Chitinase-Like Protein PpCTL1 Contributes to Maintaining Fruit Firmness by Affecting Cellulose Biosynthesis during Peach Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Fruit Firmness Determination
2.3. Measurement of Pectin and Cellulose Content
2.4. PpCTL Protein Identification, Sequence Alignment and Phylogenetic Tree Construction
2.5. Total RNA Extraction and Gene Expression Analysis
2.6. Virus-Induced Gene Silencing (VIGS)
2.7. Paraffin Section Assays
2.8. Statistical Analysis
3. Results
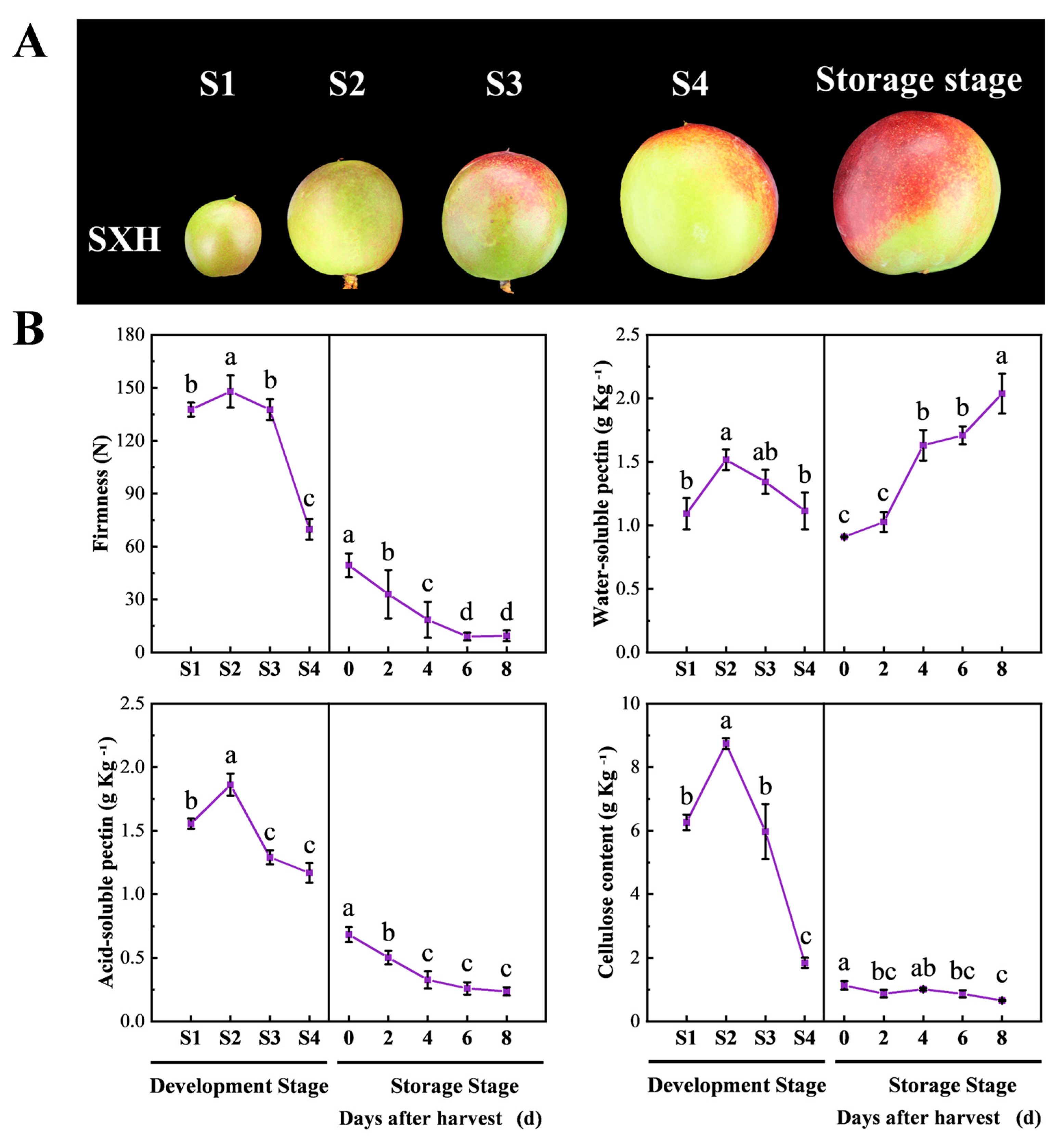
3.1. Changes in Fruit Firmness and Cell Wall Composition during Peach Fruit Development and Ripening
3.2. Correlation between Fruit Firmness and Cell Wall Composition at Different Developmental Stages
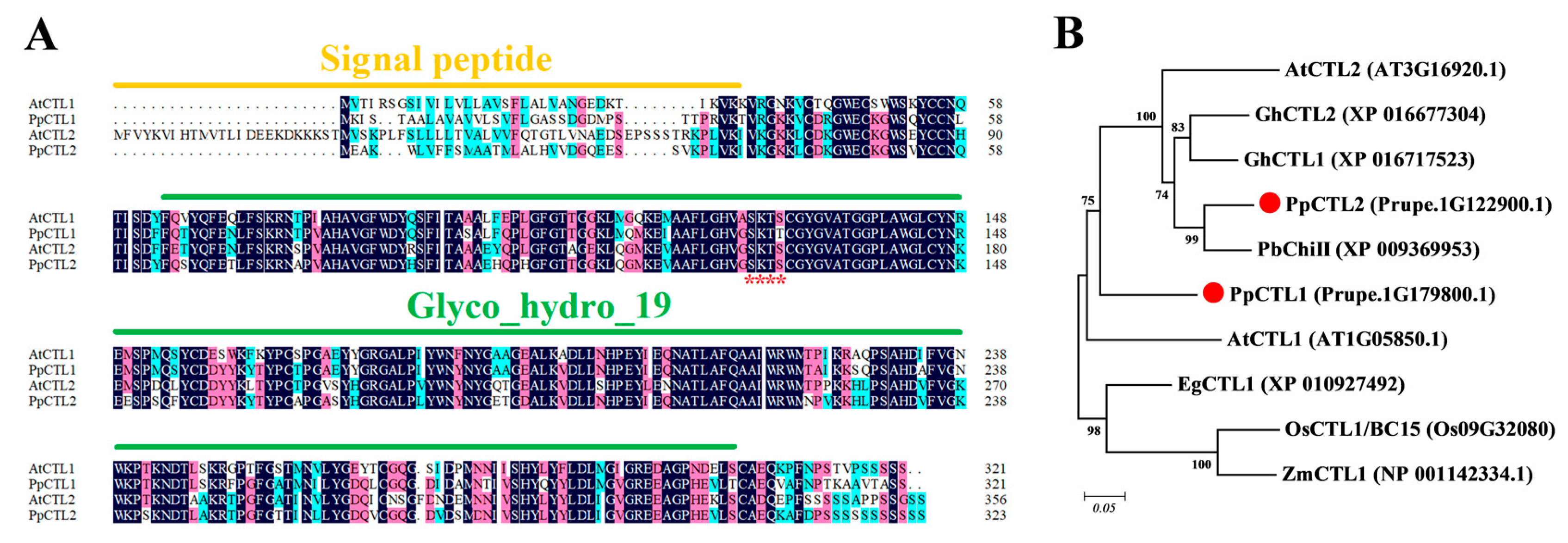
3.3. Sequence Analysis of PpCTL1 and PpCTL2
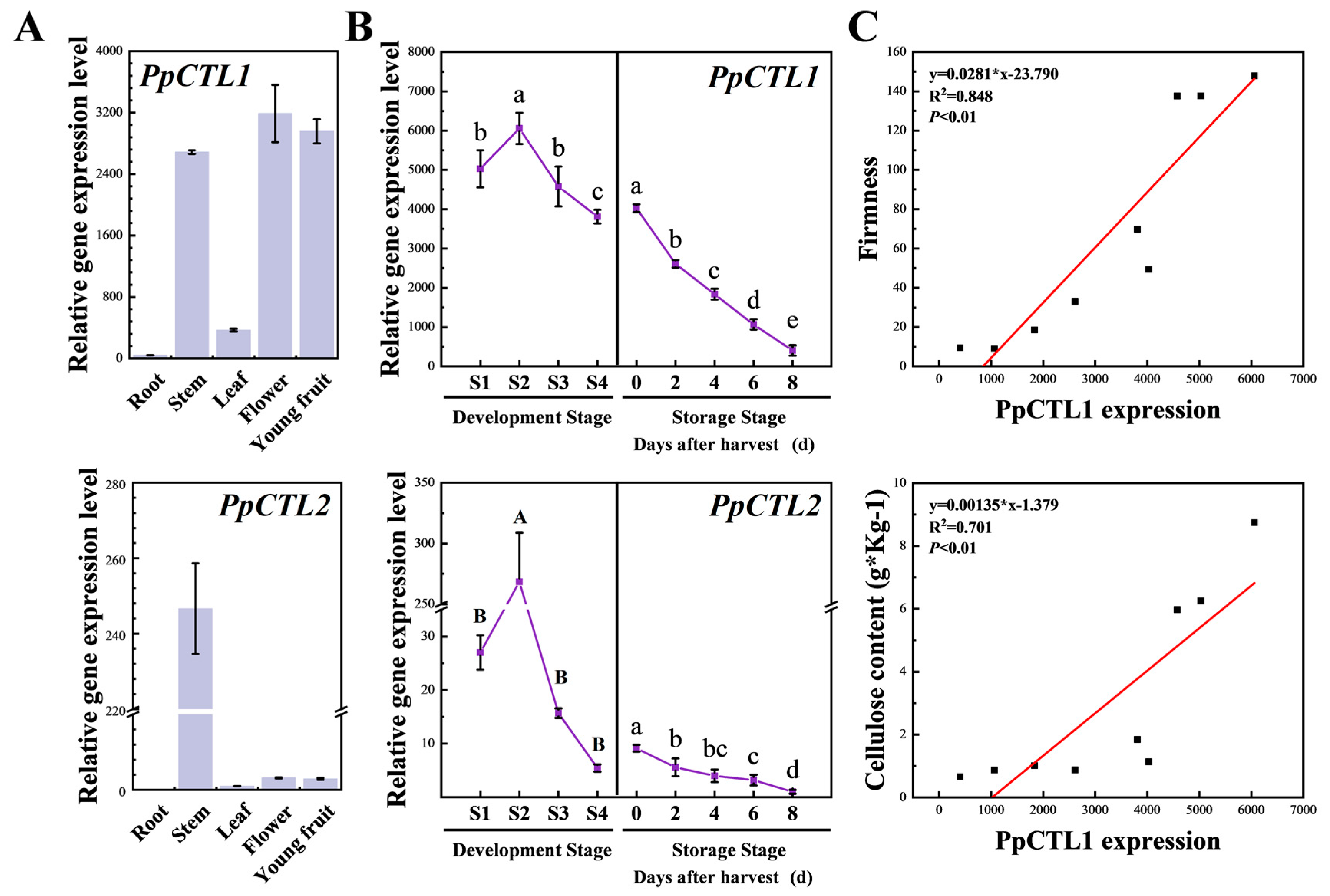
3.4. PpCTL1 Expression Is Closely Associated with Cellulose Content and Firmness
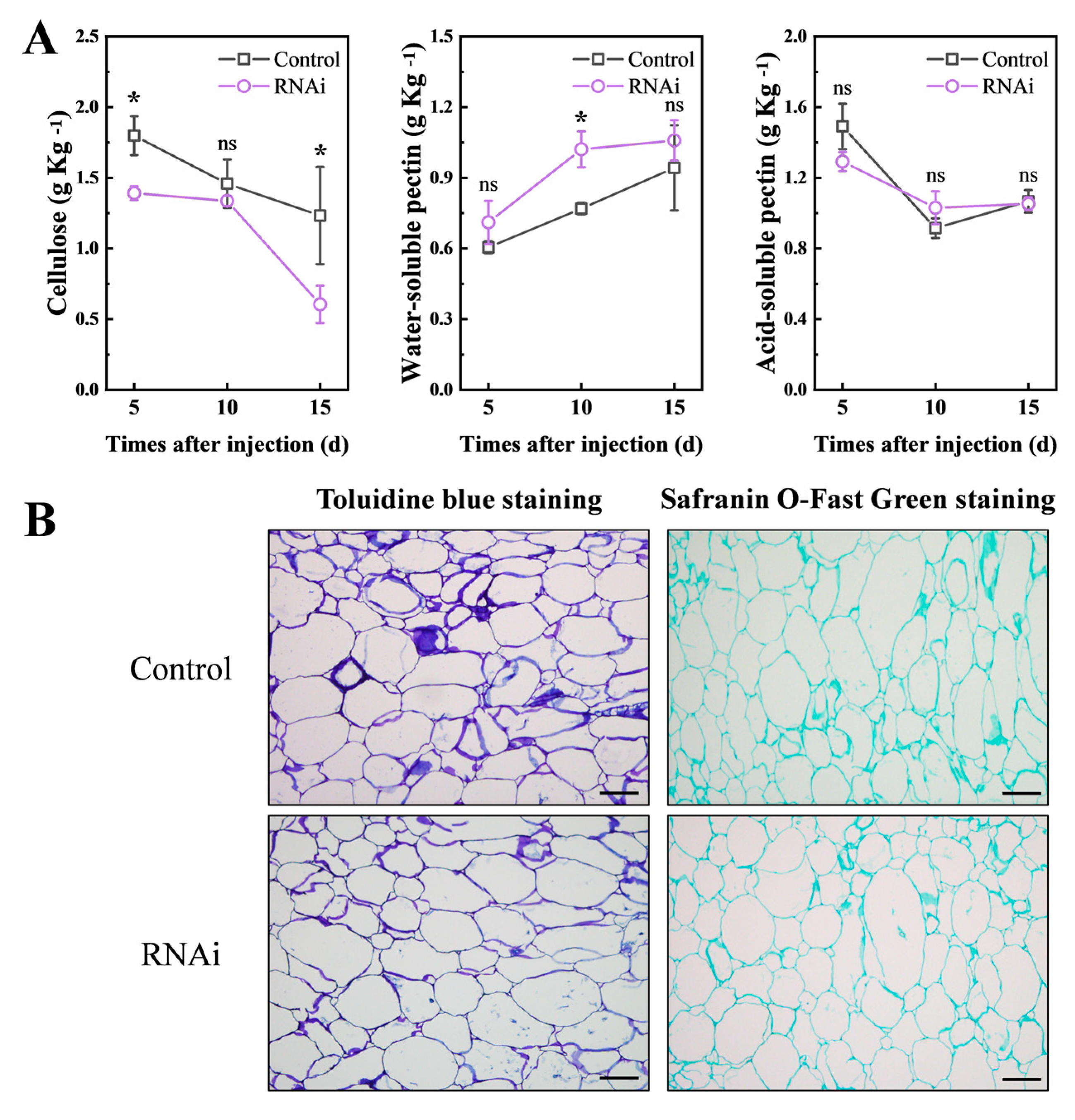
3.5. Downregulated PpCTL1 Expression Affects Fruit Firmness
3.6. PpCTL1 Impacts Cellulose Content in Fruit
4. Discussion
4.1. Cellulose May Be Responsible for the Peach Fruit Firmness during Development
4.2. Pectin Depolymerization Plays a Central Role in Peach Fruit Softening
4.3. Downregulation of PpCTL1 Suppresses Fruit Firmness by Interfering with Cellulose Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hayama, H.; Shimada, T.; Fujii, H.; Ito, A.; Kashimura, Y. Ethylene-regulation of fruit softening and softening-related genes in peach. J. Exp. Bot. 2006, 57, 4071–4077. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, B.J.; Su, G.; Zhang, M.; Grierson, D.; Chen, K.S. Transcriptional regulation of fleshy fruit texture. J. Integr. Plant Biol. 2022, 64, 1649–1672. [Google Scholar] [CrossRef] [PubMed]
- Saladie, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Ren, X.L.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [PubMed]
- Harker, F.R.; Redgwell, R.J.; Hallett, I.C.; Murray, S.H.; Carter, G. Texture of fresh fruit. In Horticultural Reviews; John Wiley and Sons: Hoboken, NJ, USA, 1997. [Google Scholar]
- Brummell, D.A. Cell wall disassembly in ripening fruit. Funct. Plant Biol. 2006, 33, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef]
- Qian, M.; Xu, Z.; Zhang, Z.; Li, Q.; Yan, X.; Liu, H.; Han, M.; Li, F.; Zheng, J.; Zhang, D.; et al. The downregulation of PpPG21 and PpPG22 influences peach fruit texture and softening. Planta 2021, 254, 1–12. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, J.; Kang, T.; Shah, K.; Li, Q.; Liu, K.; Xing, L.; Ma, J.; Zhang, D.; Zhao, C. PpePL1 and PpePL15 Are the Core Members of the Pectate Lyase Gene Family Involved in Peach Fruit Ripening and Softening. Front. Plant Sci. 2022, 13, 844055. [Google Scholar] [CrossRef]
- Witasari, L.D.; Huang, F.C.; Hoffmann, T.; Rozhon, W.; Fry, S.C.; Schwab, W. Higher expression of the strawberry xyloglucan endotransglucosylase/hydrolase genes FvXTH9 and FvXTH6 accelerates fruit ripening. Plant J. 2019, 100, 1237–1253. [Google Scholar] [CrossRef]
- Wilson, T.H.; Kumar, M.; Turner, S.R. The molecular basis of plant cellulose synthase complex organisation and assembly. Biochem. Soc. Trans. 2021, 49, 379–391. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, R.; Dong, S.; Li, Y.; Fan, C.; Wang, Y.; Xia, T.; Chen, P.; Wang, L.; Feng, S.; et al. AtCSLD3 and GhCSLD3 mediate root growth and cell elongation downstream of the ethylene response pathway in Arabidopsis. J. Exp. Bot. 2018, 69, 1065–1080. [Google Scholar] [CrossRef]
- Wang, D.; Qin, Y.; Fang, J.; Yuan, S.; Peng, L.; Zhao, J.; Li, X. A Missense Mutation in the Zinc Finger Domain of OsCESA7 Deleteriously Affects Cellulose Biosynthesis and Plant Growth in Rice. PLoS ONE 2016, 11, e0153993. [Google Scholar] [CrossRef] [PubMed]
- Jiao, S.; Hazebroek, J.P.; Chamberlin, M.A.; Perkins, M.; Sandhu, A.S.; Gupta, R.; Simcox, K.D.; Yinghong, L.; Prall, A.; Heetland, L.; et al. Chitinase-like1 Plays a Role in Stalk Tensile Strength in Maize. Plant Physiol. 2019, 181, 1127–1147. [Google Scholar] [CrossRef] [PubMed]
- Gartaula, G.; Dhital, S.; Netzel, G.; Flanagan, B.M.; Yakubov, G.E.; Beahan, C.T.; Collins, H.M.; Burton, R.A.; Bacic, A.; Gidley, M.J. Quantitative structural organisation model for wheat endosperm cell walls: Cellulose as an important constituent. Carbohydr. Polym. 2018, 196, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wen, S.; Bie, Z. Overexpression of hexose transporter CsHT3 increases cellulose content in cucumber fruit peduncle. Plant Physiol. Biochem. 2019, 145, 107–113. [Google Scholar] [CrossRef]
- He, Y.H.; Li, J.Y.; Ban, Q.Y.; Han, S.K.; Rao, J.P. Role of Brassinosteroids in Persimmon (Diospyros kaki L.) Fruit Ripening. J. Agric. Food Chem. 2018, 66, 2637–2644. [Google Scholar] [CrossRef]
- Paniagua, C.; Santiago-Domenech, N.; Kirby, A.R.; Gunning, A.P.; Morris, V.J.; Quesada, M.A.; Matas, A.J.; Mercado, J.A. Structural changes in cell wall pectins during strawberry fruit development. Plant Physiol. Biochem. 2017, 118, 55–63. [Google Scholar] [CrossRef]
- Qi, X.-d.; Wei, J.-m.; Li, H.; Zhao, D. Cell wall metabolism and related gene expression in Malus domestica Borkh. during fruit growth and softening. Fruits 2015, 70, 153–161. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Ejsmentewicz, T.; Covarrubias, M.P.; Gudenschwager, O.; Campos-Vargas, R. Changes in cell wall pectins and their relation to postharvest mesocarp softening of “Hass” avocados (Persea americana Mill.). Plant Physiol. Biochem. 2018, 128, 142–151. [Google Scholar] [CrossRef]
- Cardenas-Perez, S.; Chanona-Perez, J.J.; Guemes-Vera, N.; Cybulska, J.; Szymanska-Chargot, M.; Chylinska, M.; Koziol, A.; Gawkowska, D.; Pieczywek, P.M.; Zdunek, A. Structural, mechanical and enzymatic study of pectin and cellulose during mango ripening. Carbohydr. Polym. 2018, 196, 313–321. [Google Scholar] [CrossRef]
- Bu, J.; Yu, Y.; Aisikaer, G.; Ying, T. Postharvest UV-C irradiation inhibits the production of ethylene and the activity of cell wall-degrading enzymes during softening of tomato (Lycopersicon esculentum L.) fruit. Postharvest Biol. Technol. 2013, 86, 337–345. [Google Scholar] [CrossRef]
- Passarinho, P.A.; de Vries, S.C. Arabidopsis Chitinases: A Genomic Survey. Arab. Book 2002, 1, e0023. [Google Scholar] [CrossRef] [PubMed]
- Grover, A. Plant Chitinases: Genetic Diversity and Physiological Roles. Crit. Rev. Plant Sci. 2012, 31, 57–73. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Feng, P.; Ma, Q.; Zhang, Y.; Yang, Y.; Zhang, J. Cotton Chitinase Gene GhChi6 Improves the Arabidopsis Defense Response to Aphid Attack. Plant Mol. Biol. Rep. 2020, 39, 251–261. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Y.; Kim, S.G.; Jung, K.H.; Gupta, R.; Kim, J.; Park, Y.; Kang, K.Y.; Kim, S.T. A secreted chitinase-like protein (OsCLP) supports root growth through calcium signaling in Oryza sativa. Physiol. Plant. 2017, 161, 273–284. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, S.H.; Jung, M.S.; Kim, M.S.; Oh, J.E.; Ju, H.W.; Kim, K.I.; Vierling, E.; Lee, H.; Hong, S.W. Arabidopsis hot2 encodes an endochitinase-like protein that is essential for tolerance to heat, salt and drought stresses. Plant J. 2007, 49, 184–193. [Google Scholar] [CrossRef]
- Witmer, X.; Nonogaki, H.; Beers, E.P.; Bradford, K.J.; Welbaum, G.E. Characterization of chitinase activity and gene expression in muskmelon seeds. Seed Sci. Res. 2007, 13, 167–178. [Google Scholar] [CrossRef]
- Hermans, C.; Porco, S.; Vandenbussche, F.; Gille, S.; De Pessemier, J.; Van Der Straeten, D.; Verbruggen, N.; Bush, D.R. Dissecting the role of CHITINASE-LIKE1 in nitrate-dependent changes in root architecture. Plant Physiol. 2011, 157, 1313–1326. [Google Scholar] [CrossRef]
- van Hengel, A.J.; Guzzo, F.; van Kammen, A.; de Vries, S.C. Expression pattern of the carrot EP3 endochitinase genes in suspension cultures and in developing seeds. Plant Physiol. 1998, 117, 43–53. [Google Scholar] [CrossRef]
- Peumans, W.J.; Proost, P.; Swennen, R.L.; Van Damme, E.J. The abundant class III chitinase homolog in young developing banana fruits behaves as a transient vegetative storage protein and most probably serves as an important supply of amino acids for the synthesis of ripening-associated proteins. Plant Physiol. 2002, 130, 1063–1072. [Google Scholar] [CrossRef]
- Hossain, M.A.; Noh, H.N.; Kim, K.I.; Koh, E.J.; Wi, S.G.; Bae, H.J.; Lee, H.; Hong, S.W. Mutation of the chitinase-like protein-encoding AtCTL2 gene enhances lignin accumulation in dark-grown Arabidopsis seedlings. J. Plant Physiol. 2010, 167, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Porco, S.; Verbruggen, N.; Bush, D.R. Chitinase-like protein CTL1 plays a role in altering root system architecture in response to multiple environmental conditions. Plant Physiol. 2010, 152, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Kays, S.J.; Schroeder, B.P.; Ye, Z.H. Mutation of a chitinase-like gene causes ectopic deposition of lignin, aberrant cell shapes, and overproduction of ethylene. Plant Cell 2002, 14, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, C.; Bauer, S.; Hematy, K.; Saxe, F.; Ibanez, A.B.; Vodermaier, V.; Konlechner, C.; Sampathkumar, A.; Ruggeberg, M.; Aichinger, E.; et al. Chitinase-like1/pom-pom1 and its homolog CTL2 are glucan-interacting proteins important for cellulose biosynthesis in Arabidopsis. Plant Cell 2012, 24, 589–607. [Google Scholar] [CrossRef] [PubMed]
- Mokshina, N.; Gorshkova, T.; Deyholos, M.K. Chitinase-like (CTL) and cellulose synthase (CESA) gene expression in gelatinous-type cellulosic walls of flax (Linum usitatissimum L.) bast fibers. PLoS ONE 2014, 9, e97949. [Google Scholar] [CrossRef]
- He, Y.; Xue, J.; Li, H.; Han, S.; Jiao, J.; Rao, J. Ethylene response factors regulate ethylene biosynthesis and cell wall modification in persimmon (Diospyros kaki L.) fruit during ripening. Postharvest Biol. Technol. 2020, 168, 111255. [Google Scholar] [CrossRef]
- Qian, M.; Zhang, Y.; Yan, X.; Han, M.; Li, J.; Li, F.; Li, F.; Zhang, D.; Zhao, C. Identification and Expression Analysis of Polygalacturonase Family Members during Peach Fruit Softening. Int. J. Mol. Sci. 2016, 17, 1933. [Google Scholar] [CrossRef]
- Xu, Z.; Dai, J.; Su, W.; Wu, H.; Shah, K.; Xing, L.; Ma, J.; Zhang, D.; Zhao, C. Selection and Validation of Reliable Reference Genes for Gene Expression Studies in Different Genotypes and TRV-Infected Fruits of Peach (Prunus persica L. Batsch) during Ripening. Genes 2022, 13, 160. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Bonghi, C.; Trainotti, L.; Botton, A.; Tadiello, A.; Rasori, A.; Ziliotto, F.; Zaffalon, V.; Casadoro, G.; Ramina, A. A microarray approach to identify genes involved in seed-pericarp cross-talk and development in peach. BMC Plant Biol. 2011, 11, 107. [Google Scholar] [CrossRef]
- Zanchin, A.; Bonghi, C.; Casadoro, G.; Ramina, A.; Rascio, N. Cell Enlargement and Cell-Separation during Peach Fruit-Development. Int. J. Plant Sci. 1994, 155, 49–56. [Google Scholar] [CrossRef]
- Aspeborg, H.; Schrader, J.; Coutinho, P.M.; Stam, M.; Kallas, A.; Djerbi, S.; Nilsson, P.; Denman, S.; Amini, B.; Sterky, F.; et al. Carbohydrate-active enzymes involved in the secondary cell wall biogenesis in hybrid aspen. Plant Physiol. 2005, 137, 983–997. [Google Scholar] [CrossRef]
- Finaev, D. Some aspects of cellulose biosynthesis. Biol. Plant 2007, 51, 407–413. [Google Scholar] [CrossRef]
- Wu, B.; Zhang, B.; Dai, Y.; Zhang, L.; Shang-Guan, K.; Peng, Y.; Zhou, Y.; Zhu, Z. Brittle culm15 encodes a membrane-associated chitinase-like protein required for cellulose biosynthesis in rice. Plant Physiol. 2012, 159, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- van Hengel, A.J.; Tadesse, Z.; Immerzeel, P.; Schols, H.; van Kammen, A.; de Vries, S.C. N-acetylglucosamine and glucosamine-containing arabinogalactan proteins control somatic embryogenesis. Plant Physiol. 2001, 125, 1880–1890. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Kalaitzis, P.; Blazakis, K.N.; Zdunek, A. The role of arabinogalactan proteins (AGPs) in fruit ripening—A review. Hortic. Res. 2020, 7, 176. [Google Scholar] [CrossRef]

| Firmness | ||
|---|---|---|
| Development Stage | Softening Stage | |
| Firmness | 1 | 1 |
| WSP | 0.595 | −0.937 * |
| ASP | 0.733 | 0.998 ** |
| Cellulose | 0.970 * | 0.737 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Dai, J.; Liang, L.; Zhang, Y.; He, Y.; Xing, L.; Ma, J.; Zhang, D.; Zhao, C. Chitinase-Like Protein PpCTL1 Contributes to Maintaining Fruit Firmness by Affecting Cellulose Biosynthesis during Peach Development. Foods 2023, 12, 2503. https://doi.org/10.3390/foods12132503
Xu Z, Dai J, Liang L, Zhang Y, He Y, Xing L, Ma J, Zhang D, Zhao C. Chitinase-Like Protein PpCTL1 Contributes to Maintaining Fruit Firmness by Affecting Cellulose Biosynthesis during Peach Development. Foods. 2023; 12(13):2503. https://doi.org/10.3390/foods12132503
Chicago/Turabian StyleXu, Ze, Jieyu Dai, Liping Liang, Yonglan Zhang, Yaojun He, Libo Xing, Juanjuan Ma, Dong Zhang, and Caiping Zhao. 2023. "Chitinase-Like Protein PpCTL1 Contributes to Maintaining Fruit Firmness by Affecting Cellulose Biosynthesis during Peach Development" Foods 12, no. 13: 2503. https://doi.org/10.3390/foods12132503
APA StyleXu, Z., Dai, J., Liang, L., Zhang, Y., He, Y., Xing, L., Ma, J., Zhang, D., & Zhao, C. (2023). Chitinase-Like Protein PpCTL1 Contributes to Maintaining Fruit Firmness by Affecting Cellulose Biosynthesis during Peach Development. Foods, 12(13), 2503. https://doi.org/10.3390/foods12132503





