mango by-products applications in food industry. Trends Food Sci. Technol. 2021, 118, 179–188. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and healthy yogurts fortified with probiotics and fruit peel powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Belizón, M.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa-Fernández, E.J. Supercritical impregnation of antioxidant mango polyphenols into a multilayer pet/pp food-grade film. J. CO2 Util. 2018, 25, 56–67. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L.; Cocoletzi, H.H. Mango leaf extract incorporated chitosan antioxidant film for active food packaging. Int. J. Biol. Macromol. 2019, 126, 1234–1243. [Google Scholar]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Silva, N.H.C.S.; Cardoso, L.C.; Serrano, C.M.; Martínez de la Ossa, E.J.; Freire, C.S.R.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. A novel 1,3,4-thiadiazole modified chitosan: Synthesis, characterization, antimicrobial activity, and release study from film dressings. Appl. Biol. Chem. 2022, 65, 54. [Google Scholar] [CrossRef]
- Vidal, C.P.; Velásquez, E.; Gavara, R.; Hernández-Muñoz, P.; Muñoz-Shugulí, C.; Galotto, M.J.; de Dicastillo, C.L. Modeling the release of an antimicrobial agent from multilayer film containing coaxial electrospun polylactic acid nanofibers. J. Food Eng. 2023, 352, 111524. [Google Scholar] [CrossRef]
- Wu, H.; Wang, J.; Li, T.; Lei, Y.; Peng, L.; Chang, J.; Li, S.; Yuan, X.; Zhou, M.; Zhang, Z. Effects of cinnamon essential oil-loaded Pickering emulsion on the structure, properties and application of chayote tuber starch-based composite films. Int. J. Biol. Macromol. 2023, 240, 124444. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C. Analytical approaches for analysis of safety of modern food packaging: A review. Molecules 2020, 25, 752. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Wang, Z.; Chen, W.; Wang, J. Targeted versus nontargeted green strategies based on headspace-gas chromatography–ion mobility spectrometry combined with chemometrics for rapid detection of fungal contamination on wheat kernels. J. Agric. Food Chem. 2020, 68, 12719–12728. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Mesa, M.; Escourrou, A.; Monteau, F.; Le Bizec, B.; Dervilly-Pinel, G. Current applications and perspectives of ion mobility spectrometry to answer chemical food safety issues. Trends Anal. Chem. 2017, 94, 39–53. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Wang, S.; Mo, H.; Xu, D.; Zhou, W.; Hu, L. Early detection and monitoring for aspergillus flavus contamination in maize kernels. Food Control 2021, 121, 107636. [Google Scholar] [CrossRef]
- Rosales, J.M.; Cejudo, C.; Verano, L.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J. Supercritical impregnation of pla filaments with mango leaf extract to manufacture functionalized biomedical devices by 3D printing. Polymers 2021, 13, 2125. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Ruiz-Navajas, Y.; Viuda-Martos, M.; Sendra, E.; Perez-Alvarez, J.A.; Fernández-López, J. In vitro antibacterial and antioxidant properties of chitosan edible films incorporated with thymus moroderi or thymus piperella essential oils. Food Control 2013, 30, 386–392. [Google Scholar] [CrossRef]
- Piñeros-Hernandez, D.; Medina-Jaramillo, C.; López-Córdoba, A.; Goyanes, S. Edible cassava starch films carrying rosemary antioxidant extracts for potential use as active food packaging. Food Hydrocoll. 2017, 63, 488–495. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Melo-Banda, J.A.; Páramo-García, U.; Paraguay-Delgado, F.; García-Alamilla, R.; Martínez-Hernández, A.L.; Zapién-Castillo, S. Chitosan-starch films with natural extracts: Physical, chemical, morphological and thermal properties. Materials 2018, 11, 120. [Google Scholar] [CrossRef] [Green Version]
- Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of food packaging films to provide antioxidant properties. J. Supercrit. Fluids 2017, 128, 200–207. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Casas Cardoso, L.; Fernández-Ponce, M.T.; Mantell Serrano, C.; Martínez de la Ossa, E.J. Supercritical impregnation of olive leaf extract to obtain bioactive films effective in cherry tomato preservation. Food Packag. Shelf Life 2019, 21, 100338. [Google Scholar] [CrossRef]
- Moussa, S.H.; Tayel, A.A.; Al-Hassan, A.A.; Farouk, A. Tetrazolium/formazan test as an efficient method to determine fungal chitosan antimicrobial activity. J. Mycol. 2013, 2013, 7. [Google Scholar] [CrossRef] [Green Version]
- Binninger, A.S. Perception of Naturalness of Food Packaging and Its Role in Consumer Product. Evaluation. J. Food Prod. Mark. 2015, 23, 251–266. [Google Scholar] [CrossRef]
- Deng, X.; Srinivasan, R. When Do Transparent Packages Increase (or Decrease) Food Consumption? J. Mark. 2013, 7, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Serrano, C.M.; Bigger, S.W. Mass Transfer and Optical Properties of Active PET/PP Food-Grade Films Impregnated with Olive Leaf Extract. Polymers 2022, 14, 84. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan–polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Verano-Naranjo, L.; Toro-Barrios, N.; Pereyra, C.; Mantell, C.; Casas, L. Structural modification of polymers functionalized with mango leaf extract by supercritical impregnation: Approaching of further food and biomedical applications. Polymers 2022, 14, 2413. [Google Scholar] [CrossRef]
- Calle, J.L.P.; Vázquez-Espinosa, M.; Barea-Sepúlveda, M.; Ruiz-Rodríguez, A.; Ferreiro-González, M.; Palma, M. Novel Method Based on Ion Mobility Spectrometry Combined with Machine Learning for the Discrimination of Fruit Juices. Foods 2023, 12, 2536. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and uv blocking properties of composite chitosan films with curcumin grafted cellulose nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Sánchez-Gomar, I.; Benítez-Camacho, J.; Cejudo-Bastante, C.; Casas, L.; Moreno-Luna, R.; Mantell, C.; Durán-Ruiz, M.C. Pro-Angiogenic Effects of Natural Antioxidants Extracted from Mango Leaf, Olive Leaf and Red Grape Pomace over Endothelial Colony-Forming Cells. Antioxidants 2022, 11, 851. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial activity and composition profile of grape (vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanchez, J.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; de la Ossa, E.J.M. Impregnation of mango leaf extract into a polyester textile using supercritical carbon dioxide. J. Supercrit. Fluids 2017, 128, 208–217. [Google Scholar] [CrossRef]
- Van den Broek, L.A.; Knoop, R.J.; Kappen, F.H.; Boeriu, C.G. Chitosan films and blends for packaging material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and biodegradable starch films: A review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Xu, Y.X.; Kim, K.M.; Hanna, M.A.; Nag, D. Chitosan–starch composite film: Preparation and characterization. Ind. Crops Prod. 2005, 21, 185–192. [Google Scholar] [CrossRef]
- Bourtoom, T.; Chinnan, M.S. Preparation and properties of rice starch–chitosan blend biodegradable film. LWT-Food Sci. Technol. 2008, 41, 1633–1641. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Fernando, A.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B. Physical properties of chitosan films incorporated with natural antioxidants. Ind. Crops Prod. 2017, 107, 565–572. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Nerín, C.; Alfaro, P.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Development of new antioxidant active packaging films based on ethylene vinyl alcohol copolymer (evoh) and green tea extract. J. Agric. Food Chem. 2011, 59, 7832–7840. [Google Scholar] [CrossRef]
- Rossi-Márquez, G.; Han, J.H.; García-Almendárez, B.; Castaño-Tostado, E.; Regalado-González, C. Effect of temperature, ph and film thickness on nisin release from antimicrobial whey protein isolate edible films. J. Sci. Food Agric. 2009, 89, 2492–2497. [Google Scholar] [CrossRef]
- Verano Naranjo, L.; Cejudo Bastante, C.; Casas Cardoso, L.; Mantell Serrano, C.; Martínez de la Ossa Fernández, E.J. Supercritical impregnation of ketoprofen into polylactic acid for biomedical application: Analysis and modeling of the release kinetic. Polymers 2021, 13, 1982. [Google Scholar] [CrossRef]
- Surendren, A.; Mohanty, A.K.; Liu, Q.; Misra, M. A review of biodegradable thermoplastic starches, their blends and composites: Recent developments and opportunities for single-use plastic packaging alternatives. Green Chem. 2022, 24, 8606–8636. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Properties of wheat starch film-forming dispersions and films as affected by chitosan addition. J. Food Eng. 2013, 114, 303–312. [Google Scholar] [CrossRef]
- Talón, E.; Trifkovic, K.T.; Nedovic, V.A.; Bugarski, B.M.; Vargas, M.; Chiralt, A.; González-Martínez, C. Antioxidant edible films based on chitosan and starch containing polyphenols from thyme extracts. Carbohydr. Polym. 2017, 157, 1153–1161. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of pla-pbsa based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Maharaj, A.; Naidoo, Y.; Dewir, Y.H.; Rihan, H. Phytochemical screening, and antibacterial and antioxidant activities of Mangifera indica L. Leaves. Horticulturae 2022, 8, 909. [Google Scholar] [CrossRef]
- Quintana, S.E.; Salas, S.; García-Zapateiro, L.A. Bioactive compounds of mango (mangifera indica): A review of extraction technologies and chemical constituents. J. Sci. Food Agric. 2021, 101, 6186–6192. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.T.; Gómez, E.; Cejudo-Bastante, C.; Casas, L.; Montes, A.; Mantell, C.; de la Ossa-Fernández, E.J.M.; Pereyra, C. Development of functionalized alginate dressing with mango polyphenols by supercritical technique to be employed as an antidiabetic transdermal system. J. Supercrit. Fluids 2021, 175, 105274. [Google Scholar] [CrossRef]
- Madera-Santana, M.J.; Herrera Méndez, C.; Rodríguez, J.R. An overview of the chemical modifications of chitosan and their advantages. Green Mater. 2018, 6, 131–142. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Liu, Q.; Jin, Z.; Jiao, A. Preparation and characterization of hydroxypropyl methylcellulose/hydroxypropyl starch composite films reinforced by chitosan nanoparticles of different sizes. Mater. Today Commun. 2023, 35, 105714. [Google Scholar] [CrossRef]
- Bangyekan, C.; Aht-Ong, D.; Srikulkit, K. Preparation and properties evaluation of chitosan-coated cassava starch films. Carbohydr. Polym. 2006, 63, 61–71. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C. The effects of chitosan and grape seed extract-based edible films on the quality of vacuum packaged chicken breast fillets. Food Packag. Shelf Life 2018, 18, 13–20. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Freire, C.S.R.; Vilela, C. Chapter 9—Active packaging. In Sustainable Food Processing and Engineering Challenges; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 315–341. [Google Scholar]
- Grosso, P.; Cejudo, C.; Sánchez-Gomar, I.; Durán-Ruiz, M.C.; Moreno-Luna, R.; Casas, L.; Pereyra, C.; Mantell, C. Supercritical impregnation of mango leaf extract into pla 3D-printed devices and evaluation of their biocompatibility with endothelial cell cultures. Polymers 2022, 14, 2706. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liao, W.; Xia, W. Recent advances in chitosan based bioactive materials for food preservation. Food Hydrocoll. 2023, 140, 108612. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Gierszewska, M.; Wrona, M.; Nerin, C.; Grabska-Zielińska, S. Polylactide-based films with the addition of poly(ethylene glycol) and extract of propolis-physico-chemical and storage properties. Foods 2022, 11, 1488. [Google Scholar] [CrossRef]
- Lucera, A.; Conte, A.; Del Nobile, M.A. Chapter 25—volatile compounds usage in active packaging systems. In Antimicrobial Food Packaging; Barros-Velázquez, J., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 319–327. [Google Scholar]
- Avila-Sosa, R.; Palou, E.; Jiménez Munguía, M.T.; Nevárez-Moorillón, G.V.; Navarro Cruz, A.R.; López-Malo, A. Antifungal activity by vapor contact of essential oils added to amaranth, chitosan, or starch edible films. Int. J. Food Microbiol. 2012, 153, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Huynh, N.K.; Wilson, M.D.; Stanley, R.A. Extending the shelf life of raspberries in commercial settings by modified atmosphere/modified humidity packaging. Food Packag. Shelf Life 2023, 37, 101069. [Google Scholar] [CrossRef]
- Sun, J.; Wei, Z.; Xue, C. Preparation and characterization of multifunctional films based on pectin and carboxymethyl chitosan: Forming microchambers for high-moisture fruit preservation. Food Packag. Shelf Life 2023, 37, 101073. [Google Scholar] [CrossRef]
- Cortés, L.A.; Herrera, A.O.; Castellanos, D.A. Natural plant-based compounds applied in antimicrobial active packaging and storage of berries. J. Food Process. Preserv. 2022, 46, e16995. [Google Scholar] [CrossRef]
- Choi, I.; WHong, W.; Lee, S.S.; Han, J. Influence of acetylation and chemical interaction on edible film properties and different processing methods for food application. Food Chem. 2023, 426, 136555. [Google Scholar] [CrossRef]
- Xie, C.; Wang, F.; He, Z.; Tang, H.; Li, H.; Hou, J.; Liu, Y.; Jiang, L. Development and characterization of active packaging based on chitosan/chitin nanofibers incorporated with scallion flower extract and its preservation in fresh-cut bananas. Int. J. Biol. Macromol. 2023, 242, 125045. [Google Scholar] [CrossRef]

| Thickness (mm) | mg MLE/g Film | ||||||
|---|---|---|---|---|---|---|---|
| Volume of Film Solution | MLE Addition (% v/v) | CH | ST | CH-ST | CH | ST | CH-ST |
| 5 mL | Control | - | 0.12 ± 0.01 | 0.05 ± 0.01 | - | - | - |
| 1%MLE | - | 0.13 ± 0.02 | 0.04 ± 0.01 | - | 8.99 ± 0.16 | 36.28 ± 0.44 | |
| 3%MLE | - | 0.10 ± nd | 0.11 ± 0.03 | - | 34.60 ± 0.08 | 101.65 ± 1.75 | |
| 5%MLE | - | 0.13 ± nd | 0.13 ± 0.02 | - | 44.72 ± nd | 160.85 ± 1.15 | |
| 7.5 mL | Control | 0.08 ± 0.01 | 0.23 ± 0.05 | 0.14 ± 0.01 | - | - | - |
| 1%MLE | 0.08 ± 0.01 | 0.19 ± 0.04 | 0.11 ± 0.01 | 35.15 ± 0.07 | 8.77 ± nd | 35.54 ± 0.11 | |
| 3%MLE | 0.14 ± 0.04 | 0.19 ± 0.01 | 0.12 ± 0.02 | 97.73 ± 3.07 | 36.25 ± 0.78 | 105.92 ± 0.57 | |
| 5%MLE | 0.12 ± 0.04 | 0.20 ± 0.01 | 0.11 ± 0.02 | 141.85 ± 7.53 | 42.83 ± nd | 174.54 ± 2.25 | |
| 10 mL | Control | 0.08 ± 0.01 | - | 0.06 ± nd | - | - | - |
| 1%MLE | 0.14 ± 0.04 | - | 0.06 ± nd | 35.25 ± 0.18 | - | 35.12 ± 0.09 | |
| 3%MLE | 0.12 ± 0.04 | - | 0.16 ± 0.01 | 105.31 ± 5.77 | - | 100.85 ± 0.15 | |
| 5%MLE | 0.13 ± 0.04 | - | 0.15 ± 0.02 | 175.31 ± 2.69 | - | 168.52 ± 0.78 | |
| Film Formulation | S. aureus (%IMG/50 mg Film) | E. coli (%IMG/50 mg Film) |
|---|---|---|
| CH control | 62.83 ± 2.26 b | 74.65 ± 3.54 c |
| CH 3% MLE | 76.82 ± 1.02 c | 77.55 ± 1.60 c |
| ST control | 11.12 ± 1.30 a | 7.28 ± 2.28 a |
| ST 3% MLE | 12.86 ± 3.31 a | 8.92 ± 0.08 a |
| CH-ST control | 63.02 ± 2.35 b | 64.75 ± 1.42 b |
| CH-ST 3% MLE | 80.13 ± 0.16 c | 74.09 ± 3.19 c |
| Film Formulation | Day 3 | Day 6 | Day 13 |
|---|---|---|---|
| Control | 0/9 (0%) | 2/9 (22.2%) | 7/9 (77.8%) |
| CH 0% | 0/9 (0%) | 0/9 (0%) | 5/9 (55.5%) |
| CH 3% | 0/9 (0%) | 0/9 (0%) | 4/9 (44.4%) |
| CH-ST 0% | 0/9 (0%) | 0/9 (0%) | 4/9 (44.4%) |
| CH-ST 3% | 0/9 (0%) | 0/9 (0%) | 3/9 (33.3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cejudo, C.; Ferreiro, M.; Romera, I.; Casas, L.; Mantell, C. Functional, Physical, and Volatile Characterization of Chitosan/Starch Food Films Functionalized with Mango Leaf Extract. Foods 2023, 12, 2977. https://doi.org/10.3390/foods12152977
Cejudo C, Ferreiro M, Romera I, Casas L, Mantell C. Functional, Physical, and Volatile Characterization of Chitosan/Starch Food Films Functionalized with Mango Leaf Extract. Foods. 2023; 12(15):2977. https://doi.org/10.3390/foods12152977
Chicago/Turabian StyleCejudo, Cristina, Marta Ferreiro, Irene Romera, Lourdes Casas, and Casimiro Mantell. 2023. "Functional, Physical, and Volatile Characterization of Chitosan/Starch Food Films Functionalized with Mango Leaf Extract" Foods 12, no. 15: 2977. https://doi.org/10.3390/foods12152977
APA StyleCejudo, C., Ferreiro, M., Romera, I., Casas, L., & Mantell, C. (2023). Functional, Physical, and Volatile Characterization of Chitosan/Starch Food Films Functionalized with Mango Leaf Extract. Foods, 12(15), 2977. https://doi.org/10.3390/foods12152977









