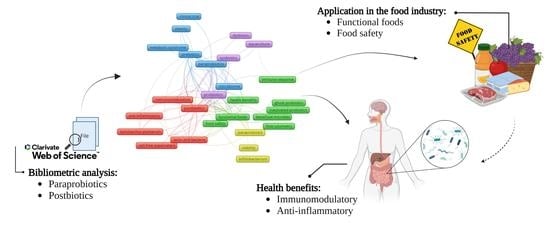
Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Bibliometric Analysis
3. Results
3.1. Description of Scientific Production and Bibliometric Analysis
3.1.1. Correlation between a Year of Publication, Authors’ Countries, Research Area, and Affiliation
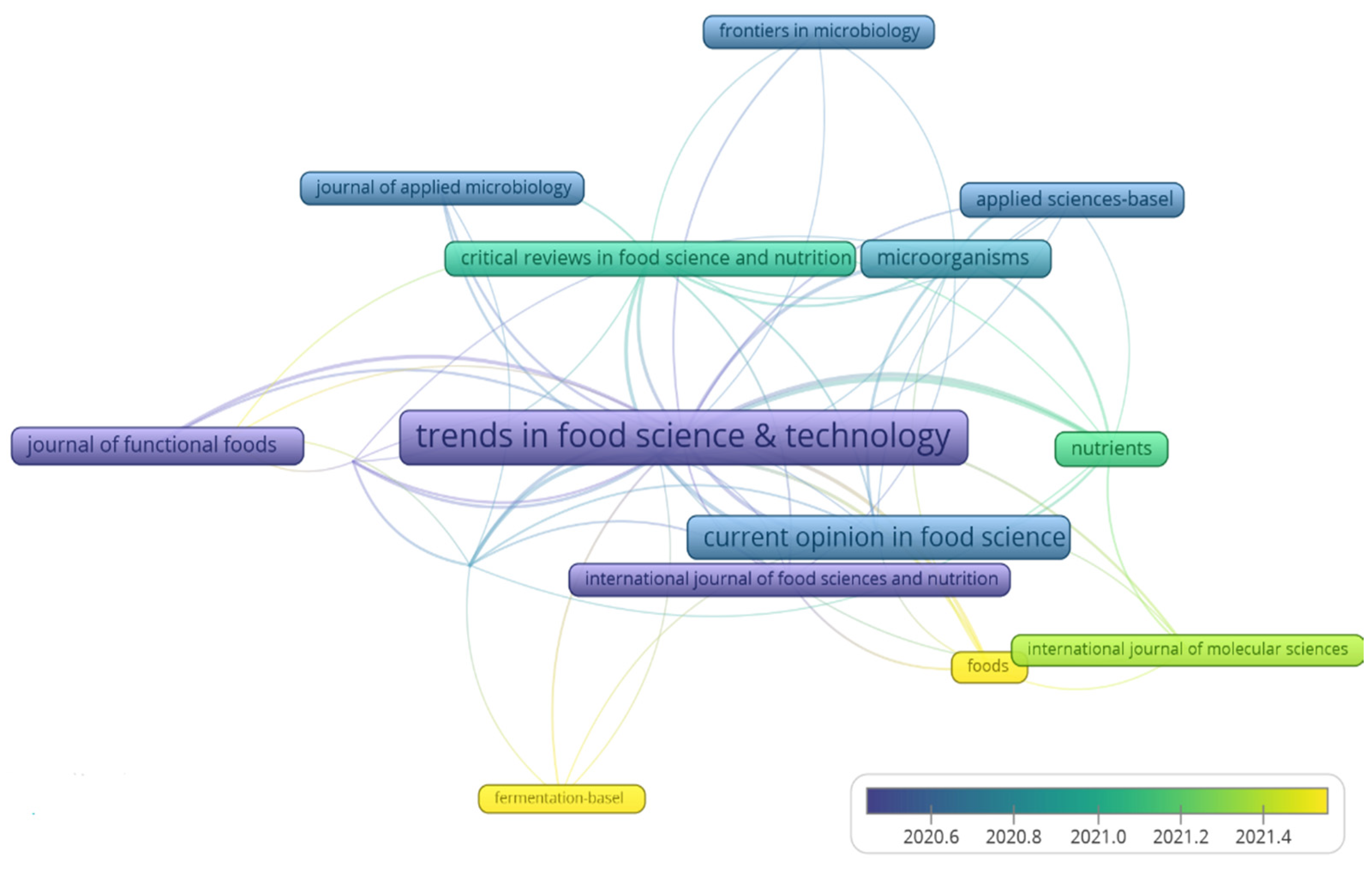
3.1.2. Most Cited Journals
3.1.3. Most Relevant Publications
3.1.4. Thematic Analysis
3.2. Use of Paraprobiotics and Probiotics as a Promise to Contribute to Adding Value and Food Safety
4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pazzini, I.A.E.; de Melo, A.M.; Ribani, R.H. Bioactive Potential, Health Benefits and Application Trends of Syzygium malaccense (Malay Apple): A Bibliometric Review. Trends Food Sci. Technol. 2021, 116, 1155–1169. [Google Scholar] [CrossRef]
- Pimentel, T.C.; Brandão, L.R.; de Oliveira, M.P.; da Costa, W.K.A.; Magnani, M. Health Benefits and Technological Effects of Lacticaseibacillus casei-01: An Overview of the Scientific Literature. Trends Food Sci. Technol. 2021, 114, 722–737. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, M.; Lu, Z. The LuxS/AI-2 System Regulates the Probiotic Activities of Lactic Acid Bacteria. Trends Food Sci. Technol. 2022, 127, 272–279. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Teame, T.; Wang, A.; Xie, M.; Zhang, Z.; Yang, Y.; Ding, Q.; Gao, C.; Olsen, R.E.; Ran, C.; Zhou, Z. Paraprobiotics and Postbiotics of Probiotic Lactobacilli, Their Positive on the Host and Action Mechanisms: A Review. Front. Nutr. 2020, 7, 570344. [Google Scholar] [CrossRef] [PubMed]
- Nataraj, B.H.; Ali, S.A.; Behare, P.V.; Yadav, H. Postbiotics-Parabiotics: The New Horizons in Microbial Biotherapy and functional Foods. Microb. Cell Fact. 2020, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- De Almada, C.N.; Almada, C.N.; Martinez, R.C.R.; Sant’Ana, A.S. Paraprobiotics: Evidences on Their Ability to Modify Biological Responses, Inactivation Methods and Perspectives on Their Application in Foods. Trends Food Sci. Technol. 2016, 58, 96–114. [Google Scholar] [CrossRef]
- Cuevas-Gonzalez, P.F.; Liceaga, A.M.; Aguilar-Toala, J.E. Postbiotics and Paraprobiotics: From Concepts to Applications. Food Res. Int. 2020, 136, 109502. [Google Scholar] [CrossRef]
- Wang, J.; Maniruzzaman, M.A. Global Bibliometric and Visualized Analysis of Bacteria-Mediated Cancer Therapy. Drug Discov. Today 2022, 27, 103297. [Google Scholar] [CrossRef]
- Rad, A.H.; Hosseini, S.; Pourjafar, H. Postbiotics as Dynamic Biological Molecules for Antimicrobial Activity: A Mini-Review. Biointerface Res. Appl. Chem. 2022, 12, 6543–6556. [Google Scholar] [CrossRef]
- Rahman, Z.; Dandekar, M.P. Implication of Paraprobiotics in Age-Associated Gut Dysbiosis and Neurodegenerative Diseases. NeuroMol. Med. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Puntillo, M.; Segli, F.; Champagne, C.P.; Raymond, Y.; Vinderola, G. Functional Microbes and Their Incorporation into Foods and Food: Probiotics and Postbiotics. Annu. Rev. Food Sci. Technol. 2022, 13, 385–407. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-Tool for Comprehensive Science Mapping Analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Anglada-Tort, M.; Sanfilippo, K.R.M. Visualizing Music Psychology: A Bibliometric Analysis of Psychology of Music, Music Perception, and Musicae Scientiae from 1973 to 2017. Music. Sci. 2019, 2, 205920431881178. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.; Rocha, R.S.; Ramos, G.L.P.A.; Xavier-Santos, D.; Pimentel, T.C.; Lorenzo, J.M.; Henrique Campelo, P.; Cristina Silva, M.; Esmerino, E.A.; Freitas, M.Q.; et al. What Are the Challenges for Ohmic Heating in the Food Industry? Insights of a Bibliometric Analysis. Food Res. Int. 2022, 157, 111272. [Google Scholar] [CrossRef] [PubMed]
- Colares, G.S.; Dell’Osbel, N.; Wiesel, P.G.; Oliveira, G.A.; Lemos, P.H.Z.; da Silva, F.P.; Lutterbeck, C.A.; Kist, L.T.; Machado, Ê.L. Floating Treatment Wetlands: A Review and Bibliometric Analysis. Sci. Total Environ. 2020, 714, 136776. [Google Scholar] [CrossRef]
- Nordin, A.H.; Ngadi, N.; Ilyas, A.R.; Nabgan, W.; Norfarhana, A.S. Starch-Based Plastics: A Bibliometric Analysis. Mater. Today Proc. 2022, 74, 519–523. [Google Scholar] [CrossRef]
- Gu, Z.; Meng, S.; Wang, Y.; Lyu, B.; Li, P.; Shang, N. A Novel Bioactive Postbiotics: From Microbiota-Derived Extracellular to Health Promoting. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef]
- Sawada, D.; Sugawara, T.; Hirota, T.; Nakamura, Y. Effects of Lactobacillus gasseri CP2305 on Mild Menopausal Symptoms In-Aged Women. Nutrients 2022, 14, 1695. [Google Scholar] [CrossRef]
- Sorensen, H.M.; Rochfort, K.D.; Maye, S.; MacLeod, G.; Brabazon, D.; Loscher, C.; Freeland, B. Exopolysaccharides of Lactic Acid Bacteria: Production, Purification and Health Benefits towards Functional Food. Nutrients 2022, 14, 2938. [Google Scholar] [CrossRef]
- Şahin, E. A Bibliometric Overview of the International Journal of Gastronomy and Food Science: To Where Is Gastronomy Research Evolving? Int. J. Gastron. Food Sci. 2022, 28, 100543. [Google Scholar] [CrossRef]
- Tur, J.A.; Bibiloni, M.M. Functional Foods. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 157–161. [Google Scholar]
- Aguilar-Toalá, J.E.; Garcia-Varela, R.; Garcia, H.S.; Mata-Haro, V.; González-Córdova, A.F.; Vallejo-Cordoba, B.; Hernández-Mendoza, A. Postbiotics: An Evolving Term within the Functional Foods Field. Trends Food Sci. Technol. 2018, 75, 105–114. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Barros, C.P.; Guimaraes, J.T.; Esmerino, E.A.; Duarte, M.C.K.H.; Silva, M.C.; Silva, R.; Ferreira, B.M.; Sant’Ana, A.S.; Freitas Monica, Q.; Cruz, A.G. Paraprobiotics and Postbiotics: Concepts and Potential Applications in dairy Products. Curr. Opin. Food Sci. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Balthazar, C.F.; Scudino, H.; Pimentel, T.C.; Esmerino, E.A.; Ashokkumar, M.; Freitas, M.Q.; Cruz, A.G. High-Intensity Ultrasound: A Novel Technology for the Development of probiotic and Prebiotic Dairy Products. Ultrason. Sonochem. 2019, 57, 12–21. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimaraes, J.T.; Yilmaz, N.; Lotfi, A. Postbiotics Produced by Lactic Acid Bacteria: The next Frontier in Food. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Diez-Gutierrez, L.; San Vicente, L.; Barron Luis Javier, R.; del Carmen Villaran, M.; Chavarri, M. Gamma-Aminobutyric Acid and Probiotics: Multiple Health Benefits and their Future in the Global Functional Food and Nutraceuticals Market. J. Funct. Foods 2020, 64, 103669. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; Almeida, F.L.C.; de Castro, R.J.S.; Sato, H.H. Isomaltulose: From Origin to Application and Its Beneficial Properties—A Bibliometric Approach. Food Res. Int. 2022, 155, 111061. [Google Scholar] [CrossRef] [PubMed]
- Fasogbon, B.M.; Adebo, O.A. A Bibliometric Analysis of 3D Food Printing Research: A Global and African Perspective. Future Foods 2022, 6, 100175. [Google Scholar] [CrossRef]
- Vargas-Albores, F.; Martinez-Cordova, L.R.; Hernandez-Mendoza, A.; Cicala, F.; Lago-Leston, A.; Martinez-Porchas, M. Therapeutic Modulation of Fish Gut Microbiota, a Feasible Strategy For? Aquaculture 2021, 544, 737050. [Google Scholar] [CrossRef]
- Rad, A.H.; Aghebati-Maleki, L.; Kafil Hossein Samadi and Abbasi, A. Molecular Mechanisms of Postbiotics in Colorectal Cancer Prevention And. Crit. Rev. Food Sci. Nutr. 2021, 61, 1787–1803. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, R.S.G.; da Cunha, D.T.; Stedefeldt, E. Food Safety Knowledge as Gateway to Cognitive Illusions of Food Handlers and the Different Degrees of Risk Perception. Food Res. Int. 2019, 116, 126–134. [Google Scholar] [CrossRef]
- Porfiri, L.; Burtscher, J.; Kangethe, R.T.; Verhovsek, D.; Cattoli, G.; Domig, K.J.; Wijewardana, V. Irradiated Non-Replicative Lactic Acid Bacteria Preserve Metabolic While Exhibiting Diverse Immune Modulation. Front. Vet. Sci. 2022, 9, 859124. [Google Scholar] [CrossRef]
- Mi, X.J.; Tran, T.H.M.; Park, H.R.; Xu, X.Y.; Subramaniyam, S.; Choi, H.S.; Kim, J.; Koh, S.C.; Kim, Y.J. Immune-Enhancing Effects of Postbiotic Produced by Bacillus velezensis-2 Isolated from Korea Foods. Food Res. Int. 2022, 152, 110911. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, H.; Wang, Y.; Cheng, R.; Pu, F.; Yang, Y.; Li, J.; Wu, S.; Shen, X.; He, F. Heat-Inactivated Lacticaseibacillus paracasei N1115 Alleviates the damage Due to Brain Function Caused by Long-Term Antibiotic Cocktail in Mice. BMC Neurosci. 2022, 23, 38. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Hashimoto, N.; Yin, T.; Sandagdorj, B.; Arakawa, C.; Inoue, T.; Suzuki, S. Heat-killed Lactobacillus brevis KB290 Attenuates Visceral Fat Accumulation Induced by High-fat Diet in Mice. J. Appl. Microbiol. 2021, 131, 1998–2009. [Google Scholar] [CrossRef]
- Almada, C.N.; Almada-Érix, C.N.; Costa, W.K.; Graça, J.S.; Cabral, L.; Noronha, M.F.; Gonçalves, A.E.S.; Santos, A.D.; Lollo, P.C.; Magnani, M.; et al. Wheat-Durum Pasta Added of Inactivated Bifidobacterium animalis Glucose and Total Cholesterol Levels and Modulates Gut in Healthy Rats. Int. J. Food Sci. Nutr. 2021, 72, 781–793. [Google Scholar] [CrossRef]
- Mohammadi, R.; Moradi, M.; Tajik, H.; Molaei, R. Potential Application of Postbiotics Metabolites from Bioprotective Culture to Fabricate Bacterial Nanocellulose Based Antimicrobial Packaging Material. Int. J. Biol. Macromol. 2022, 220, 528–536. [Google Scholar] [CrossRef]
- Chang, H.M.; Foo, H.L.; Loh, T.C.; Lim, E.T.C.; Abdul Mutalib, N.E. Comparative Studies of Inhibitory and Antioxidant Activities, and Organic Acids Compositions of Postbiotics Produced by Probiotic Lactiplantibacillus plantarum Strains Isolated from Malaysian Foods. Front. Vet. Sci. 2021, 7, 602280. [Google Scholar] [CrossRef]
- Toushik, S.H.; Park, J.H.; Kim, K.; Ashrafudoulla, M.; Ulrich, M.S.I.; Mizan, M.F.R.; Roy, P.K.; Shim, W.B.; Kim, Y.M.; Park, S.H.; et al. Antibiofilm Efficacy of Leuconostoc mesenteroides J.27-Derived Postbiotic and Food-Grade Essential Oils against Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Escherichia coli Alone and in Combination, and Their Application as a Green Preservative in the Seafood Industry. Food Res. Int. 2022, 156, 111163. [Google Scholar] [CrossRef] [PubMed]
- Toushik, S.H.; Kim, K.; Park, S.H.; Park, J.H.; Ashrafudoulla, M.; Ulrich, M.S.I.; Mizan, M.F.R.; Hossain, M.I.; Shim, W.B.; Kang, I.; et al. Prophylactic Efficacy of Lactobacillus curvatus B67-Derived Postbiotic and Quercetin, Separately and Combined, against Listeria monocytogenes and Salmonella enterica ser. Typhimurium on Processed Meat Sausage. Meat Sci. 2023, 197, 109065. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.; Qiu, L.; Taher, M.A.; Zaki, A.A.; Abou-Zeid, N.A.; Dawood, D.H.; Shalabi, O.M.A.K.; Khojah, E.; Elawady, A.A. Health Benefits of Postbiotics Produced by E. Coli Nissle 1917 in Functional Yogurt Enriched with Cape Gooseberry (Physalis peruviana L.). Fermentation 2022, 8, 128. [Google Scholar] [CrossRef]
- İncili, G.K.; Karatepe, P.; Akgöl, M.; Güngören, A.; Koluman, A.; İlhak, O.İ.; Kanmaz, H.; Kaya, B.; Hayaloğlu, A.A. Characterization of Lactic Acid Bacteria Postbiotics, Evaluation in-Vitro Antibacterial Effect, Microbial and Chemical Quality on Chicken Drumsticks. Food Microbiol. 2022, 104, 104001. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Underlying Its Probiotic Action. Int. J. Mol. Sci. 2021, 22, 12076. [Google Scholar] [CrossRef]
- Hernández, A.; Rodríguez, A.; Córdoba, M.G.; Martín, A.; Ruiz-Moyano, S. Fungal Control in Foods through Biopreservation. Curr. Opin. Food Sci. 2022, 47, 100904. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, C.; Bao, X.; Chen, F.; Guo, X. Strategies for Controlling Biofilm Formation in Food Industry. Grain Oil Sci. Technol. 2022, 5, 179–186. [Google Scholar] [CrossRef]





| Description | Results |
|---|---|
| Main information about data | |
| Timespan | 2015–2022 |
| Sources | 69 |
| Documents | 132 |
| Annual growth rate (%) | 66.18 |
| Document average age | 1.32 |
| Average citations per doc | 14.33 |
| References | 9626 |
| Document contents | |
| Keywords plus | 6637 |
| Author’s Keywords | 460 |
| Authors | |
| Authors | 620 |
| Authors of single-authored docs | 4 |
| Authors collaboration | |
| Single-authored docs | 4 |
| Co-authors per doc | 5.62 |
| International co-authorships (%) | 30.3 |
| Document types | |
| Article | 77 |
| Article; book chapter | 1 |
| Article; early access | 2 |
| Review | 47 |
| Review, early access | 5 |
| Reference | Document Type | Main Results | Average Citation per Year | Total Citation |
|---|---|---|---|---|
| Aguilar-Toalá et al. [23] | Review |
| 54.4 | 272 |
| De Almada et al. [7] | Review |
| 17.71 | 124 |
| Zendeboodi et al. [24] | Article |
| 36 | 108 |
| Nataraj et al. [6] | Review |
| 29.67 | 89 |
| Barros et al. [25] | Review |
| 20.67 | 62 |
| Guimaraes et al. [26] | Review |
| 15.5 | 62 |
| Cuevas-Gonzales et al. [8] | Review |
| 19.67 | 59 |
| Moradi et al. [27] | Review |
| 17 | 51 |
| Diez-Gutierrez et al. [28] | Review |
| 16 | 48 |
| Teame et al. [5] | Review |
| 15.33 | 46 |
| Microorganisms | Application | Highlights | References |
|---|---|---|---|
| Lacticaseibacillus N1115 | Brain dysfunction treatment | Significant relief from memory dysfunction, anxiety, and depression. | [36] |
| Lacticaseibacillus casei, Lactobacillus acidophilus, Lactiplantibacillus plantarum, and Lacticaseibacillus paracasei | Immunology modulation | The immunomodulatory activity can be altered according to the inactivation treatment. | [34] |
| Levilactobacillus brevis KB290 | Reduction of fat accumulation | Reduction of elevated serum glucose levels and insulin resistance. | [37] |
| Bifidobacterium animalis | Durum wheat pasta | Reduction in serum glucose and cholesterol levels. | [38] |
| Postbiotics de FreshQ (Lacticaseibacillus rhamnosus, Lacticaseibacillus paracasei subsp.) | Packaging material | Antimicrobial activity. | [39] |
| Lactiplantibacillus plantarum | Antioxidant agent | The composition and functional characteristics of postbiotics are influenced by the strain and composition of the culture medium. | [40] |
| Leuconostoc mesenteroides J27 | Antibiofilm | Postbiotics and essential oils can efficiently inhibit biofilm formation in seafood. | [41] |
| Latilactobacillus curvatus B67 | Antimicrobial and antibiofilm | Inhibition of pathogenic biofilm formation, which indicates potential use as an alternative bioprotective agent in the meat processing industry. | [42] |
| E. coli Nissle 1917 | Functional Yogurt | Antimicrobial, antitumor, and antioxidant activities. | [43] |
| Pediococcus. Acidilactici; Latilactobacillus sakei/Staphylococcus xylosus | chicken thighs | It reduced pathogens in chicken thighs, which could contribute to extending the shelf life of poultry meat and meat products. | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro, S.S.; Schnorr, C.E.; Pasquali, M.A.d.B. Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods 2023, 12, 2394. https://doi.org/10.3390/foods12122394
Monteiro SS, Schnorr CE, Pasquali MAdB. Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods. 2023; 12(12):2394. https://doi.org/10.3390/foods12122394
Chicago/Turabian StyleMonteiro, Shênia Santos, Carlos Eduardo Schnorr, and Matheus Augusto de Bittencourt Pasquali. 2023. "Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods" Foods 12, no. 12: 2394. https://doi.org/10.3390/foods12122394
APA StyleMonteiro, S. S., Schnorr, C. E., & Pasquali, M. A. d. B. (2023). Paraprobiotics and Postbiotics—Current State of Scientific Research and Future Trends toward the Development of Functional Foods. Foods, 12(12), 2394. https://doi.org/10.3390/foods12122394