Major Active Metabolite Characteristics of Dendrobium officinale Rice Wine Fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus Cofermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Rice Wine Fermentation with Mixed Yeasts
2.3. Basic Physical and Chemical Index Determinations
2.4. Sensory Evaluation
2.5. Determination of Active Substance Content
2.6. Nontargeted Metabolomic Assay of Rice Wine Metabolites
2.6.1. Extraction of Metabolites
2.6.2. Metabolite Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sensory and Physicochemical Analyses of Rice Wine Fermented with Mixed Yeasts
3.2. Metabolomic Analysis of Rice Wine
3.2.1. Metabolite KEGG Pathway Enrichment Analysis
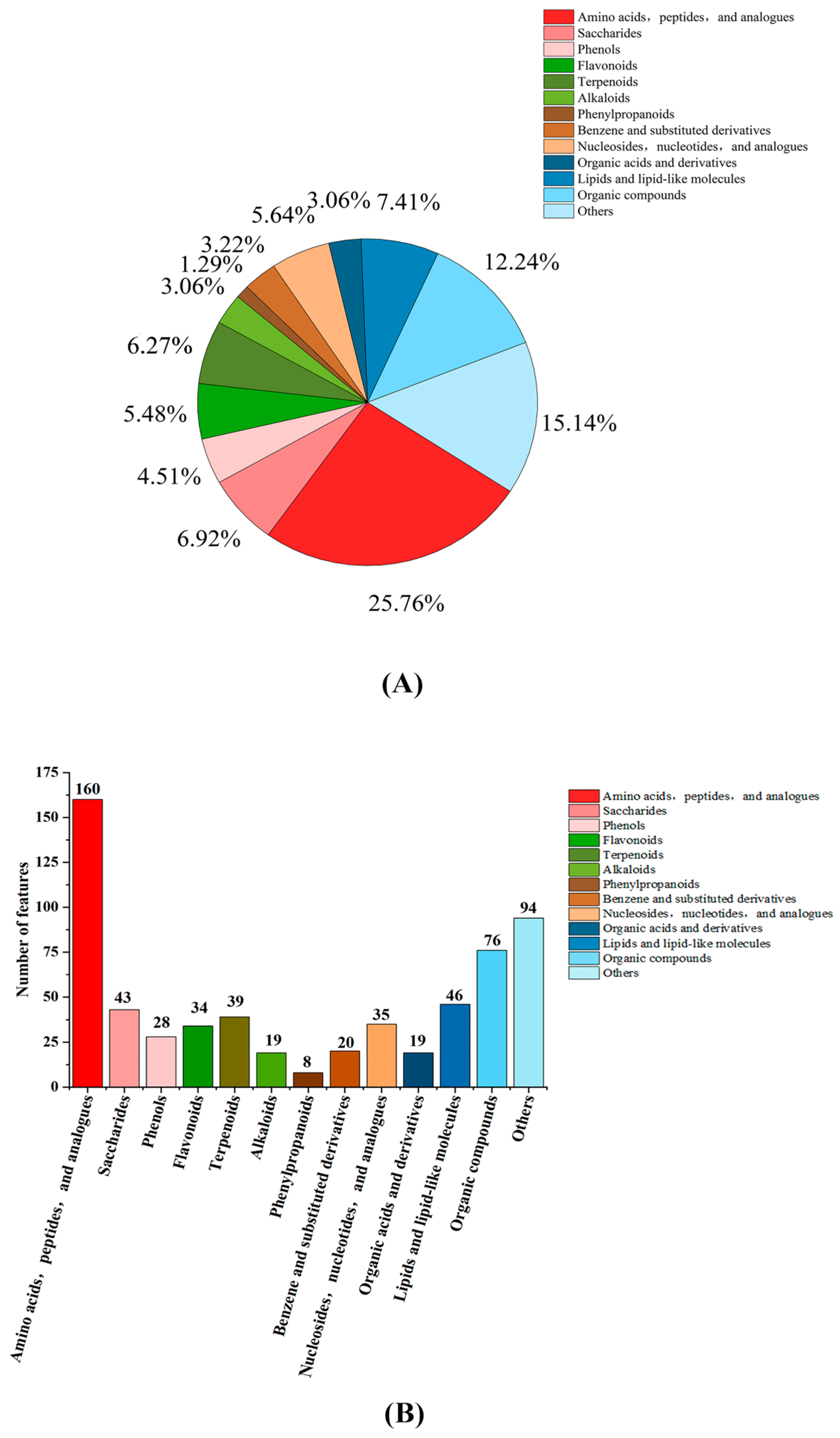
3.2.2. Statistical Analysis of Metabolite Classification
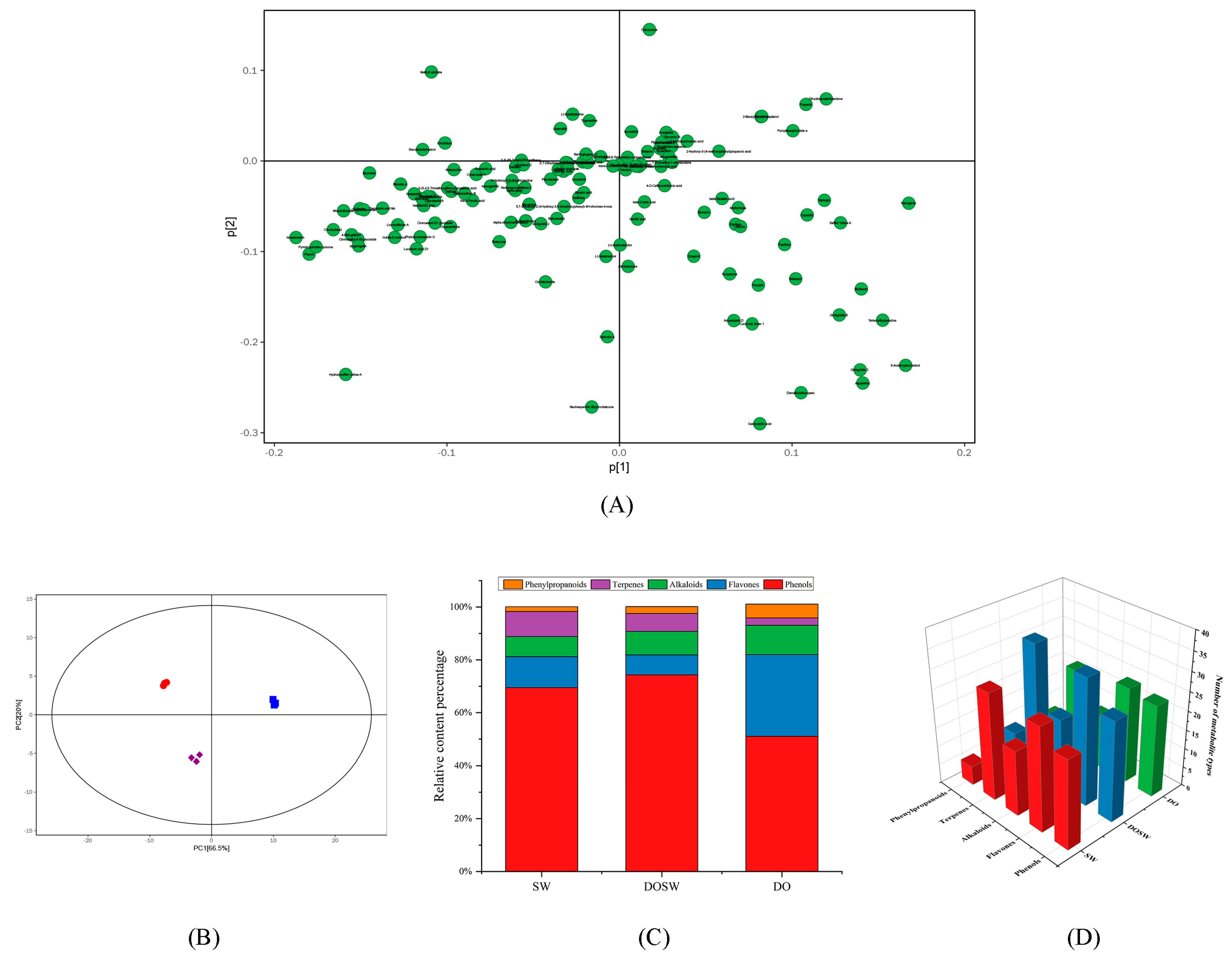
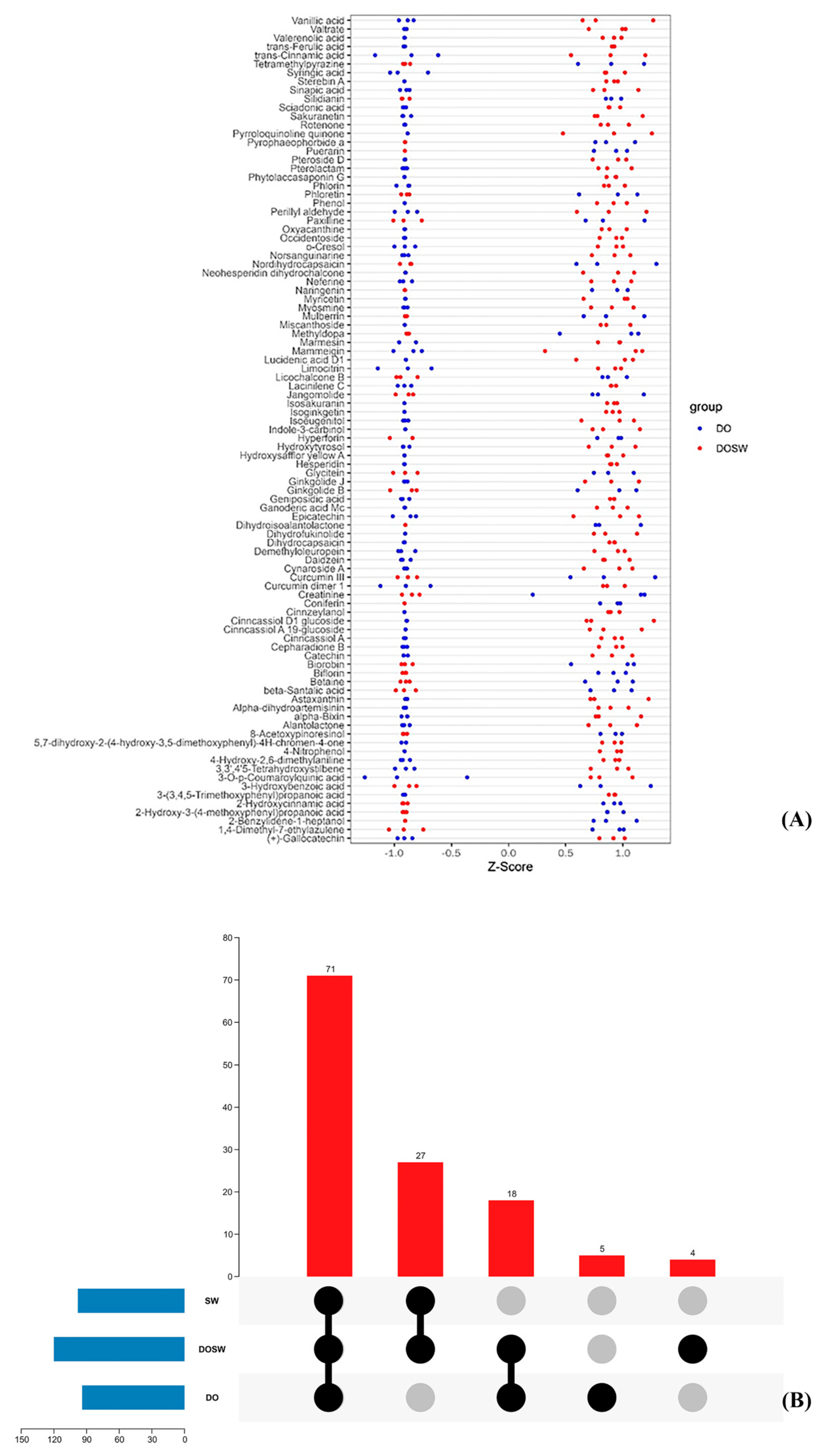
3.3. Analysis of Major Active Metabolites
3.4. Changes in The Main Active Substances in Rice Wine
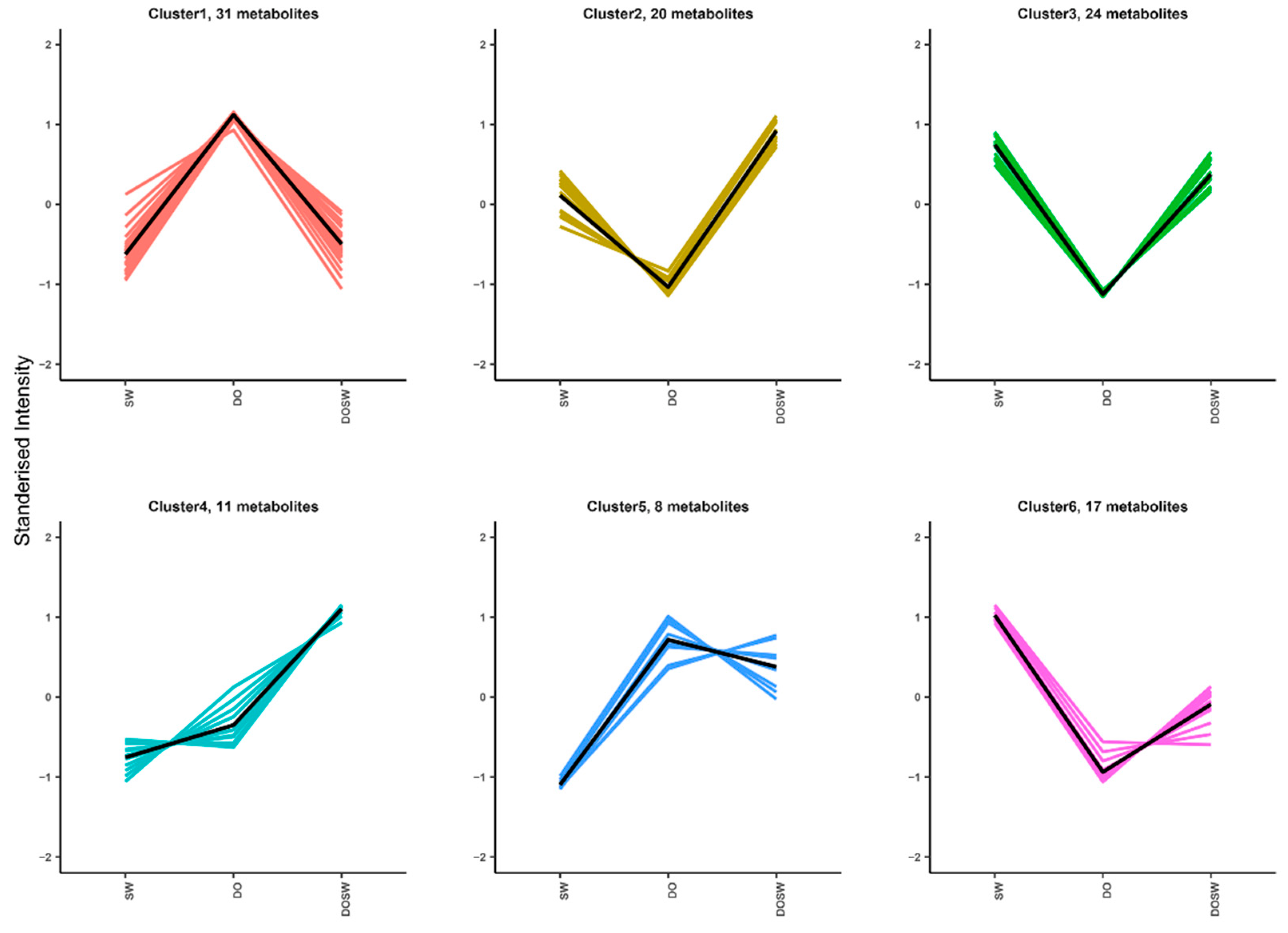
3.4.1. K-Means Analysis of The Main Active Metabolites
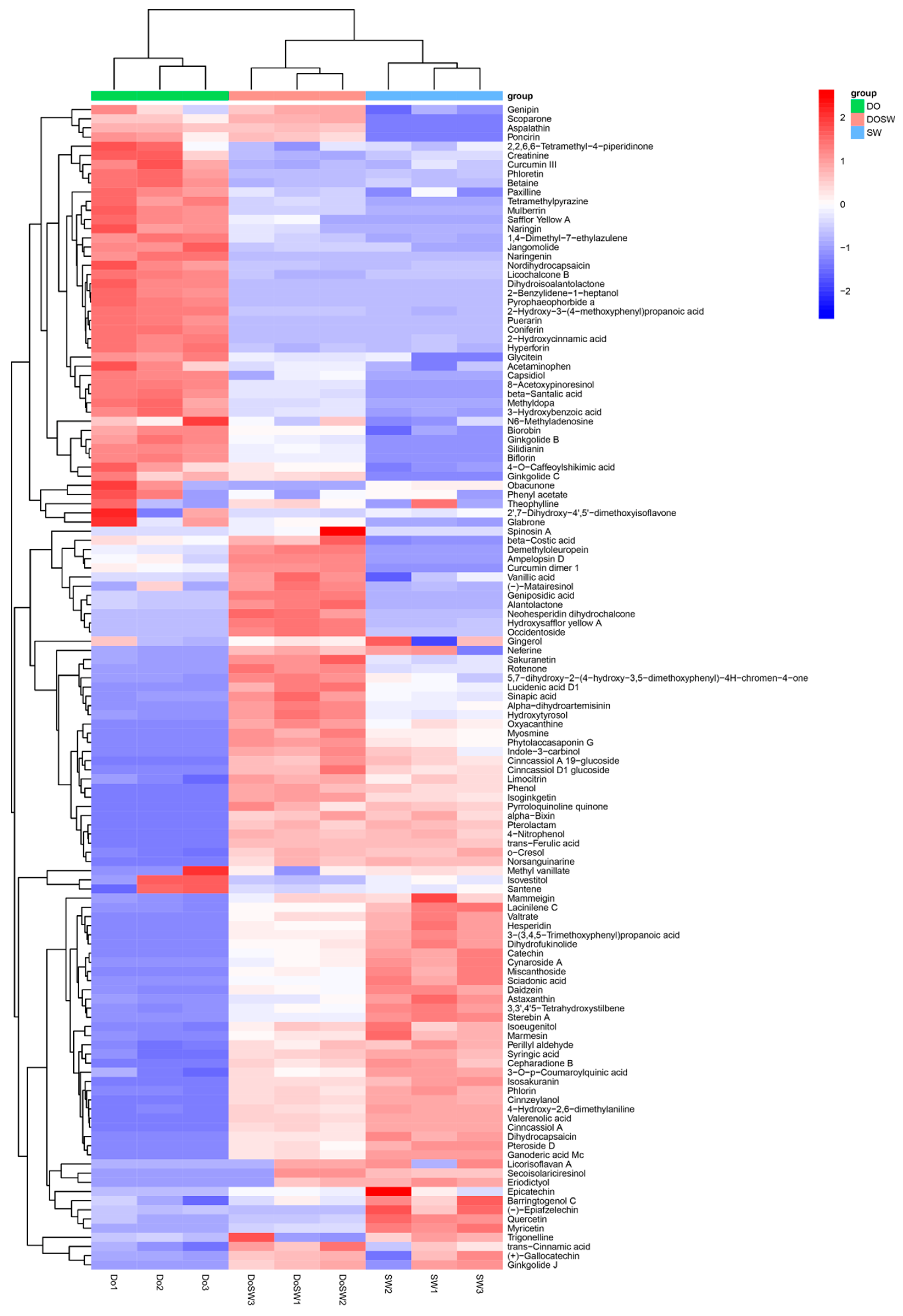
3.4.2. Cluster Analysis of The Main Active Metabolites
3.5. Screening of Major Active Differential Metabolites
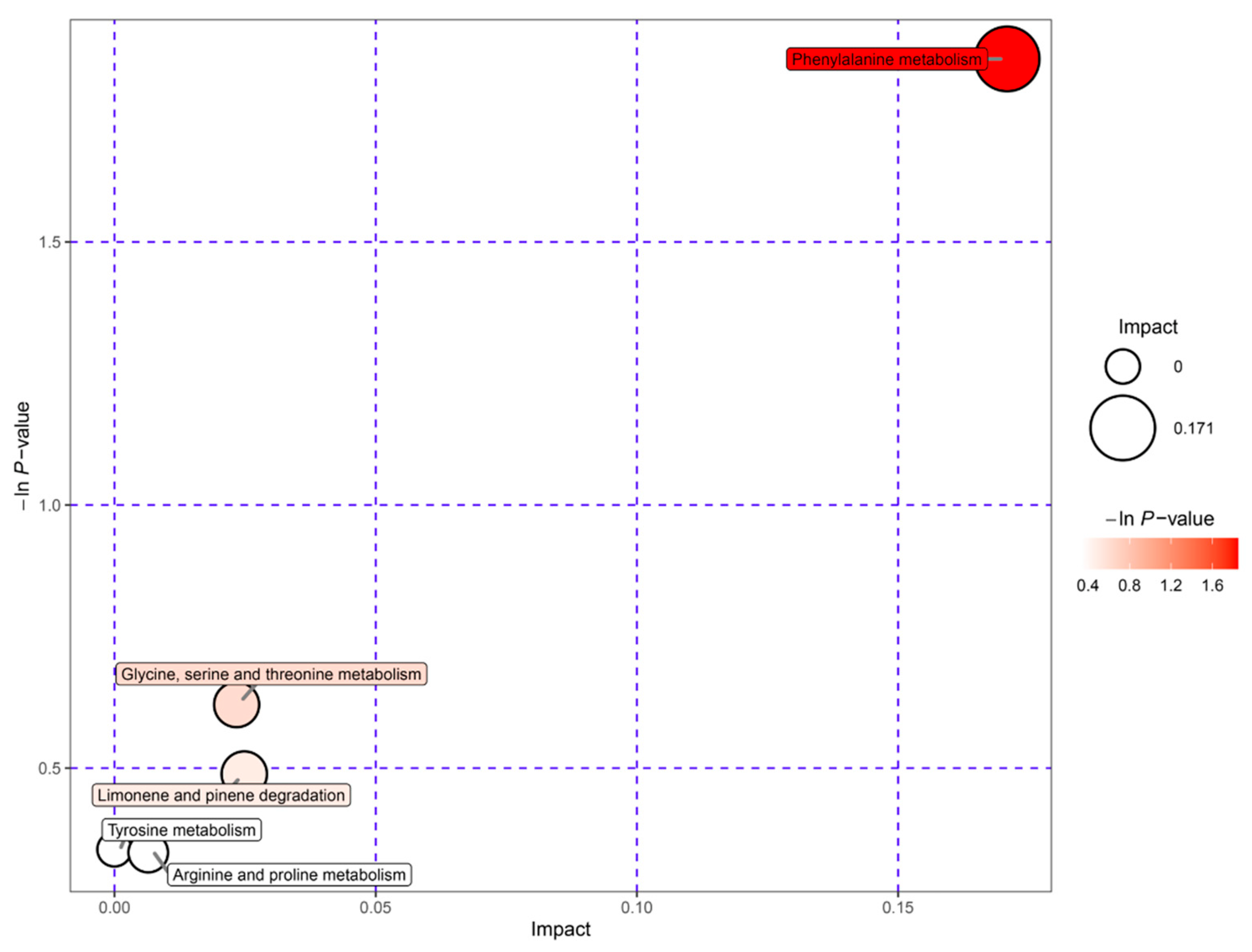
3.6. Pathway Analysis of Key Active Differential Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lencioni, L.; Romani, C.; Gobbi, M.; Comitini, F.; Ciani, M.; Domizio, P. Controlled mixed fermentation at winery scale using Zygotorulaspora florentina and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2016, 234, 36–44. [Google Scholar]
- Sanoppa, K.; Huang, T.C.; Wu, M.C. Effects of Saccharomyces cerevisiae in association with Torulaspora delbrueckii on the aroma and amino acids in longan wines. Food Sci. Nutr. 2019, 7, 2817–2826. [Google Scholar]
- Yang, Y.; Xia, Y.; Wang, G.; Yu, J.; Ai, L. Effect of mixed yeast starter on volatile flavor compounds in chinese rice wine during different brewing stages. LWT 2017, 78, 373–381. [Google Scholar]
- Morrison−Whittle, P.; Lee, S.A.; Fedrizzi, B.; Goddard, M.R. Co−evolution as tool for diversifying flavor and aroma profiles of wines. Front. Microbiol. 2018, 9, 910. [Google Scholar]
- Peng, C.; Viana, T.; Petersen, M.A.; Larsen, F.H.; Arneborg, N. Metabolic footprint analysis of metabolites that discriminate single a nd mixed yeast cultures at two key time−points during mixed culture al coholic fermentations. Metabolomics 2018, 14, 93. [Google Scholar]
- Roullier−Gall, C.; David, V.; Hemmler, D.; Schmitt−Kopplin, P.; Alexandre, H. Exploring yeast interactions through metabolic profiling. Sci. Rep. 2020, 10, 6073. [Google Scholar]
- Sun, N.; Gao, Z.; Li, S.; Chen, X.; Guo, J. Assessment of chemical constitution and aroma properties of kiwi wines obtained from pure and mixed fermentation with wickerhamomyces anomalus and saccharomyces cerevisiae. J. Sci. Food Agric. 2022, 102, 175–184. [Google Scholar]
- Feng, X.; Shi, Y.; Zhou, Z.; Ji, Z.; Zhou, W.; Chen, S.; Mao, J. Alleviation of loperamide−induced constipation with sticky rice fermented huangjiu by the regulation of serum neurotransmitters and gut microbiota. J. Sci. Food Agric. 2023, 103, 692–704. [Google Scholar]
- Shoji, M.; Sugimoto, M.; Matsuno, K.; Fujita, Y.; Mii, T.; Ayaki, S.; Takeuchi, M.; Yamaji, S.; Tanaka, N.; Takahashi, E.; et al. A novel aqueous extract from rice fermented with aspergillus oryzae and saccharomyces cerevisiae possesses an anti−influenza a virus activity. PLoS ONE 2021, 16, e0244885. [Google Scholar]
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional uses, phytochemistry, pharmacology, and quality control of dendrobium officinale kimura et. Migo. Front. Pharmacol. 2021, 12, 726528. [Google Scholar]
- Yan, X.T.; Zhang, W.; Zhang, Y.; Zhang, Z.; Chen, D.; Wang, W.; Ma, W.; Qu, H.; Qian, J.Y.; Gu, R. In vitro anti−obesity effect of shenheling extract (shle) fermented with lactobacillus fermentum grx08. Foods 2022, 11, 1221. [Google Scholar]
- Wang, C.; Tang, J.; Qiu, S. Profiling of fungal diversity and fermentative yeasts in traditional chinese xiaoqu. Front. Microbiol. 2020, 11, 2103. [Google Scholar]
- Marier, J.R.; Boulet, M.J. Direct Analysis of Lactose in Milk and Serum. J. Dairy Sci. 1959, 42, 1390–1391. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar]
- Escudero-López, B.; Cerrillo, I.; Herrero-Martín, G.; Hornero-Méndez, D.; Gil-Izquierdo, A.; Medina, S.; Ferreres, F.; Berná, G.; Martín, F.; Fernández-Pachón, M.-S. Fermented orange juice: Source of higher carotenoid and flavanone cont ents. J. Agric. Food Chem. 2013, 61, 8773–8782. [Google Scholar]
- Chassy, A.W.; Adams, D.O.; Laurie, V.F.; Waterhouse, A.L. Tracing phenolic biosynthesis in vitis vinifera via in situ c−13 labeling and liquid chromatography−diode−array detector−mass spectrometer/mass spectrometer detection. Anal. Chim. Acta 2012, 747, 51–57. [Google Scholar]
- Wagner, K.H.; Elmadfa, I. Biological relevance of terpenoids. Overview focusing on mono−, di− and tetraterpenes. Ann. Nutr. Metab. 2003, 47, 95–106. [Google Scholar]
- Zhou, Y.X.; Zhang, H.; Peng, C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014, 28, 961–975. [Google Scholar]
- Ciulu, M.; Cadiz−Gurrea, M.L.; Segura−Carretero, A. Extraction and analysis of phenolic compounds in rice: A review. Molecules 2018, 23, 2890. [Google Scholar]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti−inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar]
- Setford, P.C.; Jeffery, D.W.; Grbin, P.R.; Muhlack, R.A. Factors affecting extraction and evolution of phenolic compounds during red wine maceration and the role of process modelling. Trends Food Sci. Technol. 2017, 69, 106–117. [Google Scholar]
- Nazarni, R.; Purnama, D.A.; Umar, S.; Eni, H. The effect of fermentation on total phenolic, flavonoid and tannin content and its relation to antibacterial activity in jaruk tigarun (crataeva nurvala, buch ham). Food Res. Int. 2016, 23, 309–315. [Google Scholar]
- Wang, L.; Hu, G.; Lei, L.; Lin, L.; Wang, D.; Wu, J. Identification and aroma impact of volatile terpenes in moutai liquor. Int. J. Food Prop. 2015, 19, 1335–1352. [Google Scholar]
- Zhang, X.; Zhang, S.; Gao, B.; Qian, Z.; Liu, J.; Wu, S.; Si, J. Identification and quantitative analysis of phenolic glycosides with antioxidant activity in methanolic extract of dendrobium catenatum flowers and selection of quality control herb−markers. Food Res. Int. 2019, 123, 732–745. [Google Scholar]
- Yuan, Y.; Zuo, J.; Zhang, H.; Zu, M.; Yu, M.; Liu, S. Transcriptome and metabolome profiling unveil the accumulation of flavonoids in dendrobium officinale. Genomics 2022, 114, 110324. [Google Scholar]
- Pujari, I.; Thomas, A.; Thomas, J.; Jhawar, N.; Guruprasad, K.P.; Rai, P.S.; Satyamoorthy, K.; Babu, V.S. Cytotoxicity and radiosensitizing potency of moscatilin in cancer cells at low radiation doses of X−ray and uv−c. 3 Biotech. 2021, 11, 281. [Google Scholar]
- Chen, Y.; Wang, Y.; Liang, C.; Liu, L.; Song, X.; Zhao, Y.; Wang, J.; Niu, J. Characterization of the key bibenzyl synthase in dendrobium sinense. Int. J. Mol. Sci. 2022, 23, 6780. [Google Scholar]
- Dal Cin, V.; Tieman, D.M.; Tohge, T.; McQuinn, R.; de Vos, R.C.; Osorio, S.; Schmelz, E.A.; Taylor, M.G.; Smits−Kroon, M.T.; Schuurink, R.C.; et al. Identification of genes in the phenylalanine metabolic pathway by ectopic expression of a myb transcription factor in tomato fruit. Plant. Cell 2011, 23, 2738–2753. [Google Scholar]
- Xia, X.L. Molecular Evolution and Taxonomy Analysis of Plant Isoflavone Metabolism Pathway. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2011. [Google Scholar]
- Jiangbo, L.; Weiying, W.; Hui, Z.; Yimin, D. Analysis of flavonoid metabolic pathways and related genes in Dendrobium officinale based on transcriptome sequencing. Fujian J. Agric. Sci. 2019, 3, 1019–1025. [Google Scholar]


| Fermentation Method | Alcoholic Strength (%vol) | Reduced Sugar Content (mg/mL) | Total Sugar Content (mg/mL) | Total Acid Content (mg/mL) | pH | Sensory Scoring |
|---|---|---|---|---|---|---|
| SW | 15.10 ± 0.42 a | 16.25 ± 0.38 b | 42.88 ± 1.02 b | 3.02 ± 0.10 a | 4.65 ± 0.06 b | 75.70 ± 3.23 b |
| DOSW | 14.60 ± 0.74 a | 23.71 ± 0.14 a | 61.81 ± 3.06 a | 3.02 ± 0.05 a | 4.95 ± 0.06 a | 79.60 ± 3.98 a |
| DOSW-SW | DOSW-DO | |||
|---|---|---|---|---|
| Ion mode | ES+ | ES− | ES+ | ES− |
| Metabolites | 516 | 479 | 524 | 520 |
| Differential metabolites | 192 | 183 | 327 | 399 |
| Up-metabolites | 166 | 169 | 314 | 399 |
| up-Metabolic pathway | 50 | 48 | 57 | 66 |
| Down-metabolites | 26 | 14 | 13 | 0 |
| Down-metabolic pathway | 15 | 7 | 9 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Shi, X.; Chen, H.; Zhang, L.; Cen, L.; Li, L.; Lv, Y.; Qiu, S.; Zeng, X.; Wei, C. Major Active Metabolite Characteristics of Dendrobium officinale Rice Wine Fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus Cofermentation. Foods 2023, 12, 2370. https://doi.org/10.3390/foods12122370
Yao L, Shi X, Chen H, Zhang L, Cen L, Li L, Lv Y, Qiu S, Zeng X, Wei C. Major Active Metabolite Characteristics of Dendrobium officinale Rice Wine Fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus Cofermentation. Foods. 2023; 12(12):2370. https://doi.org/10.3390/foods12122370
Chicago/Turabian StyleYao, Li, Xueqin Shi, Hang Chen, Lin Zhang, Lanyan Cen, Lian Li, Yiyi Lv, Shuyi Qiu, Xiangyong Zeng, and Chaoyang Wei. 2023. "Major Active Metabolite Characteristics of Dendrobium officinale Rice Wine Fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus Cofermentation" Foods 12, no. 12: 2370. https://doi.org/10.3390/foods12122370
APA StyleYao, L., Shi, X., Chen, H., Zhang, L., Cen, L., Li, L., Lv, Y., Qiu, S., Zeng, X., & Wei, C. (2023). Major Active Metabolite Characteristics of Dendrobium officinale Rice Wine Fermented by Saccharomyces cerevisiae and Wickerhamomyces anomalus Cofermentation. Foods, 12(12), 2370. https://doi.org/10.3390/foods12122370






