Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties †
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Bagasse Flours
Tomato Bagasse Flour Preparation
2.2. In Vitro Digestion Simulation (GID)
2.2.1. Sample Preparation
2.2.2. In Vitro Digestion Simulation
2.3. Preliminary Evaluation of the Prebiotic Potential of Tomato SF
2.3.1. Microorganisms
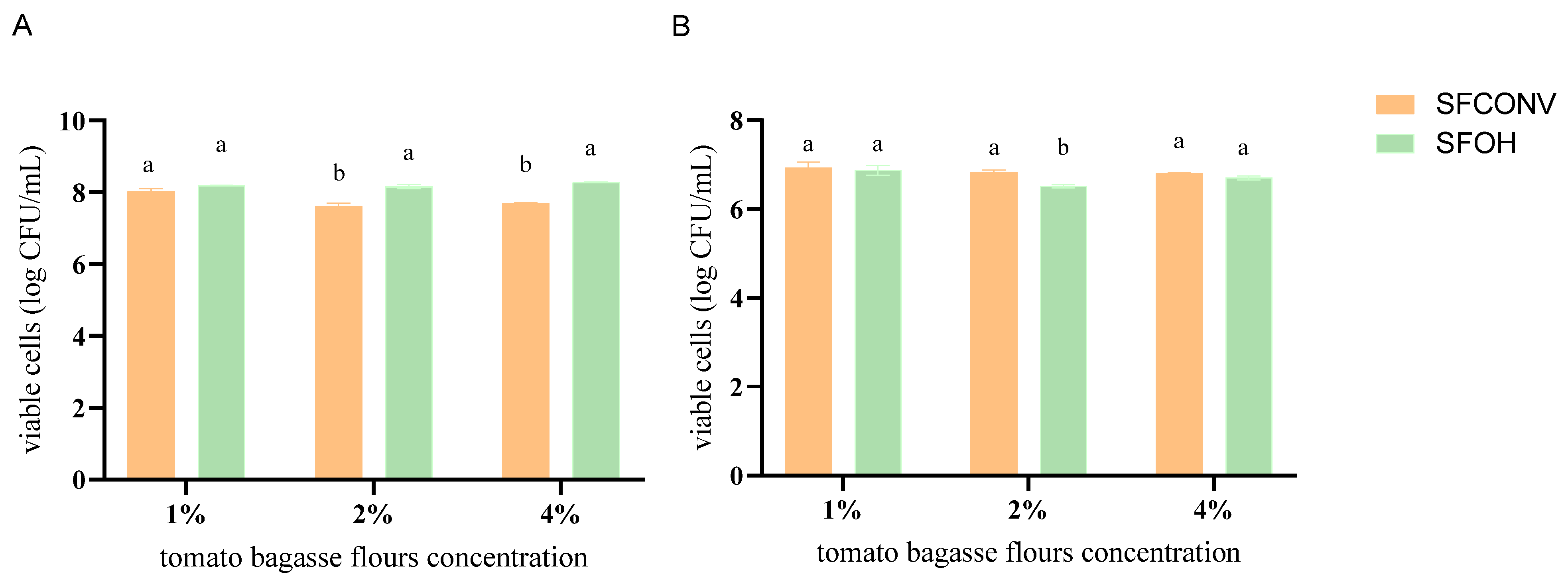
2.3.2. Selection of the Best Tomato Flour Concentration
2.4. In Vitro Fecal Fermentations
2.4.1. Collection and Preparation of Fecal Inocula
2.4.2. Nutrient Base Medium Preparation
2.4.3. Fecal Fermentations
2.4.4. Fecal Fermentation Sample Processing
2.5. Sugars and SCFA Analysis
2.6. Bacterial Population Analysis
2.6.1. DNA Extraction
2.6.2. Real-Time Quantitative Polymerase Chain Reaction—Gut Composition Analysis
2.7. Statistical Analysis
3. Results
3.1. Probiotic Effect
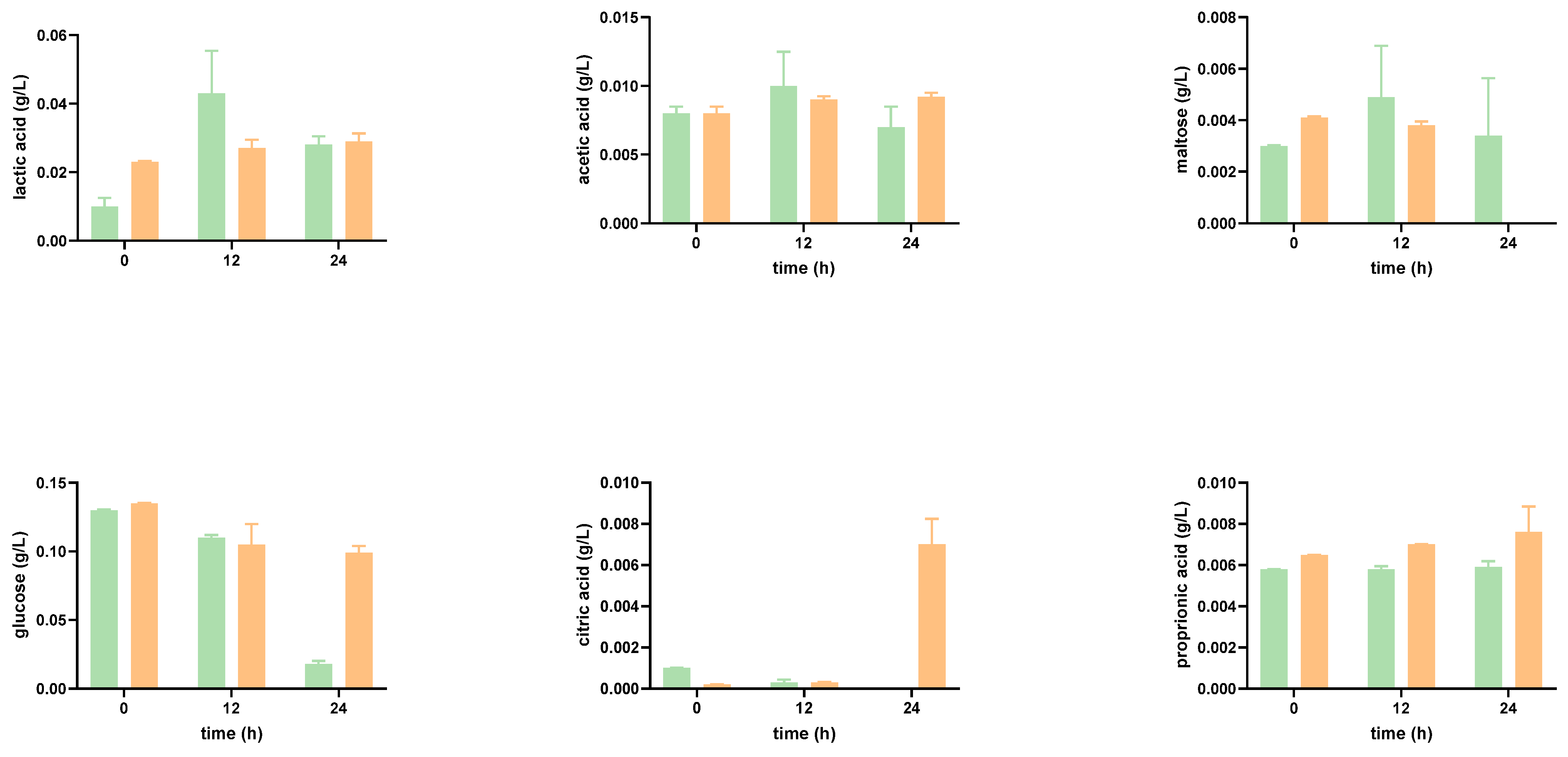
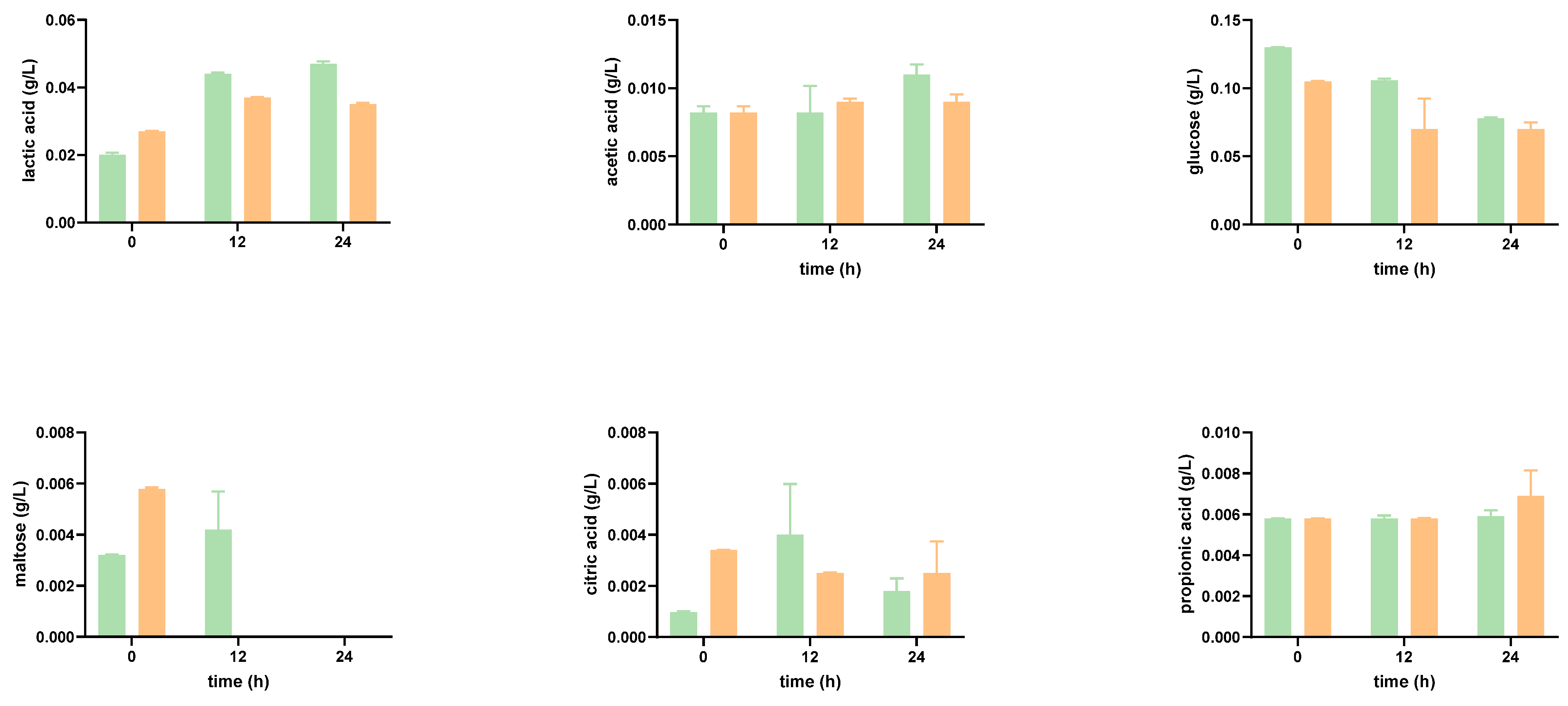
Impact of the Digested Tomato SF on Organic Acid Production
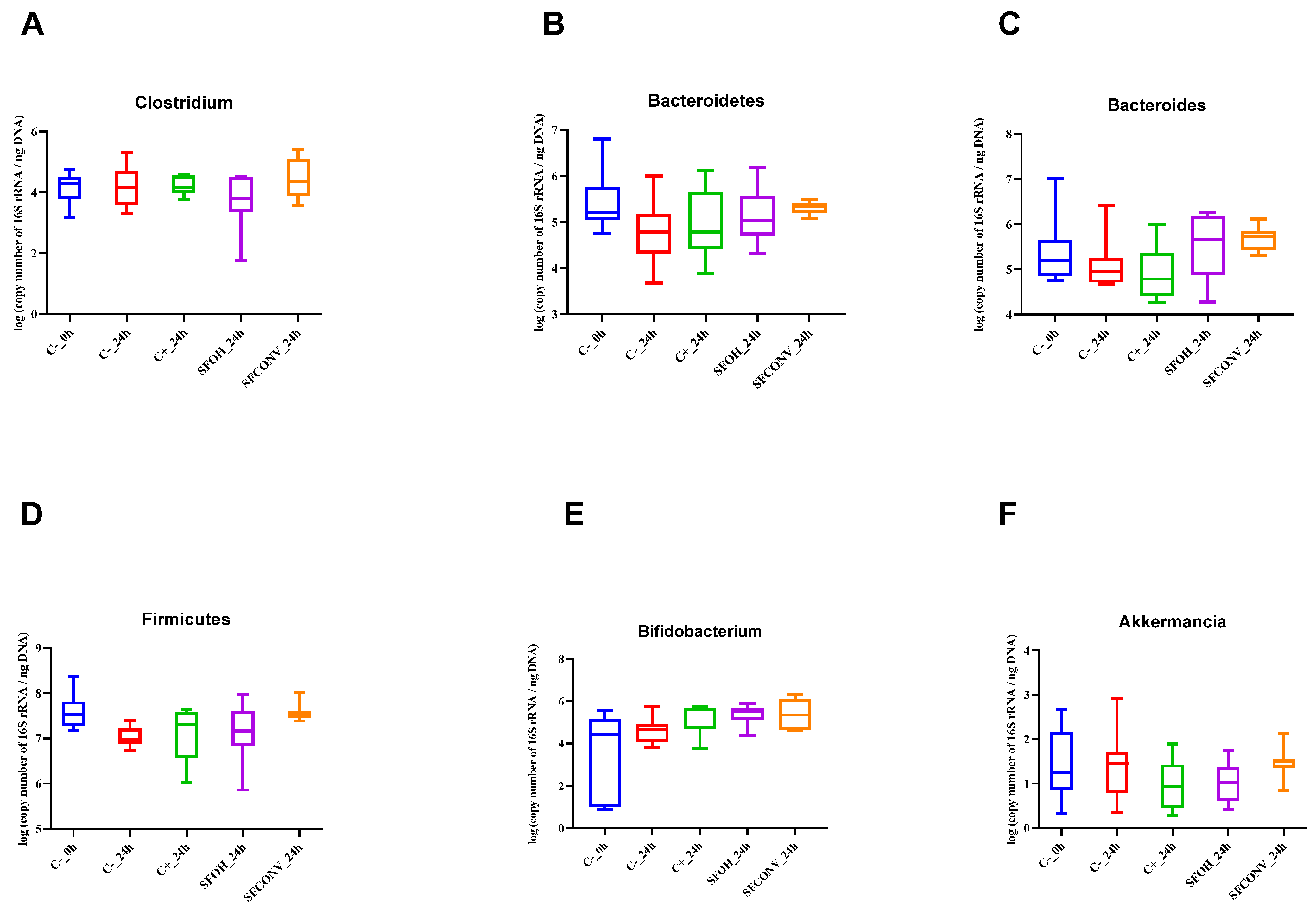
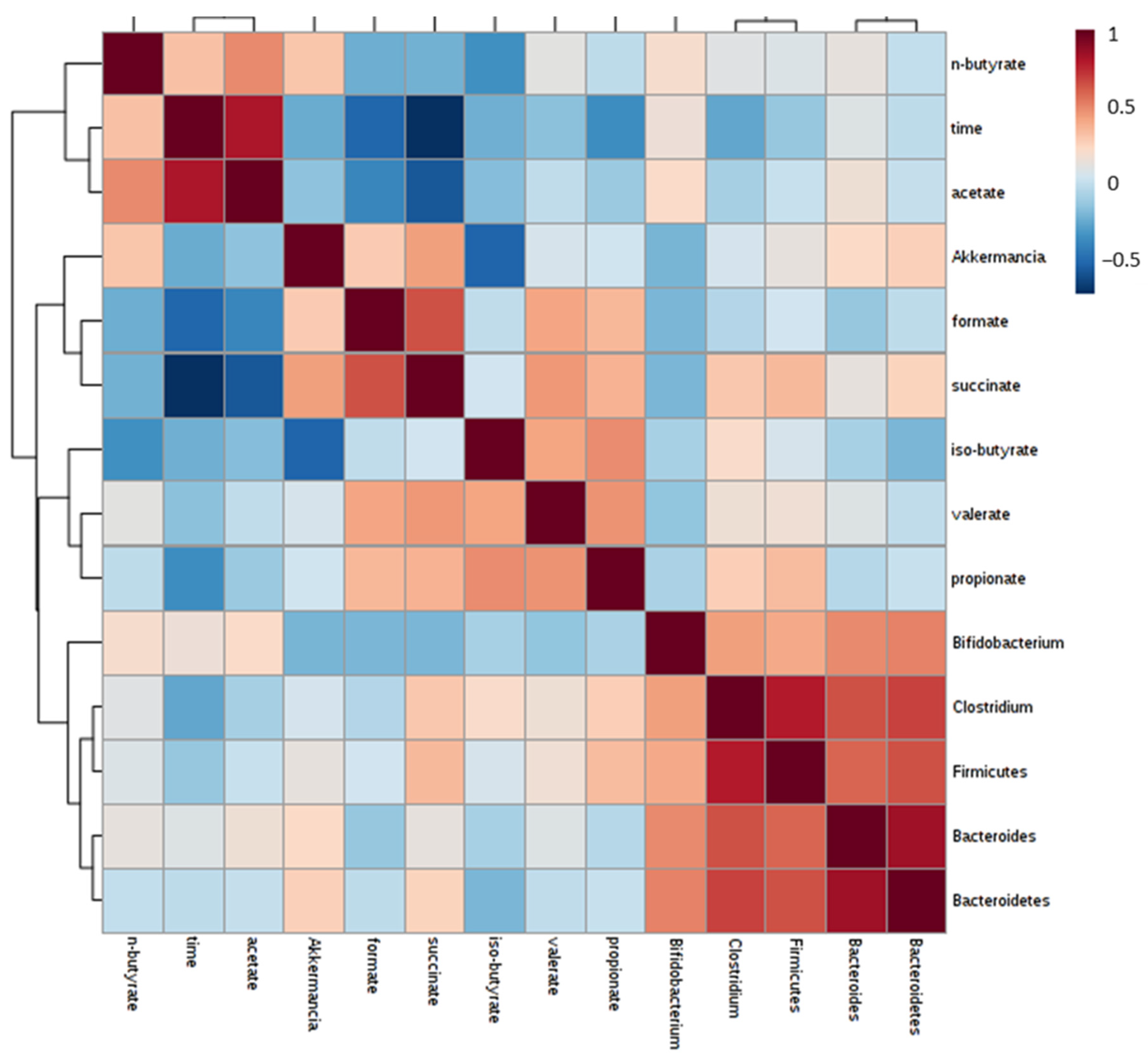
3.2. Impact of Tomato Flour after Extraction on Gut Microbiota
3.2.1. Microbial Population Modulation
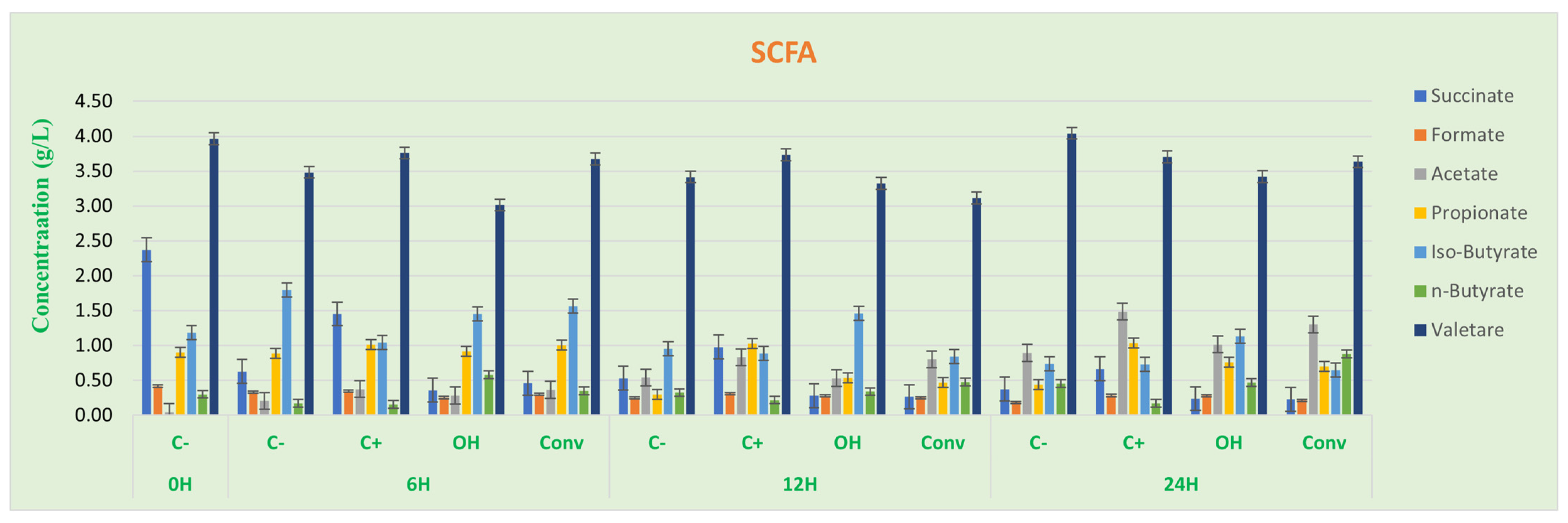
3.2.2. SCFA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, V.; Ferrão, J.; Pimentel, L.; Pintado, M.; Fernandes, T. One Health, Fermented Foods, and Gut Microbiota. Foods 2018, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Roupar, D.; Coelho, M.C.; Gonçalves, D.A.; Silva, S.P.; Coelho, E.; Silva, S.; Coimbra, M.A.; Pintado, M.; Teixeira, J.A.; Nobre, C. Evaluation of Microbial-Fructo-Oligosaccharides Metabolism by Human Gut Microbiota Fermentation as Compared to Commercial Inulin-Derived Oligosaccharides. Foods 2022, 11, 954. [Google Scholar] [CrossRef] [PubMed]
- Cait, A.; Hughes, M.R.; Antignano, F.; Cait, J.; Dimitriu, P.A.; Maas, K.R.; Reynolds, L.A.; Hacker, L.; Mohr, J.; Finlay, B.B.; et al. Microbiome-driven allergic lung inflammation is ameliorated by short-chain fatty acids. Mucosal Immunol. 2018, 11, 785–795. [Google Scholar] [CrossRef]
- Sivaprakasam, S.; Prasad, P.D.; Singh, N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 2016, 164, 144–151. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2019, 91, 157–225. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.C.; Ribeiro, T.B.; Oliveira, C.; Batista, P.; Castro, P.; Monforte, A.R.; Rodrigues, A.S.; Teixeira, J.; Pintado, M. In Vitro Gastrointestinal Digestion Impact on the Bioaccessibility and Antioxidant Capacity of Bioactive Compounds from Tomato Flours Obtained after Conventional and Ohmic Heating Extraction. Foods 2021, 10, 554. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Rangel-Hernández, J.; Morales-Sánchez, E.; Loarca-Piña, G.; Gaytán-Martínez, M. Changes on the phytochemicals profile of instant corn flours obtained by traditional nixtamalization and ohmic heating process. Food Chem. 2018, 276, 57–62. [Google Scholar] [CrossRef]
- Coelho, M.; Rodrigues, A.; Teixeira, J.; Pintado, M. Integral valorisation of tomato by-products towards bioactive compounds recovery: Human health benefits. Food Chem. 2023, 410, 135319. [Google Scholar] [CrossRef]
- Coelho, M.C.; Ghalamara, S.; Campos, D.; Ribeiro, T.B.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M. Tomato Processing By-Products Valorisation through Ohmic Heating Approach. Foods 2023, 12, 818. [Google Scholar] [CrossRef]
- Campos, D.A.; Coscueta, E.R.; Vilas-Boas, A.A.; Silva, S.; Teixeira, J.A.; Pastrana, L.M.; Pintado, M.M. Impact of functional flours from pineapple by-products on human intestinal microbiota. J. Funct. Foods 2020, 67, 103830. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Chen, T.; Long, W.; Zhang, C.; Liu, S.; Zhao, L.; Hamaker, B.R. Fiber-utilizing capacity varies in Prevotella- versus Bacteroides-dominated gut microbiota. Sci. Rep. 2017, 7, 2594. [Google Scholar] [CrossRef]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Oliveira, A.; Alexandre, E.M.; Coelho, M.; Barros, R.M.; Almeida, D.P.; Pintado, M. Peach polyphenol and carotenoid content as affected by frozen storage and pasteurization. LWT—Food Sci. Technol. 2016, 66, 361–368. [Google Scholar] [CrossRef]
- Vilella, S.B.; Vaqué, L.G. The Commission Establishes the Specific Compositional and Information Requirements for “Total Diet Replacement for Weight Control Products”: Commission Delegated Regulation (EU) 2017/1798. Eur. Food Feed. Law Rev. EFFL 2018, 13, 108–115. [Google Scholar]
- Sousa, S.; Pinto, J.; Pereira, C.; Malcata, F.X.; Pacheco, M.B.; Gomes, A.M.; Pintado, M. In vitro evaluation of yacon (Smallanthus sonchifolius) tuber flour prebiotic potential. Food Bioprod. Process. 2015, 95, 96–105. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and β-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct. 2022, 13, 12686–12696. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Tavaria, F.; Alonso, J.L.; Pintado, M. In vitro assessment of the prebiotic potential of Aloe vera mucilage and its impact on the human microbiota. Food Funct. 2015, 6, 525–531. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Walton, G.; Poveda, C.; Silva, S.; Amorim, M.; Madureira, A.R.; Pintado, M.M.; Gibson, G.; Jauregi, P. Study of in vitro digestion of Tenebrio molitor flour for evaluation of its impact on the human gut microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Watson, D.; Motherway, M.O.; Schoterman, M.; van Neerven, R.J.; Nauta, A.; van Sinderen, D. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, H.P.; Motherway, M.O.; Lakshminarayanan, B.; Stanton, C.; Ross, R.P.; Brulc, J.; Menon, R.; O’Toole, P.W.; van Sinderen, D. Carbohydrate catabolic diversity of bifidobacteria and lactobacilli of human origin. Int. J. Food Microbiol. 2015, 203, 109–121. [Google Scholar] [CrossRef]
- de Carvalho, N.M.; Teixeira, F.; Silva, S.; Madureira, A.R.; Pintado, M.E. Potential prebiotic activity of Tenebrio molitor insect flour using an optimized in vitro gut microbiota model. Food Funct. 2019, 10, 3909–3922. [Google Scholar] [CrossRef] [PubMed]
- Gaglio, R.; Alfonzo, A.; Polizzotto, N.; Corona, O.; Francesca, N.; Russo, G.; Moschetti, G.; Settanni, L. Performances of Different Metabolic Lactobacillus Groups during the Fermentation of Pizza Doughs Processed from Semolina. Fermentation 2018, 4, 61. [Google Scholar] [CrossRef]
- Crittenden, R.; Karppinen, S.; Ojanen, S.; Tenkanen, M.; Fagerström, R.; Mättö, J.; Saarela, M.; Mattila-Sandholm, T.; Poutanen, K. In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J. Sci. Food Agric. 2002, 82, 781–789. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, H.; Chen, W.; Zhong, Q.; Zhang, G.; Chen, W. Beneficial Effects of Tomato Juice Fermented by Lactobacillus Plantarum and Lactobacillus Casei: Antioxidation, Antimicrobial Effect, and Volatile Profiles. Molecules 2018, 23, 2366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Pokusaeva, K.; Fitzgerald, G.F.; van Sinderen, D. Carbohydrate metabolism in Bifidobacteria. Genes Nutr. 2011, 6, 285–306. [Google Scholar] [CrossRef]
- Mazé, A.; O’Connell-Motherway, M.; Fitzgerald, G.F.; Deutscher, J.; van Sinderen, D. Identification and Characterization of a Fructose Phosphotransferase System in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 2007, 73, 545–553. [Google Scholar] [CrossRef]
- Viborg, A.H.; Katayama, T.; Hachem, M.A.; Andersen, M.C.; Nishimoto, M.; Clausen, M.H.; Urashima, T.; Svensson, B.; Kitaoka, M. Distinct substrate specificities of three glycoside hydrolase family 42 β-galactosidases from Bifidobacterium longum subsp. infantis ATCC 15697. Glycobiology 2014, 24, 208–216. [Google Scholar] [CrossRef]
- Parche, S.; Beleut, M.; Rezzonico, E.; Jacobs, D.; Arigoni, F.; Titgemeyer, F.; Jankovic, I. Lactose-over-Glucose Preference in Bifidobacterium longum NCC2705: glcP, Encoding a Glucose Transporter, Is Subject to Lactose Repression. J. Bacteriol. 2006, 188, 1260–1265. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. Int. Rev. J. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Díaz, J.; Muñoz-Quezada, S.; Gómez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Amakiri, A.C.; Thantsha, M.S. Survival of Bifidobacterium longum LMG 13197 microencapsulated in Vegetal or Vegetal-inulin matrix in simulated gastrointestinal fluids and yoghurt. Springerplus 2016, 5, 1343. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Xie, J.; Huang, J.; Chen, L.; Gao, L.; Ou, S.; Wang, Y.; Peng, X. Plant polyphenols alter a pathway of energy metabolism by inhibiting fecal Bacteroidetes and Firmicutes in vitro. Food Funct. 2016, 7, 1501–1507. [Google Scholar] [CrossRef] [PubMed]
- Simpson, H.L.; Campbell, B.J. Review article: Dietary fibre-microbiota interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Jandhyala, S.M.; Talukdar, R.; Subramanyam, C.; Vuyyuru, H.; Sasikala, M.; Nageshwar Reddy, D. Role of the normal gut microbiota. World J. Gastroenterol. 2015, 21, 8787–8803. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]
- Rastmanesh, R. Use of Probiotics in Burn Patients to Improve Nutritional Status and Clinical Outcomes: A Hypothesis. Int. J. Probiotics Prebiotics 2011, 6, 159. [Google Scholar]
- Di Paola, M.; De Filippo, C.; Cavalieri, D.; Ramazzotti, M.; Poullet, J.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. PP90 impact of diet in shaping gut microbiota revealed by a comparative study in children from europe and rural africa. Dig. Liver Dis. 2011, 43, S445–S446. [Google Scholar] [CrossRef]
- Parkar, S.G.; Trower, T.M.; Stevenson, D.E. Fecal microbial metabolism of polyphenols and its effects on human gut microbiota. Anaerobe 2013, 23, 12–19. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Mahowald, M.A.; Ley, R.E.; A Lozupone, C.; Hamady, M.; Martens, E.C.; Henrissat, B.; Coutinho, P.M.; Minx, P.; Latreille, P.; et al. Evolution of Symbiotic Bacteria in the Distal Human Intestine. PLoS Biol. 2007, 5, e156. [Google Scholar] [CrossRef]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Zhou, K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J. Funct. Foods 2017, 33, 194–201. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Energetics and Application of Heterotrophy in Acetogenic Bacteria. Appl. Environ. Microbiol. 2016, 82, 4056–4069. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, M.C.; Costa, C.; Roupar, D.; Silva, S.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties. Foods 2023, 12, 1920. https://doi.org/10.3390/foods12091920
Coelho MC, Costa C, Roupar D, Silva S, Rodrigues AS, Teixeira JA, Pintado ME. Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties. Foods. 2023; 12(9):1920. https://doi.org/10.3390/foods12091920
Chicago/Turabian StyleCoelho, Marta C., Célia Costa, Dalila Roupar, Sara Silva, A. Sebastião Rodrigues, José A. Teixeira, and Manuela E. Pintado. 2023. "Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties" Foods 12, no. 9: 1920. https://doi.org/10.3390/foods12091920
APA StyleCoelho, M. C., Costa, C., Roupar, D., Silva, S., Rodrigues, A. S., Teixeira, J. A., & Pintado, M. E. (2023). Modulation of the Gut Microbiota by Tomato Flours Obtained after Conventional and Ohmic Heating Extraction and Its Prebiotic Properties. Foods, 12(9), 1920. https://doi.org/10.3390/foods12091920