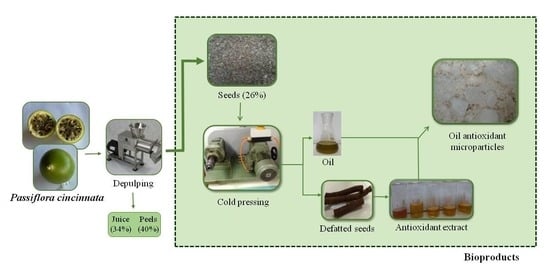
Bioproducts from Passiflora cincinnata Seeds: The Brazilian Caatinga Passion Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Seeds Oil Extraction
2.2.1. Fatty Acid Composition
2.2.2. Oxidative Stability
2.3. Antioxidant Compound Recovery from Defatted Seeds
2.3.1. Total Phenolic Content
2.3.2. Evaluation of the Antioxidant Capacity
- ABTS•+
- DPPH•
- Ferric reducing/antioxidant power (FRAP)
2.3.3. BiocompoundsProfile by UPLC–MS/MS
2.3.4. Antimicrobial Assay
2.4. Microencapsulation by Spray Dryer
2.4.1. Microparticles’ Oxidative Stability
2.4.2. X-ray Diffraction
2.4.3. Morphology of Microparticles
2.4.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4.5. Particle Size
2.4.6. Moisture Content
2.4.7. Hygroscopicity
2.4.8. Solubility
2.5. Statistical Analysis
3. Results and Discussion
3.1. Oil Extraction and Characterization
3.1.1. Yield
3.1.2. Fatty Acid Profile
3.2. Antioxidant Compound Recovery from Defatted Seeds
3.2.1. Evaluation of the Extraction Process
3.2.2. Biocompound Profile
3.2.3. Antimicrobial Action
3.3. Microparticle Characterization
3.3.1. Oxidative Stability of Oil Microparticles
3.3.2. DRX
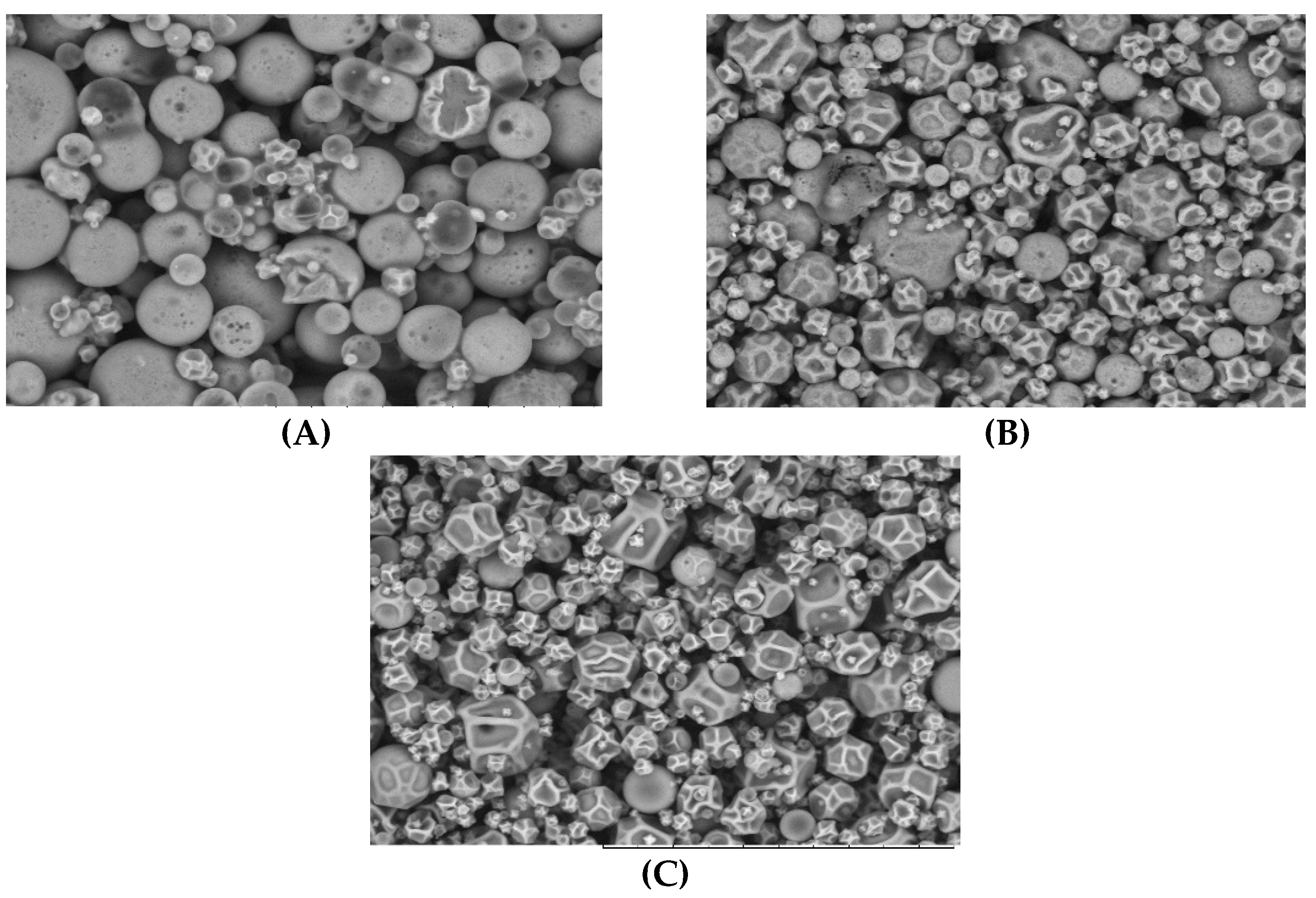
3.3.3. Morphology and Particle Size
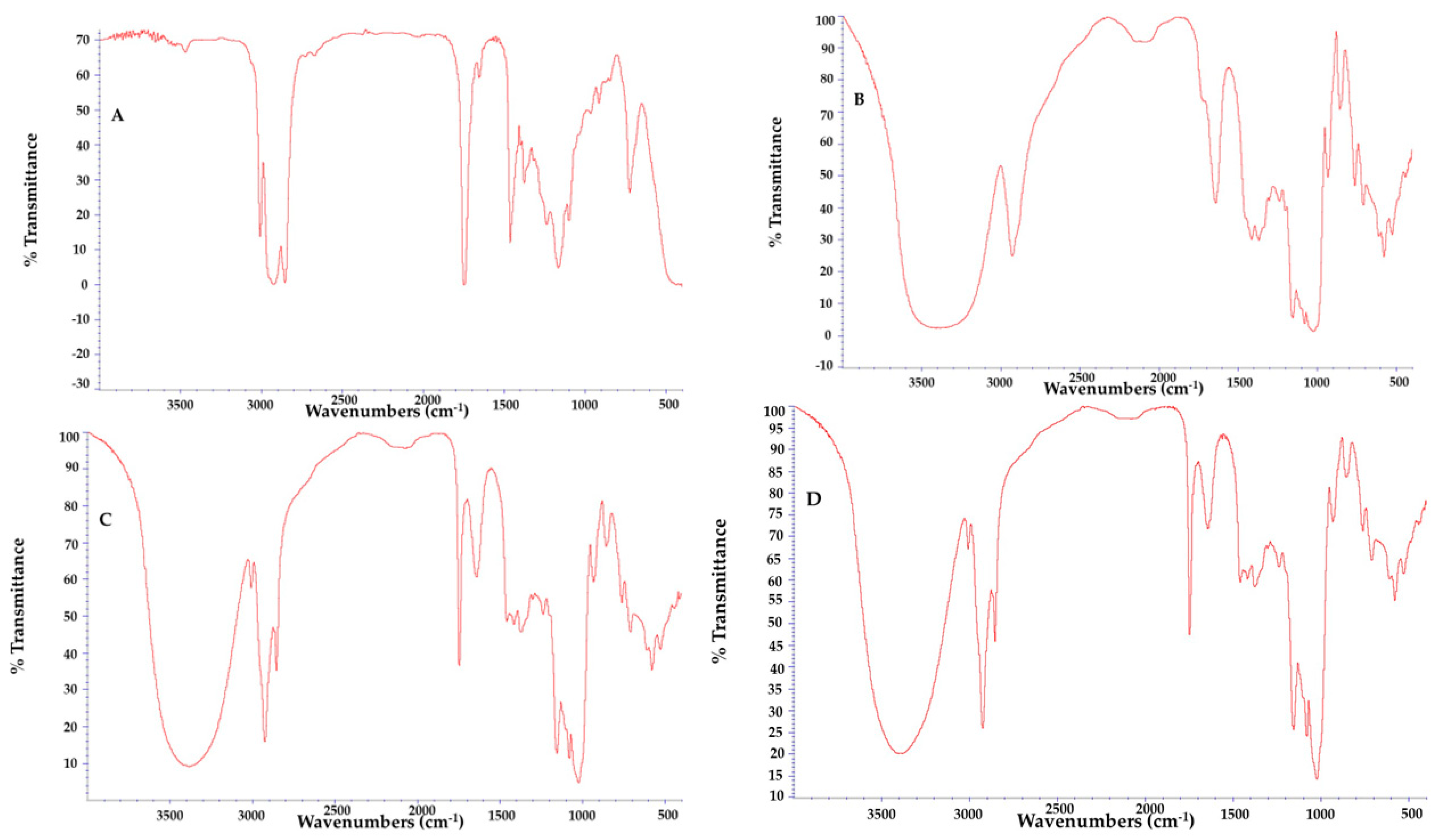
3.3.4. FTIR
3.3.5. Moisture, Solubility and Hygroscopicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brandão, L.E.M.; Nôga, D.A.M.F.; Dierschnabel, A.L.; Campêlo, C.L.D.C.; Meurer, Y.D.S.R.; Lima, R.H.; Engelberth, R.C.G.J.G.J.; Cavalcante, J.S.; Lima, C.A.; Marchioro, M.; et al. Passiflora cincinnata Extract Delays the Development of Motor Signs and Prevents Dopaminergic Loss in a Mice Model of Parkinson’s Disease. Evid. Based Complement. Altern. Med. 2017, 2017, 8429290. [Google Scholar] [CrossRef] [Green Version]
- de Lavor, É.M.; Leal, A.E.B.P.; Fernandes, A.W.C.; de Almeida Ribeiro, F.P.R.; de Menezes Barbosa, J.; e Silva, M.G.; de Andrade Teles, R.B.; da Silva Oliveira, L.F.; Silva, J.C.; Rolim, L.A.; et al. Ethanolic Extract of the Aerial Parts of Passiflora cincinnata Mast. (Passifloraceae) Reduces Nociceptive and Inflammatory Events in Mice. Phytomedicine 2018, 47, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Siebra, A.L.A.; Oliveira, L.R.; Martins, A.O.B.P.B.; Siebra, D.C.; Albuquerque, R.S.; Lemos, I.C.S.; Delmondes, G.A.; Tintino, S.R.; Figueredo, F.G.; da Costa, J.G.M.; et al. Potentiation of Antibiotic Activity by Passiflora cincinnata Mast. Front of Strains Staphylococcus aureus and Escherichia coli. Saudi J. Biol. Sci. 2018, 25, 37–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBGE. PAM—Produção Agrícola Municipal; Instituto Brasileiro de Geografia e Estatística: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Araújo, A.J.B.; Santos, N.C.; Barros, S.L.; Vilar, S.B.O.; Schmidt, F.L.; Araujo, F.P.; Azevedo, L.C. Caracterização Físico—Química e Perfil Lipídico Da Semente de Maracujá Do Mato (Passiflora cincinnata Mast.). Cad. Pesqui. Ciência Inovação 2019, 2, 14–22. [Google Scholar]
- Faleiro, F.G.; Tadeu, N.; Junqueira, V.; Braga, M.F.; deOliveira, E.J.; Peixoto, J.R.; Costa, A.M. Germoplasma e Melhoramento Genético Do Maracujazeiro: Histórico e Perspectivas. Planaltina Embrapa Cerrados 2011, 38. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/76032/1/doc-307.pdf (accessed on 24 June 2023).
- Reis, C.C.; Mamede, A.M.G.N.; Soares, A.; Freitas, S.P. Production of Lipids and Natural Antioxidants from Passion Fruit Seeds. Grasas Aceites 2020, 71, e385. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Raffo, A.; Giovannini, A.; Kiefer, J. Passion Fruit (Passiflora spp.) Seed Oil. In Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019; pp. 577–603. [Google Scholar] [CrossRef]
- Ribeiro, D.N.; Alves, F.M.S.; dos Santos Ramos, V.H.; Alves, P.; Narain, N.; Vedoy, D.R.L.; Cardozo-Filho, L.; de Jesus, E. Extraction of Passion Fruit (Passiflora cincinnata Mast.) Pulp Oil Using Pressurized Ethanol and Ultrasound: Antioxidant Activity and Kinetics. J. Supercrit. Fluids 2020, 165, 104944. [Google Scholar] [CrossRef]
- Malacrida, C.R.; Jorge, N. Yellow Passion Fruit Seed Oil (Passiflora edulis f. Flavicarpa): Physical and Chemical Characteristics. Braz. Arch. Biol. Technol. 2012, 55, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Moreira, N.X.; Curi, R.; Mancini Filho, J. Ácidos Graxos: Uma Revisão. Nutr. Rev. Soc. Bras. Aliment. Nutr. 2002, 24, 105–123. [Google Scholar]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent Advances in the Spray Drying Encapsulation of Essential Fatty Acids and Functional Oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- De Oliveira Ribeiro, L.; Freitas, S.P.; da Matta, V.M.; Jung, E.P.; Kunigami, C.N. Microencapsulation of the Extract from Euterpe edulis Co-Product: An Alternative to Add Value to Fruit Agro-Chain. Waste Biomass Valoriz. 2021, 12, 1803–1814. [Google Scholar] [CrossRef]
- Jung, E.P.; Ribeiro, L.O.; Kunigami, C.N.; Figueiredo, E.S.; Nascimento, F.S. Ripe Banana Peel Flour: A Raw Material for the Food Industry. Rev. Virtual Quim. 2019, 11, 1712–1724. [Google Scholar] [CrossRef]
- Freitas de Oliveira, C.; Giordani, D.; Lutckemier, R.; Gurak, P.D.; Cladera-Olivera, F.; Ferreira Marczak, L.D. Extraction of Pectin from Passion Fruit Peel Assisted by Ultrasound. LWT-Food Sci. Technol. 2016, 71, 110–115. [Google Scholar] [CrossRef]
- Ribeiro, L.d.O.; de Freitas, B.P.; Lorentino, C.M.A.; Frota, H.F.; dos Santos, A.L.S.; Moreira, D.d.L.; do Amaral, B.S.; Jung, E.P.; Kunigami, C.N. Umbu Fruit Peel as Source of Antioxidant, Antimicrobial and α-Amylase Inhibitor Compounds. Molecules 2022, 27, 410. [Google Scholar] [CrossRef] [PubMed]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–168. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese Medicinal Plants: Dependence of Final Antioxidant Capacity and Phenol Content on Extraction Features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Hidalgo, M.; Sánchez-Moreno, C.; de Pascual-Teresa, S. Flavonoid–Flavonoid Interaction and Its Effect on Their Antioxidant Activity. Food Chem. 2010, 121, 691–696. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Da Silva James, N.K.; Castro, L.P.S.; Freitas, S.P.; Nogueira, R.I. Increasing Energy Efficiency in Microencapsulation of Soybean Oil by Spray Drying. Braz. J. Dev. 2019, 5, 8082–8095. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of the Association of Analytical Chemists International; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Cai, Y.Z.; Corke, H. Production and Properties of Spray-Dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- Cano-Chauca, M.; Stringheta, P.C.; Ramos, A.M.; Cal-Vidal, J. Effect of the Carriers on the Microstructure of Mango Powder Obtained by Spray Drying and Its Functional Characterization. Innov. Food Sci. Emerg. Technol. 2005, 6, 420–428. [Google Scholar] [CrossRef]
- Lopes, R.M.; Sevilha, A.C.; Faleiro, F.G.; Silva, D.B.D.; Vieira, R.F.; Agostini-Costa, T.D.S. Estudo Comparativo Do Perfil de Ácidos Graxos Em Semente de Passifloras Nativas Do Cerrado Brasileiro. Rev. Bras. Frutic. 2010, 32, 498–506. [Google Scholar] [CrossRef]
- De Paula, R.C.M.; Soares, A.G.; Freitas, S.P. Volatile Coumponds in Passion Fruit Seed Oil (Passiflora setacea BRS Pérola Do Cerrado and Passiflora alata BRS Doce Mel). Chem. Eng. Trans. 2015, 44, 103–108. [Google Scholar] [CrossRef]
- Pradhan, R.C.; Mishra, S.; Naik, S.N.; Bhatnagar, N.; Vijay, V.K. Oil Expression from Jatropha Seeds Using a Screw Press Expeller. Biosyst. Eng. 2011, 109, 158–166. [Google Scholar] [CrossRef]
- Astrup, A.V.; Bazinet, R.; Brenna, J.T.; Calder, P.C.; Crawford, M.A.; Dangour, A.; Donahoo, W.T.; Elmadfa, I.; Galli, C.; Gerber, M.; et al. Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation. FAO Food Nutr. Pap. 2010, 91, 1–166. [Google Scholar]
- Cacace, J.E.; Mazza, G. Mass Transfer Process during Extraction of Phenolic Compounds from Milled Berries. J. Food Eng. 2003, 59, 379–389. [Google Scholar] [CrossRef]
- Leal, A.E.B.P.; de Oliveira, A.P.; dos Santos, R.F.; Soares, J.M.D.; de Lavor, E.M.; Pontes, M.C.; de Lima, J.T.; Santos, A.D.d.C.; Tomaz, J.C.; de Oliveira, G.G.; et al. Determination of Phenolic Compounds, in Vitro Antioxidant Activity and Characterization of Secondary Metabolites in Different Parts of Passiflora cincinnata by HPLC-DAD-MS/MS Analysis. Nat. Prod. Res. 2020, 34, 995–1001. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the Extraction of Polyphenols from Grape Seed Meal by Aqueous Ethanol Solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Alcântara, M.A.; de Lima Brito Polari, I.; de Albuquerque Meireles, B.R.L.; de Lima, A.E.A.; da Silva Junior, J.C.; de Andrade Vieira, É.; dos Santos, N.A.; de Magalhães Cordeiro, A.M.T. Effect of the Solvent Composition on the Profile of Phenolic Compounds Extracted from Chia Seeds. Food Chem. 2019, 275, 489–496. [Google Scholar] [CrossRef]
- Hanhineva, K.; Rogachev, I.; Aura, A.-M.; Aharoni, A.; Poutanen, K.; Mykkänen, H. Identification of Novel Lignans in the Whole Grain Rye Bran by Non-Targeted LC–MS Metabolite Profiling. Metabolomics 2012, 8, 399–409. [Google Scholar] [CrossRef]
- Eklund, P.C.; Backman, M.J.; Kronberg, L.Å.; Smeds, A.I.; Sjöholm, R.E. Identification of Lignans by Liquid Chromatography-Electrospray Ionization Ion-Trap Mass Spectrometry. J. Mass Spectrom. 2007, 43, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Kuhnle, G.G.C.; Dell’Aquila, C.; Aspinall, S.M.; Runswick, S.A.; Joosen, A.M.C.P.; Mulligan, A.A.; Bingham, S.A. Phytoestrogen Content of Fruits and Vegetables Commonly Consumed in the UK Based on LC–MS and 13C-Labelled Standards. Food Chem. 2009, 116, 542–554. [Google Scholar] [CrossRef]
- Van Linh, N.; Trung Tuong, N.; Xuan Phong, P.; Trang, D.T.; Nhiem, N.X.; Hoai An, D.; Huu Tai, B. New Phenylethanoid and Other Compounds From Passiflora foetida L., with Their Nitric Oxide Inhibitory Activities. Nat. Prod. Commun. 2022, 17, 1934578X2211411. [Google Scholar] [CrossRef]
- Favela-Hernández, J.M.J.; García, A.; Garza-González, E.; Rivas-Galindo, V.M.; Camacho-Corona, M.R. Antibacterial and Antimycobacterial Lignans and Flavonoids from Larrea tridentata. Phyther. Res. 2012, 26, 1957–1960. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chang, C.; Hong, C.; Zhang, H.; Huang, J.; Jin, Q.; Wang, X. Phenolic Compounds as Stabilizers of Oils and Antioxidative Mechanisms under Frying Conditions: A Comprehensive Review. Trends Food Sci. Technol. 2019, 92, 33–45. [Google Scholar] [CrossRef]
- Pereira de Oliveira, J.; Almeida, O.P.; Campelo, P.H.; Carneiro, G.; de Oliveira Ferreira Rocha, L.; Santos, J.H.P.M.; Gomes da Costa, J.M. Tailoring the Physicochemical Properties of Freeze-Dried Buriti Oil Microparticles by Combining Inulin and Gum Arabic as Encapsulation Agents. LWT-Food Sci. Technol. 2022, 161, 113372. [Google Scholar] [CrossRef]
- Botrel, D.A.; De Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of Wall Matrix Systems on the Properties of Spray-Dried Microparticles Containing Fish Oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Tonon, R.V.; Grosso, C.R.F.F.; Hubinger, M.D. Influence of Emulsion Composition and Inlet Air Temperature on the Microencapsulation of Flaxseed Oil by Spray Drying. Food Res. Int. 2011, 44, 282–289. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Identificação Espectrométrica de Compostos Orgânicos; livros técnicos e científicos: Rio de Janeiro, Brazil, 2013; ISBN 978-85-216-3637-3. [Google Scholar]
- Zhang, L.; Zeng, X.; Fu, N.; Tang, X.; Sun, Y.; Lin, L. Maltodextrin: A Consummate Carrier for Spray-Drying of Xylooligosaccharides. Food Res. Int. 2018, 106, 383–393. [Google Scholar] [CrossRef]
- Hijo, A.A.C.T.; da Costa, J.M.G.; Silva, E.K.; Azevedo, V.M.; Yoshida, M.I.; Borges, S.V. Physical and Thermal Properties of Oregano (Origanum vulgare L.) Essential Oil Microparticles: Oregano Essential Oil Microparticles. J. Food Process. Eng. 2015, 38, 1–10. [Google Scholar] [CrossRef]
- Nurhadi, B.; Andoyo, R.; Mahani; Indiarto, R. Study the Properties of Honey Powder Produced from Spray Drying and Vacuum Drying Method. Int. Food Res. J. 2012, 19, 907–912. [Google Scholar]
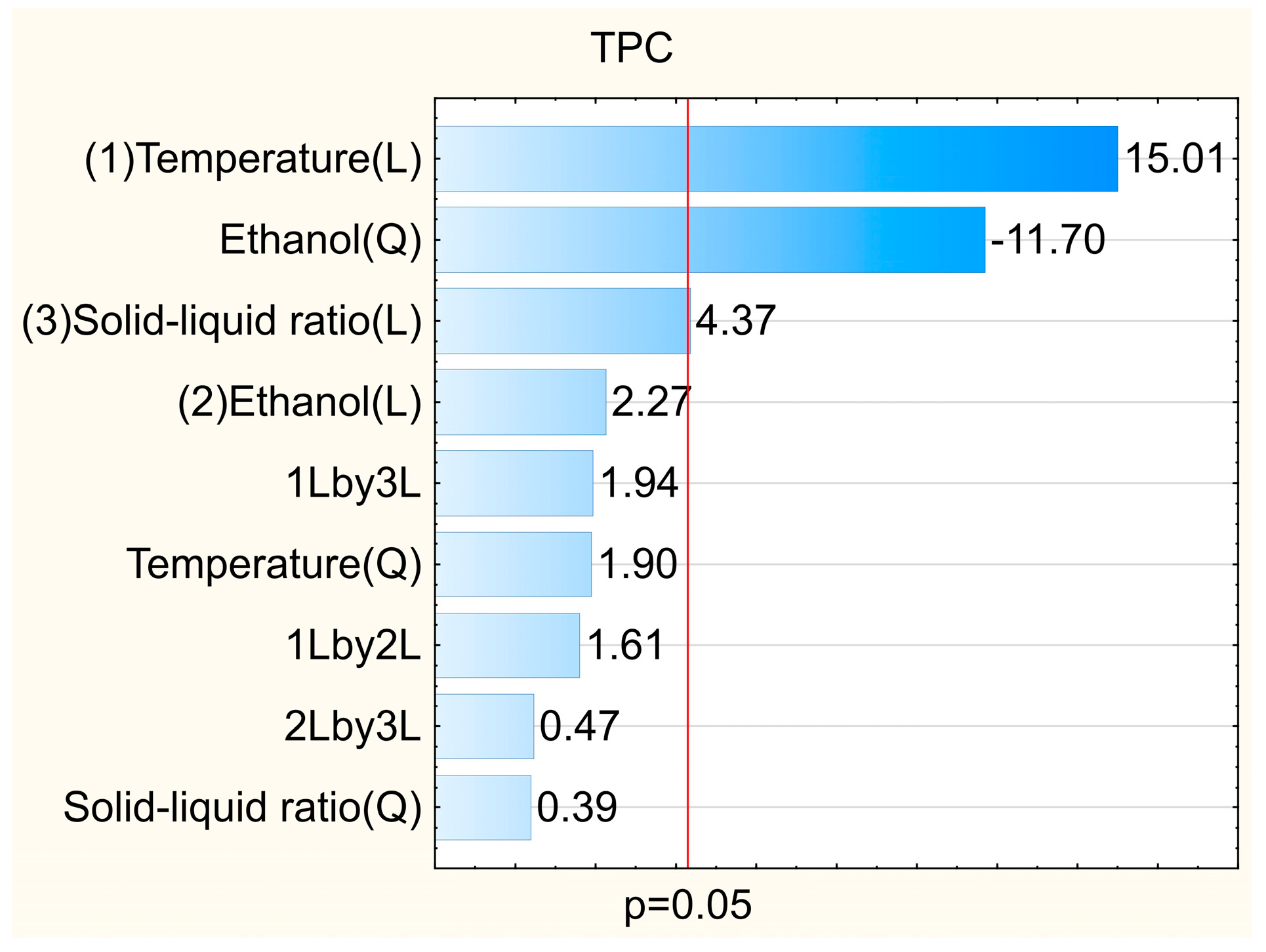
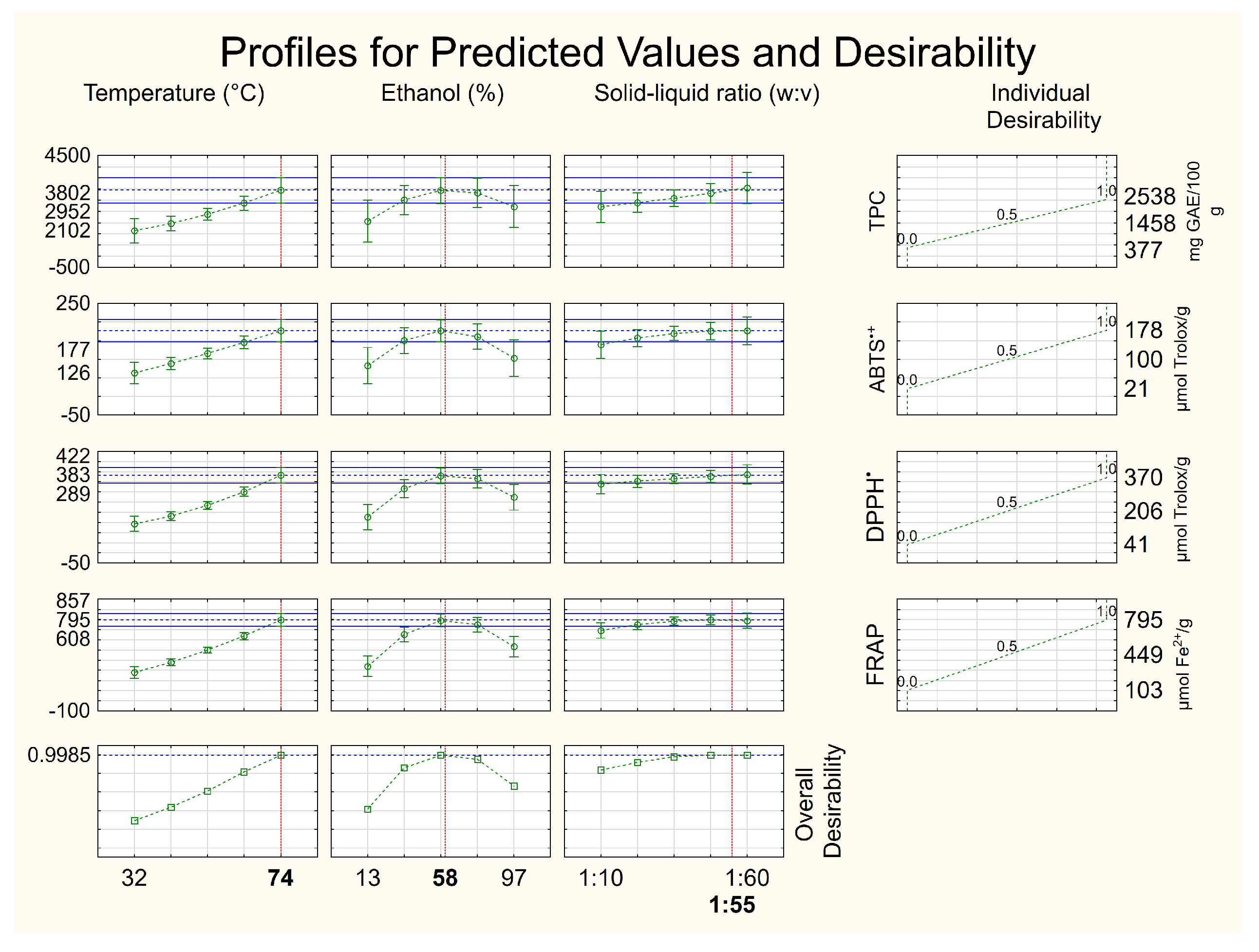


| Trials | Temperature (°C) | Ethanol (%) | Solid/Liquid Ratio (g mL−1) | TPC 1 | ABTS•+ 2 | DPPH• 2 | FRAP 3 |
|---|---|---|---|---|---|---|---|
| 1 | 40 (−1) | 30 (−1) | 1:20 (−1) | 845 ± 25 | 43 ± 2 | 111 ± 3 | 223 ± 8 |
| 2 | 40 (−1) | 30 (−1) | 1:50 (+1) | 912 ± 31 | 51 ± 1 | 107 ± 5 | 216 ± 4 |
| 3 | 40 (−1) | 80 (+1) | 1:20 (−1) | 1016 ± 27 | 64 ± 3 | 148 ± 6 | 284 ± 4 |
| 4 | 40 (−1) | 80 (+1) | 1:50 (+1) | 1220 ± 25 | 69 ± 1 | 153 ± 6 | 304 ± 6 |
| 5 | 65 (+1) | 30 (−1) | 1:20 (−1) | 1371 ± 57 | 80 ± 2 | 182 ± 8 | 395 ± 8 |
| 6 | 65 (+1) | 30 (−1) | 1:50 (+1) | 1799 ± 119 | 105 ± 2 | 214 ± 12 | 462 ± 19 |
| 7 | 65 (+1) | 80 (+1) | 1:20 (−1) | 1870 ± 35 | 114 ± 2 | 280 ± 11 | 567 ± 18 |
| 8 | 65 (+1) | 80 (+1) | 1:50 (+1) | 2302 ± 31 | 130 ± 2 | 292 ± 10 | 614 ± 17 |
| 9 | 32 (−1.68) | 55 (0) | 1:35 (0) | 991 ± 42 | 63 ± 4 | 128 ± 7 | 303 ± 6 |
| 10 | 74 (+1.68) | 55 (0) | 1:35 (0) | 2538 ± 141 | 178 ± 6 | 370 ± 20 | 795 ± 5 |
| 11 | 53 (0) | 13 (−1.68) | 1:35 (0) | 720 ± 18 | 45 ± 1 | 92 ± 5 | 211 ± 2 |
| 12 | 53 (0) | 97 (+1.68) | 1:35 (0) | 377 ± 17 | 21 ± 1 | 41 ± 0 | 103 ± 1 |
| 13 | 53 (0) | 55 (0) | 1:10 (−1.68) | 1451 ± 69 | 99 ± 4 | 210 ± 5 | 474 ± 17 |
| 14 | 53 (0) | 55 (0) | 1:60 (+1.68) | 1809 ± 80 | 111 ± 8 | 226 ± 5 | 462 ± 16 |
| 15 | 53 (0) | 55 (0) | 1:35 (0) | 1810 ± 124 | 121 ± 1 | 235 ± 1 | 515 ± 6 |
| 16 | 53 (0) | 55 (0) | 1:35 (0) | 1616 ± 57 | 117 ± 1 | 222 ± 4 | 527 ± 1 |
| 17 | 53 (0) | 55 (0) | 1:35 (0) | 1637 ± 64 | 110 ± 3 | 234 ± 3 | 504 ± 3 |
| Fatty Acids | Present Work | Lopes et al. [25] | Araújo et al. [5] |
|---|---|---|---|
| Palmitic (C16:0) | 12.14 ± 1.00 | 10.2 | 9.2 |
| Stearic (C 18:0) | 1.09 ± 0.26 | 2.9 | 3.0 |
| Oleic (C 18:1) | 8.43 ± 1.32 | 11.3 | 15.4 |
| Linoleic (C 18:2) | 78.34 ± 2.22 | 74.3 | 70.3 |
| Linolenic (C 18:3) | - | 0.6 | 0.6 |
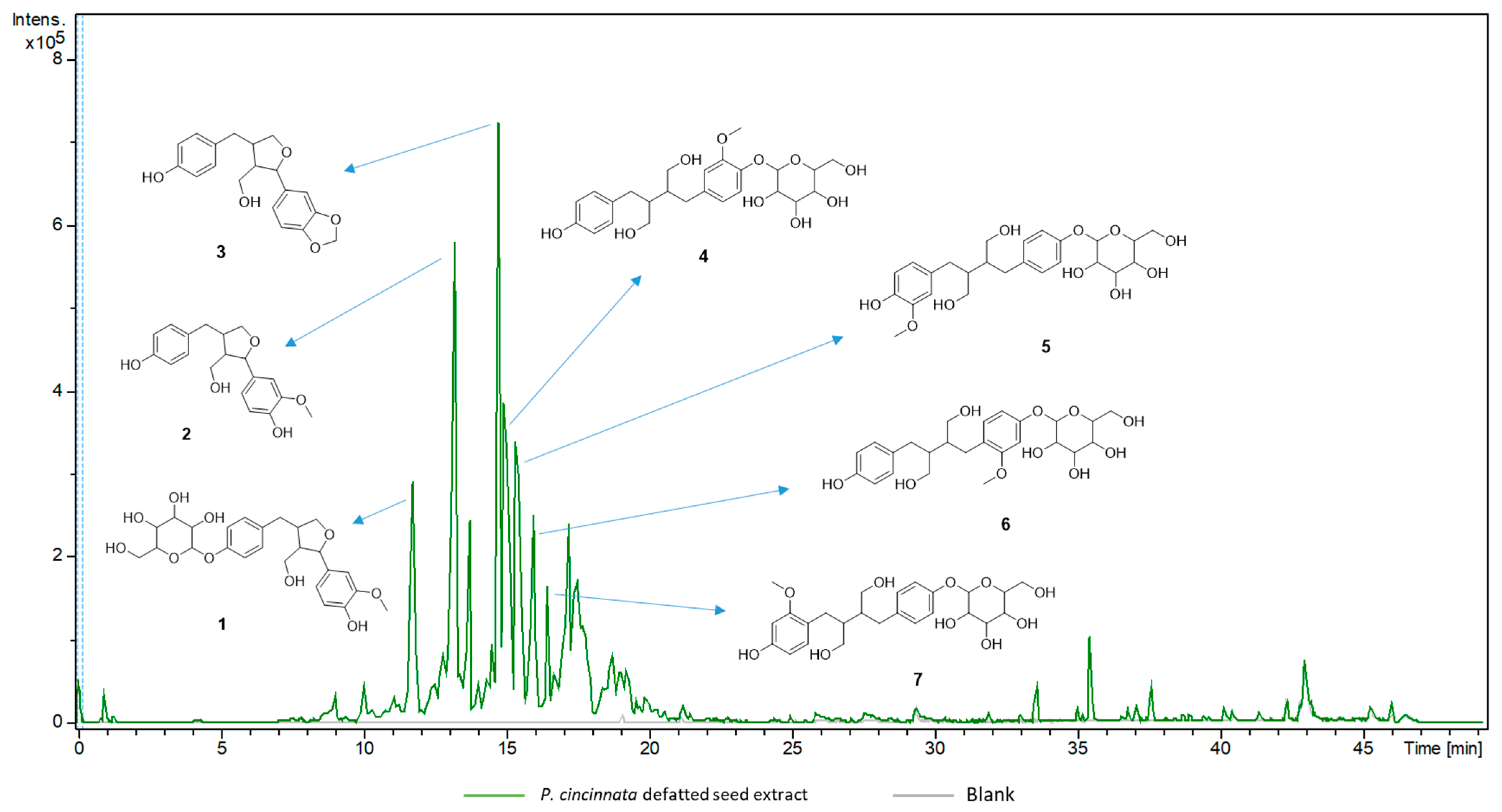
| Compound Name | TR (min) | Molecular Formula | Adduct Ion | Experimental m/z | Main MS2Fragment Ions | Relative Percentage (%) |
|---|---|---|---|---|---|---|
| 3-demethoxy-8-dehydroxy-berchemol 4-O-glucoside (1) | 11.8 | C25H32O10 | [M − H]− | 491.2162 | 165.0786, 147.0655, 135.0650 | 5.9 |
| 3-demethoxy-8-dehydroxy-berchemol (2) | 13.2 | C19H22O5 | [M − H]− | 329.1431 | 165.0793, 147.0660, 146.0572, 135.0659, 129.0540 | 10.7 |
| 2-(1,3-benzodioxol-5-yl)tetrahydro-4-[(4-hydroxyphenyl)methyl]-3-furanmethanol (3) | 14.8 | C19H20O5 | [M − H]− | 327.1280 | 163.0624, 162.0538, 147.0657, 135.0649, | 8.5 |
| Secoisolariciresinol 4-O-xylopyranoside (4) | 15.0 | C25H34O10 | [M − H]− | 493.2113 | 329.1427, 299.1287, 179.0591, 165.0777, 147.0656, 137.0443 | 10.6 |
| 3-demethoxy-secoisolariciresinol 4-O-glucoside (5) | 15.5 | C25H34O10 | [M − H]− | 493.2108 | 329.1429, 299.1292, 165.0779, 147.0661, 137.0445 | 9.8 |
| 2-methoxy-bisdemethoxy- secoisolariciresinol 4-O-glucoside (6) | 16.0 | C25H34O10 | [M − H]− | 493.2107 | 165.0781, 147.0658, 135.0650 | 4.9 |
| 2′-methoxy-bisdemethoxy- secoisolariciresinol 4-O-glucoside (7) | 16.5 | C25H34O10 | [M − H]− | 493.2102 | 165.0780, 147.0657, 135.0651 | 2.6 |
| 3-demethoxy-secoisolariciresinol 4-O-glucosil glucoside (8) | 14.8 | C31H44O15 | [M − H]− | 655.2615 | 165.0778, 163.0622, 162.0543 | 7.2 |
| Isovitexin 2″-O-arabinoside (9) | 11.5 | C26H28O14 | [M + H]+ | 565.1555 | 283.0595, 313.0702, 397.0917, 415.1010, 433.1253 | <0.5 |
| Adonivernith (10) | 10.8 | C26H28O15 | [M + H]+ | 581.1507 | 299.0564, 329.0659, 413.0844, 431.1016, 449.1073 | <0.5 |
| Microorganisms | MBC/MFC [mg GAE mL−1] a |
|---|---|
| Gram-positive bacteria | |
| Bacillus subtilis 168 LMD 74.6 | 0.602 |
| Staphylococcus aureus ATCC 29213 | 0.302 |
| Staphylococcus epidermidis ATCC 12228 | 0.302 |
| Gram-negative bacteria | |
| Acinetobacter baumannii ATCC 19606 | 0.602 |
| Escherichia coli ATCC 25922 | 0.602 |
| Klebsiella pneumoniae ATCC13883 | 0.602 |
| Psedomonas aeruginosa ATCC 27853 | ND |
| Fungi | |
| Candida albicans ATCC 90028 | ND |
| Candida tropicalis ATCC 750 | ND |
| Samples | Induction Time (Hours) |
|---|---|
| Pure oil | 5.37± 0.18 b |
| Oil + antioxidant extract microencapsulated | 6.97± 0.73 a |
| Oil microencapsulated | 5.27± 0.53 b |
| Samples | Average Particle Size (μm) | Span Value | Moisture (%) | Hygroscopicity (%) | Solubility (%) |
|---|---|---|---|---|---|
| Oil, phenolic extract and wall material microencapsulated | 20.63 ±0.81 b | 1.46 ±0.11 b | 4.83 ±0.13 b | 7.17 ± 0.10 b | 76.97 ±0.21 a |
| Oil and wall material microencapsulated | 16.40 ±0.54 a | 2.37 ±0.16 a | 4.10 ±0.06 c | 7.53 ±0.04 b | 77.03 ±0.36 a |
| Wall material microencapsulated | 15.50 ±0.05 a | 2.22 ±0.06 a | 6.16 ±0.12 a | 10.75 ±0.77 a | 72.87 ±1.74 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reis, C.C.; Freitas, S.P.; Lorentino, C.M.A.; Fagundes, T.d.S.F.; da Matta, V.M.; dos Santos, A.L.S.; Moreira, D.d.L.; Kunigami, C.N.; Jung, E.P.; Ribeiro, L.d.O. Bioproducts from Passiflora cincinnata Seeds: The Brazilian Caatinga Passion Fruit. Foods 2023, 12, 2525. https://doi.org/10.3390/foods12132525
Reis CC, Freitas SP, Lorentino CMA, Fagundes TdSF, da Matta VM, dos Santos ALS, Moreira DdL, Kunigami CN, Jung EP, Ribeiro LdO. Bioproducts from Passiflora cincinnata Seeds: The Brazilian Caatinga Passion Fruit. Foods. 2023; 12(13):2525. https://doi.org/10.3390/foods12132525
Chicago/Turabian StyleReis, Carolina Cruzeiro, Suely Pereira Freitas, Carolline Margot Albanez Lorentino, Thayssa da Silva Ferreira Fagundes, Virgínia Martins da Matta, André Luis Souza dos Santos, Davyson de Lima Moreira, Claudete Norie Kunigami, Eliane Przytyk Jung, and Leilson de Oliveira Ribeiro. 2023. "Bioproducts from Passiflora cincinnata Seeds: The Brazilian Caatinga Passion Fruit" Foods 12, no. 13: 2525. https://doi.org/10.3390/foods12132525
APA StyleReis, C. C., Freitas, S. P., Lorentino, C. M. A., Fagundes, T. d. S. F., da Matta, V. M., dos Santos, A. L. S., Moreira, D. d. L., Kunigami, C. N., Jung, E. P., & Ribeiro, L. d. O. (2023). Bioproducts from Passiflora cincinnata Seeds: The Brazilian Caatinga Passion Fruit. Foods, 12(13), 2525. https://doi.org/10.3390/foods12132525