Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Fresh Produce
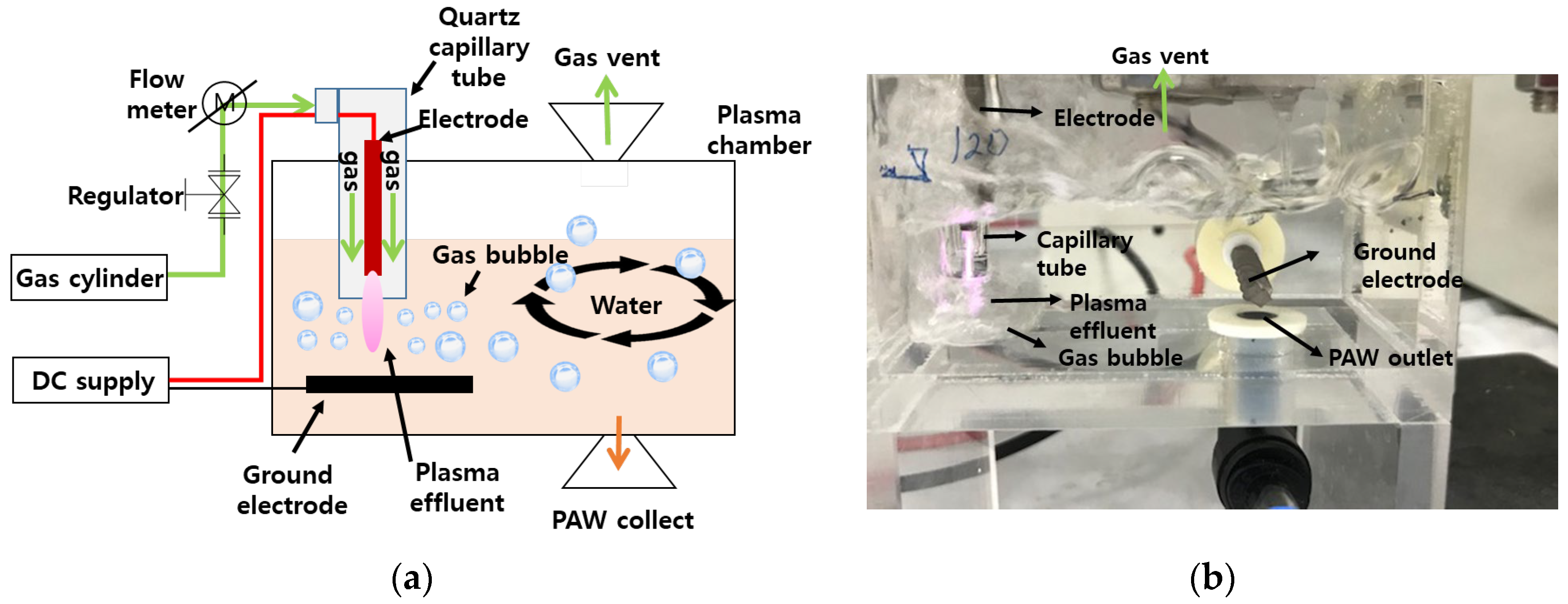
2.3. Preparation of PAW
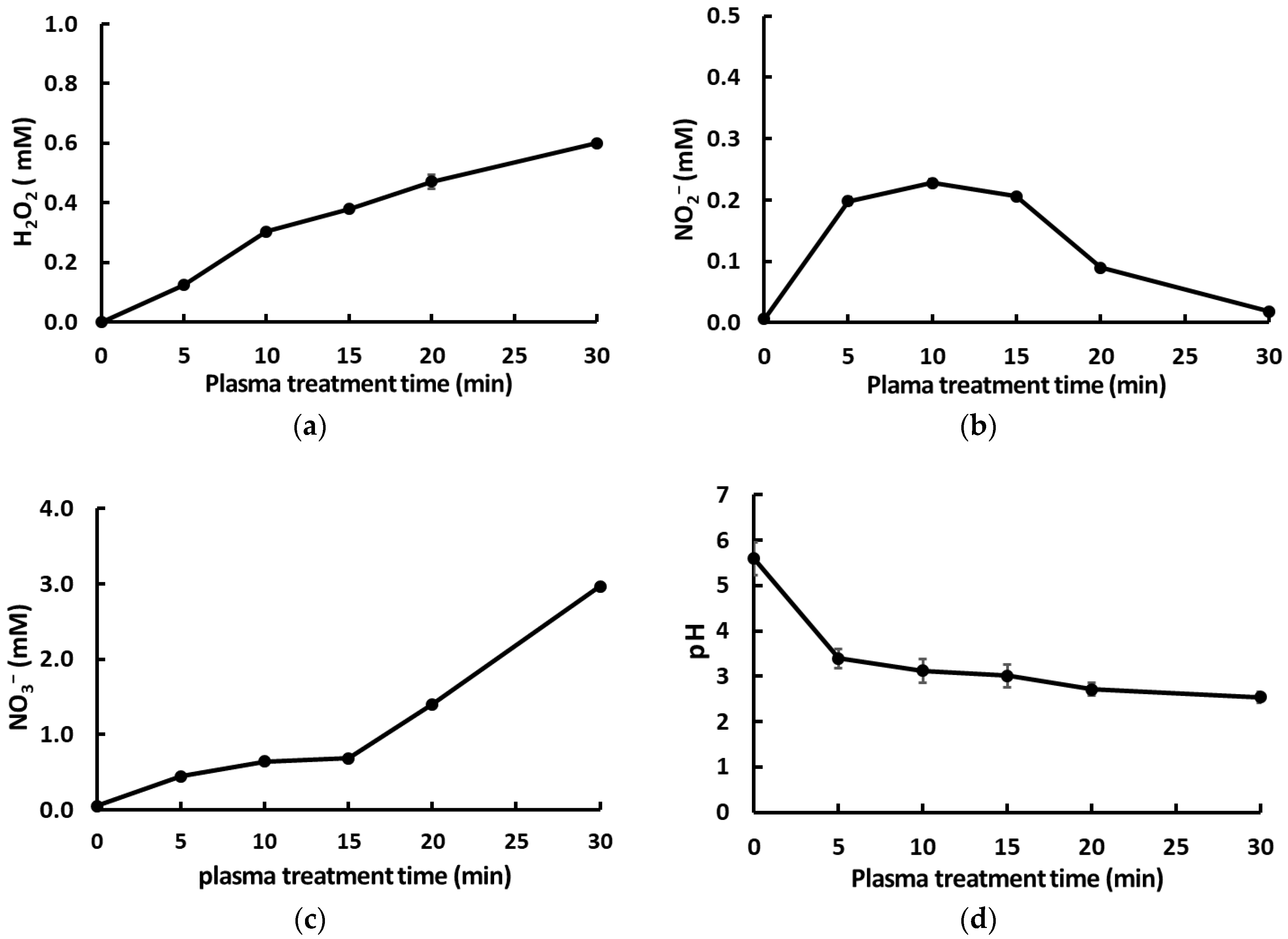
2.4. Quantification of pH Values and the Dissolved Concentrations of H2O2, NO2−, and NO3− in PAW
2.5. Optimal Conditions for PAW Treatment
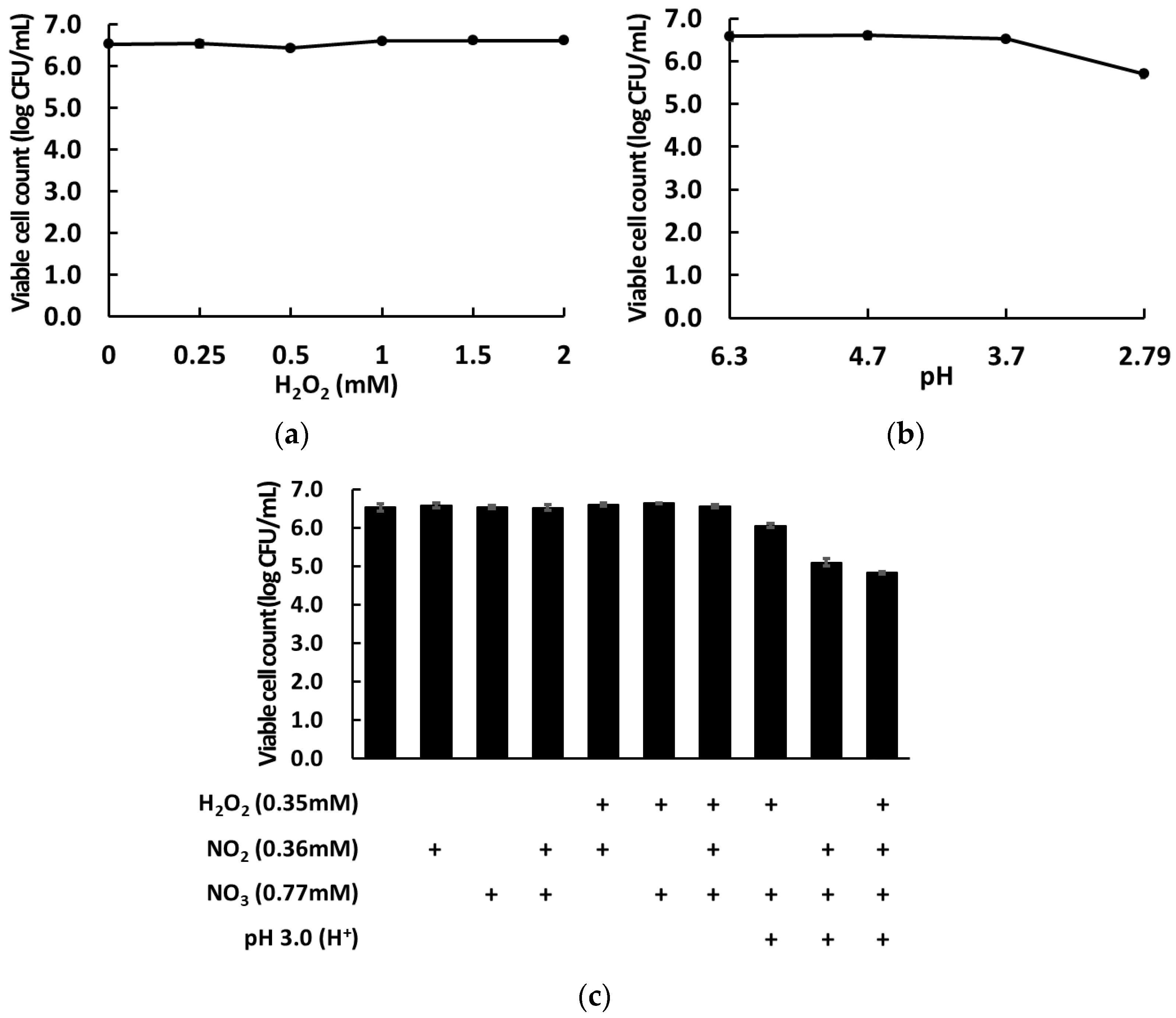
2.6. Microbial Inactivation Effects of Artificially Generated Reactive Species
2.7. PAW-Induced Inactivation of Foodborne Pathogens Inoculated on the Surface of Cherry Tomatoes
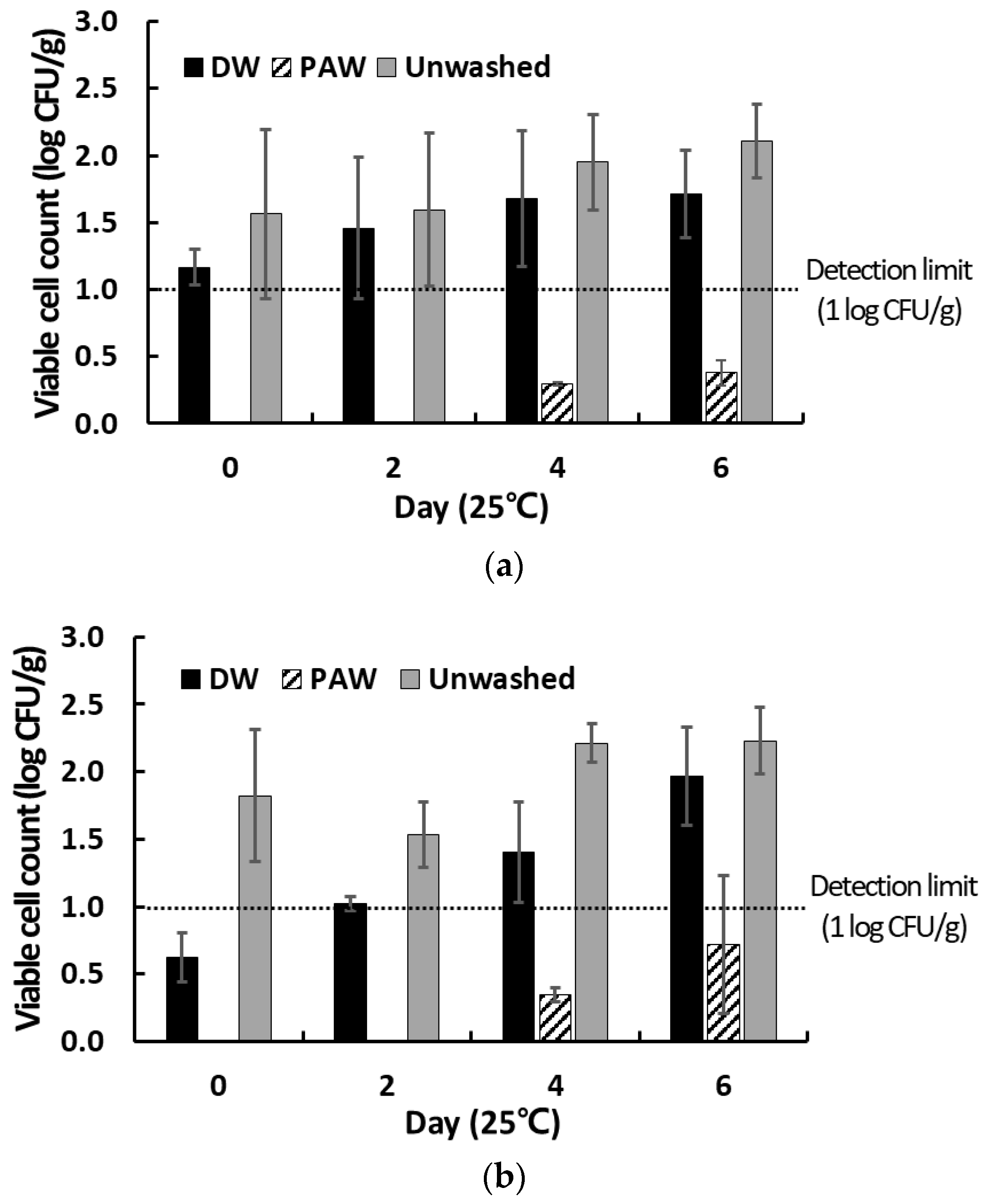
2.8. Changes in the Counts of Native Microbes Present on the Surface of PAW-Washed Cherry Tomatoes during Storage
2.9. Cytotoxicity Test
2.10. Statistical Analyses
3. Results and Discussion
3.1. Optimal Characteristics of PAW Required for Efficient Microbial Inactivation
3.2. Microbial Inactivation Based on the Composition of Artificial Reactive Species
3.3. Inactivation of Foodborne Pathogens Present on the Surface of Cherry Tomatoes and in the Wash Solution
3.4. Inactivation of Native Microbes Present on the Surface of Cherry Tomatoes
3.5. Cytotoxicity of PAW
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, L.; Zhang, M.; Bhandari, B.; Gao, Z.X. Recent developments in novel shelf life extension technologies of fresh-cut fruits and vegetables. Trends Food Sci. Technol. 2017, 64, 23–38. [Google Scholar]
- Patrignani, F.; Siroli, L.; Serrazanetti, D.I.; Gardini, F.; Lanciotti, R. Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci. Technol. 2015, 46, 311–319. [Google Scholar]
- Balali, J.I.; Yar, D.D.; Dela, V.G.A.; Adjei-kusi, P. Microbial contamination, an increasing threat to the consumption of fresh fruits and vegetables in Today’s world. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [PubMed]
- Bartz, F.E.; Lickness, J.S.; Heredia, N.; Fabiszewski de Aceituno, A.; Newman, K.L.; Hodge, D.W.; Jaykus, L.-A.; García, S.; Leon, J.S. Contamination of fresh produce by microbial indicators on farms and in packing facilities: Elucidation of environmental routes. Appl. Environ. Microbiol. 2017, 83, e02984-16. [Google Scholar]
- Bennett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [PubMed] [Green Version]
- Beuchat, L.R. Pathogenic microorganisms associated with fresh produce. J. Food Prot. 1996, 59, 204–216. [Google Scholar] [CrossRef]
- Müller, L.; Kjelsø, C.; Frank, C.; Jensen, T.; Torpdahl, M.; Søborg, B.; Dorleans, F.; Rabsch, W.; Prager, R.; Gossner, C. Outbreak of Salmonella Strathcona caused by datterino tomatoes, Denmark, 2011. Epidemiol. Infect. 2016, 144, 2802–2811. [Google Scholar] [CrossRef]
- Mansur, A.R.; Oh, D.H. Combined effect of thermosonication and slightly acidic electrolyzed water to reduce foodborne pathogens and spoilage microorganisms on fresh-cut kale. J. Food Sci. 2015, 80, 1277–1284. [Google Scholar]
- Sun, S.H.; Kim, S.J.; Kwak, S.J.; Yoon, K.S. Efficacy of sodium hypochlorite and acidified sodium chlorite in preventing browning and microbial growth on fresh-cut produce. Prev. Nutr. Food Sci. 2012, 17, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Banach, J.L.; Bokhorst-van, V.; de Veen, H.; Van Overbeek, L.; Van der Zouwen, P.; Van der Fels-Klerx, H.; Groot, M.N. The efficacy of chemical sanitizers on the reduction of Salmonella Typhimurium and Escherichia coli affected by bacterial cell history and water quality. Food Control 2017, 81, 137–146. [Google Scholar]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. A comparative study on the effectiveness of chlorine dioxide gas, ozone gas and e-beam irradiation treatments for inactivation of pathogens inoculated onto tomato, cantaloupe and lettuce seeds. Int. J. Food Microbiol. 2011, 146, 203–206. [Google Scholar]
- Yoon, J.-H.; Lee, S.-H. Comparison of the effectiveness of decontaminating strategies for fresh fruits and vegetables and related limitations. Crit. Rev. Food Sci. Nutr. 2018, 58, 3189–3208. [Google Scholar]
- Joshi, I.; Salvi, D.; Schaffner, D.W.; Karwe, M.V. Characterization of microbial inactivation using plasma-activated water and plasma-activated acidified buffer. J. Food Prot. 2018, 81, 1472–1480. [Google Scholar]
- Ma, M.; Zhang, Y.; Lv, Y.; Sun, F. The key reactive species in the bactericidal process of plasma activated water. J. Phys. D Appl. Phys. 2020, 53, 185207. [Google Scholar]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of foodborne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar]
- Xiang, Q.; Kang, C.; Niu, L.; Zhao, D.; Li, K.; Bai, Y. Antibacterial activity and a membrane damage mechanism of plasma-activated water against Pseudomonas deceptionensis CM2. LWT-Food Sci. Technol. 2018, 96, 395–401. [Google Scholar]
- Tian, Y.; Ma, R.; Zhang, Q.; Feng, H.; Liang, Y.; Zhang, J.; Fang, J. Assessment of the physicochemical properties and biological effects of water activated by nonthermal plasma above and beneath the water surface. Plasma Process. Polym. 2015, 12, 439–449. [Google Scholar]
- Guo, J.; Huang, K.; Wang, X.; Lyu, C.; Yang, N.; Li, Y.; Wang, J. Inactivation of yeast on grapes by plasma-activated water and its effects on quality attributes. J. Food Prot. 2017, 80, 225–230. [Google Scholar]
- Hou, C.Y.; Lai, Y.C.; Hsiao, C.P.; Chen, S.Y.; Liu, C.T.; Wu, J.S.; Lin, C.M. Antibacterial activity and the physicochemical characteristics of plasma activated water on tomato surfaces. LWT-Food Sci. Technol. 2021, 149, 111879. [Google Scholar]
- Mitra, S.; Veerana, M.; Choi, E.; Park, G. Effects of pre-treatment using plasma on the antibacterial activity of mushroom surfaces. Foods 2021, 10, 1888. [Google Scholar]
- Kasih, T.P.; Danil, D.; Geraldine, E.; Widyaningrum, D. Effect of plasma activated water (PAW) in maintaining the quality of cherry tomatoes. Earth Environ. Sci. 2022, 998, 012063. [Google Scholar]
- Laurita, R.; Gozzi, G.; Tappi, S.; Capelli, F.; Bisag, A.; Laghi, G.; Gherardi, M.; Cellini, B.; Abouelenein, D.; Vittori, S.; et al. Effect of plasma activated water (PAW) on rocket leaves decontamination and nutritional value. Innov. Food Sci. Emerg. Technol. 2021, 73, 102805. [Google Scholar]
- Liu, C.; Chen, C.; Jiang, A.; Sun, X.; Guan, Q.; Hu, W. Effects of plasma-activated water on microbial growth and storage quality of fresh-cut apple. Innov. Food Sci. Emerg. Techn. 2020, 59, 102256. [Google Scholar]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar]
- Li, H.; Zhang, Q.; Jin, X.; Zou, X.; Wang, Y.; Hao, D.; Fu, F.; Jiao, W.; Zhang, C.; Lin, H.; et al. Dysifragilone A inhibits LPS-induced RAW264. 7 macrophage activation by blocking the p38 MAPK signaling pathway. Mol. Med. Rep. 2018, 17, 674–682. [Google Scholar]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar]
- Zhou, R.; Zhou, R.; Wang, P.; Xian, Y.; Mai-Prochnow, A.; Lu, X.; Cullen, P.J.; Ostrikov, K.; Bazaka, K. Plasma-activated water: Generation, origin of reactive species and biological applications. J. Phys. D Appl. Phys. 2020, 53, 303001. [Google Scholar]
- Zhou, Y.; Jiang, T.; Zhang, L.; Wang, S.; Zhou, R.; Cui, X.; Fang, Z. High reactivity and bactericidal effects of plasma-activated mist from hydrogen peroxide solution. Plasma Process. Polym. 2023, 20, 2200140. [Google Scholar]
- Lin, C.-M.; Hsiao, C.-P.; Lin, H.-S.; Liou, J.S.; Hsieh, C.-W.; Wu, J.-S.; Hou, C.-Y. The antibacterial efficacy and mechanism of plasma-activated water against Salmonella Enteritis (ATCC 13076) on shell eggs. Foods 2020, 9, 1491. [Google Scholar]
- Bolouki, N.; Kuan, W.-H.; Huang, W.-Y.; Hsieh, J.-H. Characterizations of a plasma-water system generated by repetitive microsecond pulsed discharge with air, nitrogen, oxygen, and argon gases species. Appl. Sci. 2021, 11, 6158. [Google Scholar] [CrossRef]
- Ercan, U.K.; Wang, H.; Ji, H.; Fridman, G.; Brooks, A.D.; Joshi, S.G. Nonequilibrium plasma-activated antimicrobial solutions are broad-spectrum and retain their efficacies for extended period of time. Plasma Process. Polym. 2013, 10, 544–555. [Google Scholar]
- Dimitrakellis, P.; Giannoglou, M.; Xanthou, Z.M.; Gogolides, E.; Taoukis, P.; Katsaros, G. Application of plasma-activated water as an antimicrobial washing agent of fresh leafy produce. Plasma Process. Polym. 2021, 18, e2100030. [Google Scholar]
- Heaselgrave, W.; Andrew, P.W.; Kilvington, S. Acidified nitrite enhances hydrogen peroxide disinfection of Acanthamoeba, bacteria and fungi. J. Antimicrob. Chemother. 2010, 65, 1207–1214. [Google Scholar]
- Zhu, L.; Gunn, C.; Beckman, J.S. Bactericidal activity of peroxynitrite. Arch. Biochem. Biophys. 1992, 298, 452–457. [Google Scholar]
- Kumar, A.; Škoro, N.; Gernjak, W.; Povrenović, D.; Puač, N. Direct and indirect treatment of organic dye (Acid blue 25) solutions by using cold atmospheric plasma jet. Front. Phys. 2022, 10, 835635. [Google Scholar]
- Naïtali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined effects of long-living chemical species during microbial inactivation using atmospheric plasma-treated water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar]
- Tarabová, B.; Lukeš, P.; Hammer, M.U.; Jablonowski, H.; von Woedtke, T.; Reuter, S.; Machala, Z. Fluorescence measurements of peroxynitrite/peroxynitrous acid in cold air plasma treated aqueous solutions. Phys. Chem. Chem. Phys. 2019, 21, 8883–8896. [Google Scholar]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar]
- Hong, S.; Park, H.; Cho, K.; Kang, S. Development of washing and sterilization system for leafy vegetables. Eng. Agric. Environ. Food 2010, 3, 87–92. [Google Scholar] [CrossRef]
- Kilonzo-Nthenge, A.; Liu, S.; Yannam, S.; Patras, A. Atmospheric Cold Plasma Inactivation of Salmonella and Escherichia coli on the Surface of Golden Delicious Apples. Front. Nutr. 2018, 5, 120. [Google Scholar] [PubMed]
- Zhang, H.; Tikekar, R.V. Air microbubble assisted washing of fresh produce: Effect on microbial detachment and inactivation. Postharvest Biol. Technol. 2021, 181, 111687. [Google Scholar]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J.; Jaroslav, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar]
- Machala, z.; Tarabová, B.; Hensel, K.; Spetklikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in water electro-splayed through transient spark discharge in air and their bactericidal effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar]
- Boehm, D.; Heslin, C.; Cullen, P.J.; Bourke, P. Cytotoxic and mutagenic potential of solutions exposed to cold atmospheric plasma. Sci. Rep. 2016, 24, 21464. [Google Scholar]
- Boehm, D.; Curtin, J.; Cullen, P.J.; Bourke, P. Hydrogen peroxide and beyond—The potential of high voltage plasma activated liquids against cancerous cells. Anticancer Agents Med. Chem. 2017, 17, 1. [Google Scholar] [CrossRef] [Green Version]
- Van Boxem, W.; Van der Paal, J.; Gorbanev, Y.; Vanuytsel, S.; Smits, E.; Dewilde, S.; Bogaerts, A. Anti-cancer capacity of plasma-treated PBS: Effect of chemical composition on cancer cell cytotoxicity. Sci. Rep. 2017, 7, 16478. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.; Choi, S.-W.; Yoo, M.; Chang, H.-J.; Lee, N. Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution. Foods 2023, 12, 2461. https://doi.org/10.3390/foods12132461
Lee G, Choi S-W, Yoo M, Chang H-J, Lee N. Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution. Foods. 2023; 12(13):2461. https://doi.org/10.3390/foods12132461
Chicago/Turabian StyleLee, Gaeul, Sung-Wook Choi, Miyoung Yoo, Hyun-Joo Chang, and Nari Lee. 2023. "Effects of Plasma-Activated Water Treatment on the Inactivation of Microorganisms Present on Cherry Tomatoes and in Used Wash Solution" Foods 12, no. 13: 2461. https://doi.org/10.3390/foods12132461





