A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications
Abstract
:1. Introduction
2. Chemical Composition of Pea
2.1. Proximate Composition
2.2. Starch
2.3. Dietary Fiber
2.4. Protein
2.5. Lipids
2.6. Minerals and Vitamins
2.7. Polyphenols
2.7.1. Total Phenolic Content
2.7.2. Flavonoids
| Family | Compounds | Plant Part | Methods | References |
|---|---|---|---|---|
| Flavonols | Isorhamnetin 3-rutinoside, isorhamnetin glycoside, quercetin, quercetin 3-galattoside, rutin, quercetin triglucoside, quercetin diglucoside, kaempferol triglucoside, quercetin caffeoyl triglucoside, quercetin coumaroyl triglucoside, quercetin sinapoyl triglucoside, quercetin feruloyl triglucoside, isorhamnetin glycoside, kaempferol glucoside, kaempferol coumaroyl, kaempferol, dihydromyricetin, kaempferol 3-O-rutinoside-4′-glucoside, dihydroquercetin, myricetin 3-O-rhamnoside, kaempferol 3-O-glucoside, kaempferol hexoside, kaempferol-7-O-glucoside, kaempferol-7-O-rutinoside, kaempferol-3-O-rhamnoside, kaempferol dihexoside, isorhamnetin, dihydrokaempferol, kaempferol 3-O-glucopyranoside, fisetin, kaempferol 3-O-neohesperidoside, kaempferol 3-O-sophorotrioside, kaempferol 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrioside, galangin, morin, quercetin 3-O-β-D-glucopyranoside, quercetin 3-O-sophorotrioside, quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-caffeoyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-feruloyl)-sophorotrioside, quercetin 3-O-(6″″-O-trans-sinapoyl)-sophorotrioside, quercetin 3-O-(6″″-O-(4-hydroxy)-trans-cinnamoyl)-sophorotrioside, Pisumflflavonoside II [quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrio-side 7-O-β-D-glucopyranoside], Pisumflflavonoside II [quercetin 3-O-(6″″-O-trans-p-coumaroyl)-sophorotrio- side 7-O-β-D-glucopyranoside] | Seed, seed coat, pod, sprout, leaf | LC-MS, LC-ESI-MS, LC-ESI-MS/MS, UHPLC-MS, UHPLC-LTQ-MS, UHPLC-Q-HRMS | [21,71,72,73,74,75,77,78,79,80] |
| Flavones | Phloretin, apigenin, luteolin-7-O-glucoside, eriodictyol glycoside, apigenin-7-O-glucoside, luteolin, luteolin 8′-O-glucoside, vitexin, luteolin 3′,7-di-O-glucoside, apigenin-6.8-di-C-glucoside, luteolin-8′-C-glucoside, tricin | Seed, seed coat, pod | LC-MS, LC-ESI-MS, LC-ESI-MS/MS, UHPLC-MS | [21,71,72,74,75,76,78] |
| Flavanols | Catechin, (epi) catechin, gallocatechin, (epi) gallocatechin, fisetin, catechin gallate | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS/MS, UHPLC-MS, UHPLC-LTQ-MS, UHPLC-Q-HRMS | [21,71,73,75,76,77,78,79] |
| Flavanones | Eriodictyol, naringenin, naringin, hesperidin, melitidin, pinocembrin, liquiritigenin, hesperetin | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS, UHPLC-MS, UHPLC-Q-HRMS, UHPLC-LTQ-MS | [21,72,74,75,76,77,79] |
| Isoflavones | Genistein, daidzein, cirsiliol, prunetin, afrormosina, formononetin, isoformononetin, pseudobaptigenina, sayanedin, | Seed, seed coat, pod, sprout | LC-ESI-MS, UHPLC-MS, UHPLC-LTQ-MS | [21,71,74,75,79] |
| Anthocyanins | Cyanidin 3-sambubioside-5-glucoside, cyanidin 3-sophoroside-5-glucoside, delphinidin 3-sambubioside-5-glucoside, delphinidin 3-sophoroside-5-glucoside, delphinidin 3-O-(2-O-β-D-xylopyranosyl-β-D-galactopyranoside)-5-O-β-D-glucopyranoside, delphinidin 3-O-(2-O-β-D-xylopyranosyl-β-D-galactopyranoside)-5-O-(6-O-acetyl)-β-D-glucopyranoside, pelargonadin 3-glucoside, cyanidin 3,5-di-O-glucoside, malvidine-3-O-glucoside, | Seed, seed coat, pod | LC-MS, UHPLC-MS | [21,75,76] |
| Phenolic acids | gallic acid, vanillin, syringic acid, quinic acid, protocatechuic acid, chlorogenic acid, 4-o-caffeoylquinic acid, p-coumaric acid, trans-ferulic acid, trans-cinnamic acid, p-hydroxybenzoic acid, dicaffeoyl quinic acid, caffeic acid, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid, vanillin acid, ferulic acid, coumaroyl quinic acid, 5-feruloylquinic acid, vanillic acid-4-β-D-glucoside, cinnamic acid, o-coumaric acid, 2,3-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, ferulic acid, gentisic acid, m-hydroxybenzoic acid, p-hydroxybenzoic acid, 4-hydroxy-3-methoxybenzoic acid, p-hydroxyphenylacetic acid, rosmarinic acid, salicylic acid, sinapic acid, tannic acid, veratric acid | Seed, seed coat, pod, sprout | LC-MS, LC-ESI-MS/MS, LC-ESI-MS, UHPLC-MS, UHPLC-LTQ-MS | [21,70,73,74,75,76,78,80] |
2.7.3. Phenolic Acids
2.8. Other Beneficial Components
2.9. Anti-Nutritional Factors
3. Processing of Pea and Its Components
3.1. Processing of the Whole Pea Seeds
3.1.1. Drying
3.1.2. Milling
3.1.3. Soaking
3.1.4. Cooking
3.2. Modification of Pea Starches
3.3. Modification of Pea Proteins
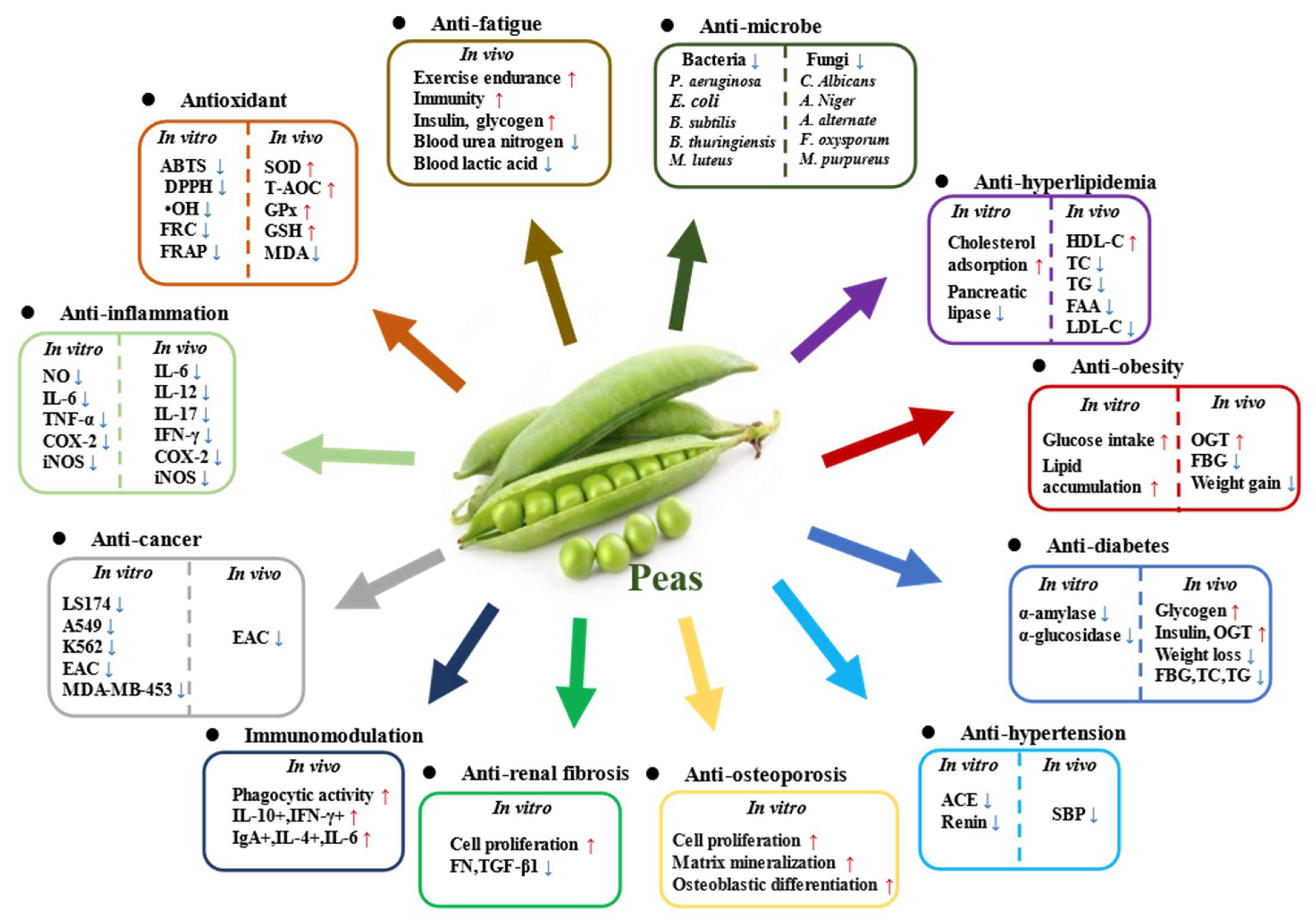
4. Health Benefits of Pea and Its Components
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Effect
4.3. Regulation of Metabolic Syndrome
4.4. Antimicrobial Effect
4.5. Anti-Renal Fibrosis Effect
4.6. Other Beneficial Effects
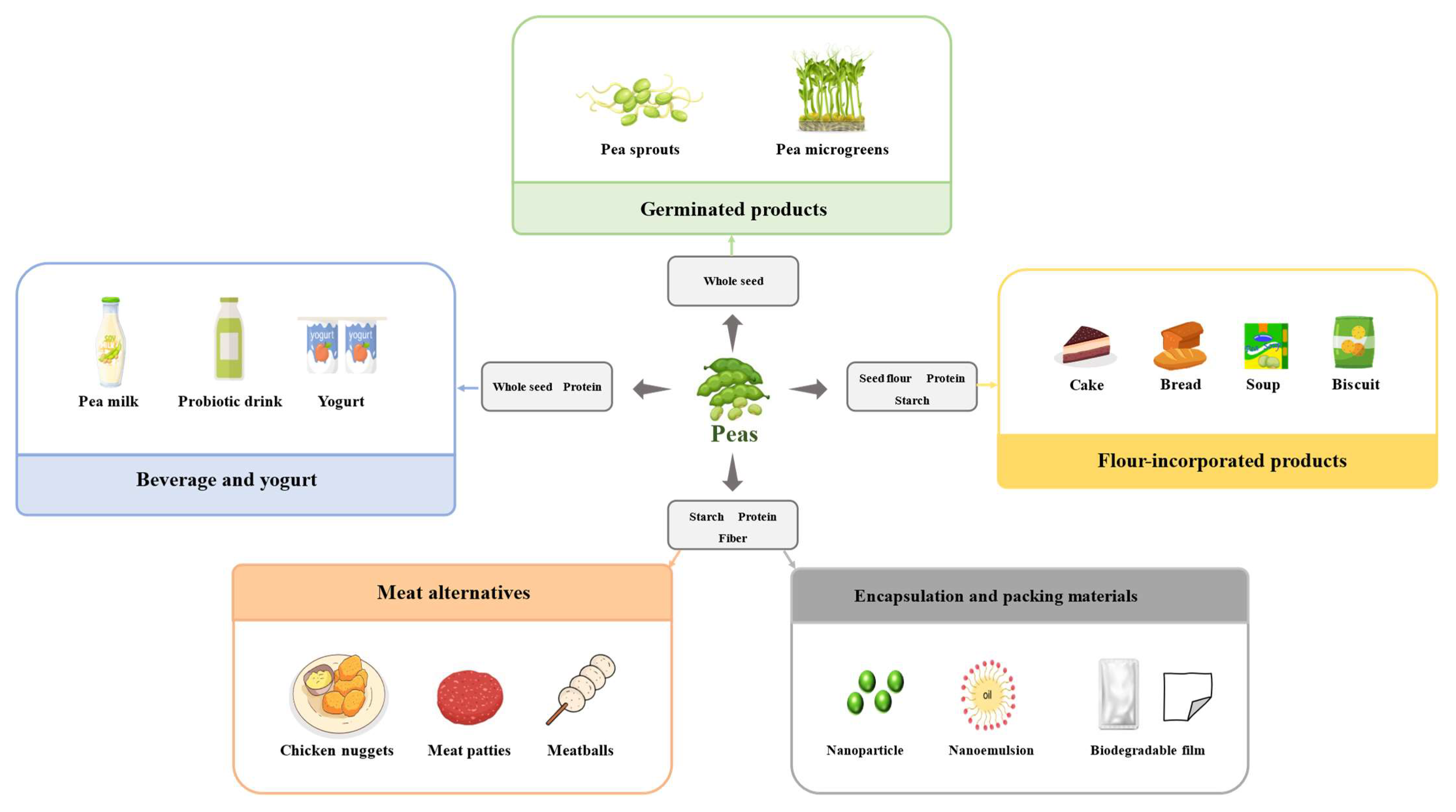
5. Applications of Pea and Its Components
5.1. Pea Beverages and Yoghurts
5.2. Germinated Pea Products
5.3. Pea Flour-Incorporated Products
5.4. Meat Alternatives
5.5. Encapsulation and Packing Materials
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fahmi, R.; Ryland, D.; Sopiwnyk, E.; Aliani, M. Sensory and Physical Characteristics of Pan Bread Fortified with Thermally Treated Split Yellow Pea (Pisum sativum L.) Flour. J. Food Sci. 2019, 84, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Akhov, L.; Ashe, P.; Lewis, C.; Deibert, L.; Irina Zaharia, L.; Forseille, L.; Xiang, D.; Datla, R.; Nosworthy, M. Comprehensive Compositional Assessment of Bioactive Compounds in Diverse Pea Accessions. Food Res. Int. 2023, 165, 112455. [Google Scholar] [CrossRef] [PubMed]
- Kumari, T.; Deka, S.C. Potential Health Benefits of Garden Pea Seeds and Pods: A Review. Legume Sci. 2021, 3, e82. [Google Scholar] [CrossRef]
- Liu, M.; Wu, N.-N.; Yu, G.-P.; Zhai, X.-T.; Chen, X.; Zhang, M.; Tian, X.-H.; Liu, Y.-X.; Wang, L.-P.; Tan, B. Physicochemical Properties, Structural Properties, and in vitro Digestibility of Pea Starch Treated with High Hydrostatic Pressure. Starch-Starke 2018, 70, 9. [Google Scholar] [CrossRef]
- Raghunathan, R.; Hoover, R.; Waduge, R.; Liu, Q.; Warkentin, T.D. Impact of Molecular Structure on the Physicochemical Properties of Starches Isolated from Different Field Pea (Pisum sativum L.) Cultivars Grown in Saskatchewan, Canada. Food Chem. 2017, 221, 1514–1521. [Google Scholar] [CrossRef]
- Gao, L.C.; Wu, Y.X.; Wan, C.X.; Wang, P.K.; Yang, P.; Gao, X.L.; Eeckhout, M.; Gao, J.F. Structural and Physicochemical Properties of Pea Starch Affected by Germination Treatment. Food Hydrocolloid 2022, 124, 107303. [Google Scholar] [CrossRef]
- Santos, C.S.; Carbas, B.; Castanho, A.; Vasconcelos, M.W.; Patto MC, V.; Domoney, C.; Brites, C. Variation in Pea (Pisum sativum L.) Seed Quality Traits Defined by Physicochemical Functional Properties. Foods 2019, 8, 570. [Google Scholar] [CrossRef] [Green Version]
- Devi, J.; Sanwal, S.K.; Koley, T.K.; Mishra, G.P.; Karmakar, P.; Singha, P.M.; Singh, B. Variations in the Total Phenolics and Antioxidant Activities among Garden Pea (Pisum sativum L.) Genotypes Differing for Maturity Duration, Seed and Flower Traits and their Association with the Yield. Sci. Hortic. 2019, 244, 141–150. [Google Scholar] [CrossRef]
- Shi, L.; Arntfield, S.D.; Nickerson, M. Changes in Levels of Phytic Acid, Lectins and Oxalates during Soaking and Cooking of Canadian Pulses. Food Res. Int. 2018, 107, 660–668. [Google Scholar] [CrossRef]
- Wojdylo, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. Microgreens as Novel Functional Foods: Variation of Nutritional and Phytochemical Profiles and their in vitro Bioactive Properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.-L.; Rajjou, L. Improving the Nutritional Quality of Pulses via Germination. Food Rev. Int. 2022, 1–34. [Google Scholar] [CrossRef]
- Babbitt, C.W.; Babbitt, G.A.; Oehman, J.M. Behavioral Impacts on Residential Food Provisioning, Use, and Waste during the COVID-19 Pandemic. Sustain. Prod. Consum. 2021, 28, 315–325. [Google Scholar] [CrossRef]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the health benefits of peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Fan, Y.S.; Wang, X.W.; Xia, M.; Cai, Y. In Vitro and In Vivo Digestibility of Pea and Chickpea Powder Prepared by Cooking and Drying Treatment. Int. J. Food Prop. 2020, 23, 1187–1199. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Dehghan, M.; Mente, A.; Bangdiwala, S.I.; Rangarajan, S.; Srichaikul, K.; Mohan, V.; Avezum, A.; Díaz, R.; Rosengren, A.; et al. Glycemic Index, Glycemic Load, and Cardiovascular Disease and Mortality. N. Engl. J. Med. 2021, 384, 1312–1322. [Google Scholar] [CrossRef]
- Thakur, S.; Scanlon, M.G.; Tyler, R.T.; Milani, A.; Paliwal, J. Pulse Flour Characteristics from a Wheat Flour Miller’s Perspective: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 775–797. [Google Scholar] [CrossRef] [Green Version]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y.P. The Health Benefits, Functional Properties, Modifications, and Applications of Pea (Pisum sativum L.) Protein: Current Status, Challenges, and Perspectives. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Kan, L.; Nie, S.; Hu, J.; Wang, S.; Bai, Z.; Wang, J.; Zhou, Y.; Jiang, J.; Zeng, Q.; Song, K.; et al. Comparative Study on the Chemical Composition, Anthocyanins, Tocopherols and Carotenoids of Selected Legumes. Food Chem. 2018, 260, 317–326. [Google Scholar] [CrossRef]
- Ye, S.X.; Shah, B.R.; Li, J.; Liang, H.S.; Zhan, F.C.; Geng, F.; Li, B. A Critical Review on Interplay Between Dietary Fibers and Gut Microbiota. Trends Food Sci. Technol. 2022, 124, 237–249. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Diapari, M.; Jha, A.B.; Tar’an, B.; Arganosa, G.; Warkentin, T.D. Genetic Diversity of Nutritionally Important Carotenoids in 94 Pea and 121 Chickpea Accessions. J. Food Compos. Anal. 2015, 43, 49–60. [Google Scholar] [CrossRef]
- Fahim, J.R.; Attia, E.Z.; Kamel, M.S. The Phenolic Profile of Pea (Pisum sativum): A Phytochemical and Pharmacological Overview. Phytochem. Rev. 2019, 18, 173–198. [Google Scholar] [CrossRef]
- Chen, S.-K.; Lin, H.-F.; Wang, X.; Yuan, Y.; Yin, J.-Y.; Song, X.-X. Comprehensive Analysis in the Nutritional Composition, Phenolic Species and in vitro Antioxidant Activities of Different Pea Cultivars. Food Chem. X 2023, 17, 100599. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining Protein Nutrition through Plant-Based Foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary Intake of Total, Animal, and Plant Proteins and Risk of All Cause, Cardiovascular, and Cancer Mortality: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef]
- Ewy, M.W.; Patel, A.; Abdelmagid, M.G.; Elfadil, O.M.; Bonnes, S.L.; Salonen, B.R.; Hurt, R.T.; Mundi, M.S. Plant-Based Diet: Is it as Good as an Animal-Based Diet When it Comes to Protein? Curr. Nutr. Rep. 2022, 11, 337–346. [Google Scholar] [CrossRef]
- Arif, U.; Ahmed, M.J.; Rabbani, M.A.; Arif, A.A. Assessment of Genetic Diversity in Pea (Pisum sativum L.) Landraces Based on Physico-Chemical and Nutritive Quality Using Cluster and Principal Component Analysis. Pak. J. Bot. 2020, 52, 575–580. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Ragaee, S.; Rabalski, I.; Warkentin, T.; Vandenberg, A. Nutrient Content and Viscosity of Saskatchewan-Grown Pulses in Relation to their Cooking Quality. Can. J. Plant. Sci. 2019, 99, 67–77. [Google Scholar] [CrossRef]
- Aluko, R.E.; Girgih, A.T.; He, R.; Malomo, S.; Li, H.; Offengenden, M.; Wu, J.P. Structural and Functional Characterization of Yellow Field Pea Seed (Pisum sativum L.) Protein-Derived Antihypertensive Peptides. Food Res. Int. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Shanthakumar, P.; Klepacka, J.; Bains, A.; Chawla, P.; Dhull, S.B.; Najda, A. The Current Situation of Pea Protein and its Application in the Food Industry. Molecules 2022, 27, 5354. [Google Scholar] [CrossRef]
- Ge, J.; Sun, C.X.; Mata, A.; Corke, H.; Gan, R.Y.; Fang, Y.P. Physicochemical and pH-Dependent Functional Properties of Proteins Isolated from Eight Traditional Chinese Beans. Food Hydrocolloid 2021, 112, 106288. [Google Scholar] [CrossRef]
- Wang, N.; Hatcher, D.W.; Warkentin, T.D.; Toews, R. Effect of Cultivar and Environment on Physicochemical and Cooking Characteristics of Field Pea (Pisum sativum). Food Chem. 2010, 118, 109–115. [Google Scholar] [CrossRef]
- Chen, X.Y.; Ma, X.W.; Wen, J.Y.; Liu, X.C.; Yu, X.R.; Xiong, F. Morphological, Structural and Functional Properties of Starches from Different Legume Resources. Legume Res. 2021, 44, 818–823. [Google Scholar] [CrossRef]
- Ren, Y.K.; Setia, R.; Warkentin, T.D.; Ai, Y.F. Functionality and Starch Digestibility of Wrinkled and Round Pea Flours of Two Different Particle Sizes. Food Chem. 2021, 336, 127711. [Google Scholar] [CrossRef]
- Ren, Y.; Yuan, T.Z.; Chigwedere, C.M.; Ai, Y. A Current Review of Structure, Functional Properties, and Industrial Applications of Pulse Starches for Value-Added Utilization. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3061–3092. [Google Scholar] [CrossRef]
- Petropoulou, K.; Salt, L.; Warren, F. A Seed Trait Studied by Gregor Mendel in Pisum sativum L. (Pea): Potential Prevention of Type 2 Diabetes. In Legumes for Global Food Security; Nova Science Publishers: New York, NY, USA, 2017; Chapter 6; pp. 129–155. [Google Scholar]
- Chung, H.J.; Liu, Q. Physicochemical Properties and In Vitro Digestibility of Flour and Starch from Pea (Pisum sativum L.) Cultivars. Int. J. Biol. Macromol. 2012, 50, 131–137. [Google Scholar] [CrossRef]
- Wang, M.; Wichienchot, S.; He, X.; Fu, X.; Huang, Q.; Zhang, B. In Vitro Colonic Fermentation of Dietary Fibers: Fermentation Rate, Short-Chain Fatty Acid Production and Changes in Microbiota. Trends Food Sci. Technol. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Wang, S.J.; Sharp, P.; Copeland, L. Structural and Functional Properties of Starches from Field Peas. Food Chem. 2011, 126, 1546–1552. [Google Scholar] [CrossRef]
- Liu, C.; Wang, S.J.; Copeland, L.; Wang, S. Physicochemical Properties and in vitro Digestibility of Starches from Field Peas Grown in China. LWT-Food Sci. Technol. 2015, 64, 829–836. [Google Scholar] [CrossRef]
- Guo, K.; Lin, L.S.; Fan, X.X.; Zhang, L.; Wei, C.X. Comparison of Structural and Functional Properties of Starches from Five Fruit Kernels. Food Chem. 2018, 257, 75–82. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T.; Oladebeye, A.O.; Oluwafemi, A.D.; Akinsola, A.F.; Imanah, O.E. Developments in the Isolation, Composition, and Physicochemical Properties of Legume Starches. Crit. Rev. Food Sci. Nutr. 2021, 61, 2938–2959. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; De Siqueira, G.L.D.A.; Lacerda, L.G.; Schnitzler, E.; Demiate, I.M. Physicochemical, Structural and Thermal Properties of Oxidized, Acetylated and Dual-Modified Common Bean (Phaseolus vulgaris L.) Starch. Food Sci. Technol. 2018, 38, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Wani, I.A.; Sogi, D.S.; Gill, B.S. Physico-Chemical Properties of Acetylated Starches from Indian Black Gram (Phaseolus mungo L.) Cultivars. J. Food Sci. Technol. 2015, 52, 4078–4089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, S.K.; Jiang, H.X.; Ai, Y.F.; Jane, J.L. Physicochemical Properties and Digestibility of Common Bean (Phaseolus vulgaris L.) Starches. Carbohydr. Polym. 2014, 108, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.T.; Wang, Y.J.; Wang, M.X.; Jane, J.L.; Du, S.K. Physicochemical Properties and In Vitro Digestibility of Legume Starches. Food Hydrocolloid 2017, 63, 249–255. [Google Scholar] [CrossRef]
- Andrabi, S.N.; Wani, I.A.; Gani, A.; Hamdani, A.M.; Masoodi, F.A. Comparative Study of Physico-Chemical and Functional Properties of Starch Extracted from Two Kidney Bean (Phaseolus vulgaris L.) and Green Gram Cultivars (Vigna radiata L.) Grown in India. Starch-Starke 2016, 68, 416–426. [Google Scholar] [CrossRef]
- Li, L.Y.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y.F. Characteristics of Pea, Lentil and Faba Bean Starches Isolated from Air-Classified Flours in Comparison with Commercial Starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef]
- Xu, M.J.; Saleh AS, M.; Liu, Y.; Jing, L.Z.; Zhao, K.; Wu, H.; Zhang, G.Q.; Yang, S.O.; Li, W.H. The Changes in Structural, Physicochemical, and Digestive Properties of Red Adzuki Bean Starch after Repeated and Continuous Annealing Treatments. Starch-Starke 2018, 70, 1700322. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Tian, X.L.; Wang, P.; Jiang, H.; Li, W.H. Compositional, Morphological, and Physicochemical Properties of Starches from Red Adzuki Bean, Chickpea, Faba Bean, and Baiyue Bean Grown in China. Food Sci. Nutr. 2019, 7, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Chen, X.; Dong, L.; Nan, X.; Ji, W.; Wang, S.; Sun, W.; Zhou, Q. Modification of Pea Dietary Fiber by Ultrafine Grinding and Hypoglycemic Effect in Diabetes Mellitus Mice. J. Food Sci. 2021, 86, 1273–1282. [Google Scholar] [CrossRef]
- Mayengbam, S.; Lambert, J.E.; Parnell, J.A.; Tunnicliffe, J.M.; Nicolucci, A.C.; Han, J.; Sturzenegger, T.; Shearer, J.; Mickiewicz, B.; Vogel, H.J.; et al. Impact of Dietary Fiber Supplementation on Modulating Microbiota-Host-Metabolic Axes in Obesity. J. Nutr. Biochem. 2019, 64, 228–236. [Google Scholar] [CrossRef]
- Brummer, Y.; Kaviani, M.; Tosh, S.M. Structural and Functional Characteristics of Dietary Fibre in Beans, Lentils, Peas and Chickpeas. Food Res. Int. 2015, 67, 117–125. [Google Scholar] [CrossRef]
- Wu, G.J.; Liu, D.; Wan, Y.J.; Huang, X.J.; Nie, S.P. Comparison of Hypoglycemic Effects of Polysaccharides from Four Legume Species. Food Hydrocolloid 2019, 90, 299–304. [Google Scholar] [CrossRef]
- Zhang, S.J.; Hu, T.T.; Chen, Y.Y.; Wang, S.Y.; Kang, Y.F. Analysis of the Polysaccharide Fractions Isolated from Pea (Pisum sativum L.) at Different Levels of Purification. J. Food Biochem. 2020, 44, e13248. [Google Scholar] [CrossRef]
- Eslinger, A.J.; Eller, L.K.; Reimer, R.A. Yellow Pea Fiber Improves Glycemia and Reduces Clostridium Leptum in Diet-Induced Obese Rats. Nutr. Res. 2014, 34, 714–722. [Google Scholar] [CrossRef]
- Lu, Z.X.; He, J.F.; Zhang, Y.C.; Bing, D.J. Composition, Physicochemical Properties of Pea Protein and its Application in Functional Foods. Crit. Rev. Food Sci. Nutr. 2020, 60, 2593–2605. [Google Scholar] [CrossRef]
- Luo, X.; Fei, Y.; Xu, Q.; Lei, T.; Mo, X.; Wang, Z.; Zhang, L.; Mou, X.; Li, H. Isolation and Identification of Antioxidant Peptides from Tartary Buckwheat Albumin (Fagopyrum tataricum Gaertn.) and their Antioxidant Activities. J. Food Sci. 2020, 85, 611–617. [Google Scholar] [CrossRef]
- Popp, J.; Trendelenburg, V.; Niggemann, B.; Randow, S.; Völker, E.; Vogel, L.; Reuter, A.; Spiric, J.; Schiller, D.; Beyer, K.; et al. Pea (Pisum sativum) Allergy in Children: Pis S 1 is an Immunodominant Major Pea Allergen and Presents Ige Binding Sites with Potential Diagnostic Value. Clin. Exp. Allergy 2020, 50, 625–635. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.L.; Marsh, J.T.; Koppelman, S.J.; Kabourek, J.L.; Johnson, P.E.; Baumert, J.L. A Perspective on Pea Allergy and Pea Allergens. Trends Food Sci. Technol. 2021, 116, 186–198. [Google Scholar] [CrossRef]
- Han, F.; Moughan, P.J.; Li, J.T.; Pang, S.J. Digestible Indispensable Amino Acid Scores (Diaas) of Six Cooked Chinese Pulses. Nutrients 2020, 12, 3831. [Google Scholar] [CrossRef]
- Zhao, H.F.; Shen, C.; Wu, Z.J.; Zhang, Z.; Xu, C.M. Comparison of Wheat, Soybean, Rice, and Pea Protein Properties for Effective Applications in Food Products. J. Food Biochem. 2020, 44, e13157. [Google Scholar] [CrossRef]
- Hall, A.E.; Moraru, C.I. Structure and Function of Pea, Lentil and Faba Bean Proteins Treated by High Pressure Processing and Heat Treatment. LWT-Food Sci. Technol. 2021, 152, 112349. [Google Scholar] [CrossRef]
- Ciurescu, G.; Toncea, I.; Ropota, M.; Habeanu, M. Seeds Composition and Their Nutrients Quality of Some Pea (Pisum sativum L.) and Lentil (Lens culinaris Medik.) Cultivars. Rom. Agric. Res. 2018, 35, 101–108. [Google Scholar] [CrossRef]
- Nadeem, M.A.; Cilesiz, Y.; Yüce, İ.; Baloch, F.S.; Karaköy, T. Macro and Micronutrients Diversity in the Seeds of Field Pea Germplasm. Pak. J. Bot. 2021, 53, 1655–1664. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.D.; Guan, X.; Huang, K.; Li, S.; Liu, J.; Yu, W.W.; Duan, R.Q. Protective Effects of Mung Bean (Vigna radiata L.) and Pea (Pisum sativum L.) against High-Fat-Induced Oxidative Stress. Food Sci. Nutr. 2019, 7, 4063–4075. [Google Scholar] [CrossRef] [Green Version]
- Padhi, E.M.T.; Liu, R.; Hernandez, M.; Tsao, R.; Ramdath, D.D. Total Polyphenol Content, Carotenoid, Tocopherol and Fatty Acid Composition of Commonly Consumed Canadian Pulses and their Contribution to Antioxidant Activity. J. Funct. Food 2017, 38, 602–611. [Google Scholar] [CrossRef]
- Gan, R.-Y.; Wang, M.-F.; Lui, W.-Y.; Wu, K.; Dai, S.-H.; Sui, Z.-Q.; Corke, H. Diversity in Antioxidant Capacity, Phenolic Contents, and Flavonoid Contents of 42 Edible Beans from China. Cereal Chem. 2017, 94, 291–297. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Su, W.J.; Qin, Y.; Wang, L.Y.; Kang, Y.F. Phenotypic Diversity of Pea (Pisum sativum L.) Varieties and the Polyphenols, Flavonoids, and Antioxidant Activity of their Seeds. Cienc. Rural. 2020, 50, e20190196. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, J.; Wei, Z.; Shahidi, F. Effect of in vitro Digestion on Phenolics and Antioxidant Activity of Red and Yellow Colored Pea Hulls. Food Chem. 2021, 337, 127606. [Google Scholar] [CrossRef]
- Borges-Martinez, E.; Gallardo-Velazquez, T.; Cardador-Martinez, A.; Moguel-Concha, D.; Osorio-Revilla, G.; Ruiz-Ruiz, J.C.; Martinez, C.J. Phenolic Compounds Profile and Antioxidant Activity of Pea (Pisum sativum L.) and Black Bean (Phaseolus L.) sprouts. Food Sci. Technol. 2022, 42, e45920. [Google Scholar] [CrossRef]
- Castaldo, L.; Izzo, L.; Gaspari, A.; Lombardi, S.; Rodriguez-Carrasco, Y.; Narvaez, A.; Grosso, M.; Ritieni, A. Chemical Composition of Green Pea (Pisum sativum L.) Pods Extracts and their Potential Exploitation as Ingredients in Nutraceutical Formulations. Antioxidants 2022, 11, 105. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.; Obaid, W.A.; Elgamal, A.M.; Daoud, R.; Sobeh, M.; El Raey, M.A. Pea (Pisum sativum) Peel Extract Attenuates Dox-Induced Oxidative Myocardial Injury. Biomed. Pharm. 2021, 143, 112120. [Google Scholar] [CrossRef]
- Guo, F.H.; Tsao, R.; Wang, X.Y.; Jiang, L.; Sun, Y.; Xiong, H. Phenolics of Yellow Pea (Pisum sativum L.) Hulls, their Plasma and Urinary Metabolites, Organ Distribution, and In Vivo Antioxidant Activities. J. Agric. Food Chem. 2021, 69, 5013–5025. [Google Scholar] [CrossRef]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave Drying Effects on Drying Kinetics, Bioactive Compounds and Antioxidant Activity of Green Peas (Pisum sativum L.). Food Biosci. 2018, 25, 32–38. [Google Scholar] [CrossRef]
- Elessawy, F.M.; Bazghaleh, N.; Vandenberg, A.; Purves, R.W. Polyphenol Profile Comparisons of Seed Coats of Five Pulse Crops Using a Semi-Quantitative Liquid Chromatography-Mass Spectrometric Method. Phytochem. Anal. 2020, 31, 458–471. [Google Scholar] [CrossRef]
- Jha, A.B.; Purves, R.W.; Elessawy, F.M.; Zhang, H.X.; Vandenberg, A.; Warkentin, T.D. Polyphenolic Profile of Seed Components of White and Purple Flower Pea Lines. Crop. Sci. 2019, 59, 2711–2719. [Google Scholar] [CrossRef] [Green Version]
- Guo, F.H.; Tsao, R.; Li, C.Y.; Wang, X.Y.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Green Pea (Pisum sativum L.) Hull Polyphenol Extracts Ameliorate Dss-Induced Colitis through Keap1/Nrf2 Pathway and Gut Microbiota Modulation. Foods 2021, 10, 2765. [Google Scholar] [CrossRef]
- Mejri, F.; Ben Khoud, H.; Njim, L.; Baati, T.; Selmi, S.; Martins, A.; Serralheiro, M.L.M.; Rauter, A.P.; Hosni, K. In Vitro and In Vivo Biological Properties of Pea Pods (Pisum sativum L.). Food Biosci. 2019, 32, 100482. [Google Scholar] [CrossRef]
- Carpentier, J.; Conforto, E.; Chaigneau, C.; Vendeville, J.E.; Maugard, T. Microencapsulation and Controlled Release of Alpha-Tocopherol by Complex Coacervation between Pea Protein and Tragacanth Gum: A Comparative Study with Arabic and Tara Gums. Innov. Food Sci. Emerg. Technol. 2022, 77, 102951. [Google Scholar] [CrossRef]
- Nazir, N.; Nisar, M.; Ahmad, S.; Wadood, S.F.; Jan, T.; Zahoor, M.; Ahmad, M.; Ullah, A. Characterization of Phenolic Compounds in Two Novel Lines of Pisum sativum L. Along with their in vitro Antioxidant Potential. Environ. Sci. Pollut. Res. 2020, 27, 7639–7646. [Google Scholar] [CrossRef]
- Troszynska, A.; Ciska, E. Phenolic Compounds of Seed Coats of White and Coloured Varieties of Pea (Pisum sativum L.) and their Total Antioxidant Activity. Czech J. Food Sci. 2002, 20, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Costantini, M.; Summo, C.; Centrone, M.; Rybicka, I.; D’agostino, M.; Annicchiarico, P.; Caponio, F.; Pavan, S.; Tamma, G.; Pasqualone, A. Macro- and Micro-Nutrient Composition and Antioxidant Activity of Chickpea and Pea Accessions. Pol. J. Food Nutr. Sci. 2021, 71, 177–185. [Google Scholar] [CrossRef]
- Sharma, A. A Review on Traditional Technology and Safety Challenges with Regard to Antinutrients in Legume Foods. J. Food Sci. Technol. 2021, 58, 2863–2883. [Google Scholar] [CrossRef] [PubMed]
- Sinkovic, L.; Pipan, B.; Sibul, F.; Nemes, I.; Horecki, A.T.; Meglic, V. Nutrients, Phytic Acid and Bioactive Compounds in Marketable Pulses. Plants 2023, 12, 170. [Google Scholar] [CrossRef]
- Hugman, J.; Wang, L.F.; Beltranena, E.; Htoo, J.K.; Zijlstra, R.T. Nutrient Digestibilityof Heat-Processed Field Pea in Weaned Pigs. Anim. Feed. Sci. Technol. 2021, 274, 114891. [Google Scholar] [CrossRef]
- Moore, K.L.; Rodríguez-Ramiro, I.; Jones, E.R.; Jones, E.J.; Rodríguez-Celma, J.; Halsey, K.; Domoney, C.; Shewry, P.R.; Fairweather-Tait, S.; Balk, J. The Stage of Seed Development Influences Iron Bioavailability in Pea (Pisum sativum L.). Sci. Rep. 2018, 8, 6865. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Chang, S.K.C. Comparative Study on Antiproliferation Properties and Cellular Antioxidant Activities of Commonly Consumed Food Legumes against Nine Human Cancer Cell Lines. Food Chem. 2012, 134, 1287–1296. [Google Scholar] [CrossRef]
- Kamalasundari, S.; Babu, R.; Umamaheswari, T. Effect of Domestic Processing Methods on Anti-Nutritional Factors and its Impact on the Bio-Availability Proteins and Starch in Commonly Consumed Whole Legumes. Asian J. Dairy. Food Res. 2019, 38, 67–72. [Google Scholar] [CrossRef]
- Moussou, N.; Ouazib, M.; Wanasundara, J.; Zaidi, F.; Rubio, L.A. Nutrients and Non-Nutrients Composition and in vitro Starch Digestibility of Five Algerian Legume Seed Flours. Int. Food Res. J. 2019, 26, 1339–1349. [Google Scholar]
- Sharma, K.; Kumar, V.; Kaur, J.; Tanwar, B.; Goyal, A.; Sharma, R.; Gat, Y.; Kumar, A. Health Effects, Sources, Utilization and Safety of Tannins: A Critical Review. Toxin Rev. 2021, 40, 432–444. [Google Scholar] [CrossRef]
- Fraga-Corral, M.; Otero, P.; Echave, J.; Garcia-Oliveira, P.; Carpena, M.; Jarboui, A.; Nuñez-Estevez, B.; Simal-Gandara, J.; Prieto, M.A. By-Products of Agri-Food Industry as Tannin-Rich Sources: A Review Of Tannins’ Biological Activities and their Potential for Valorization. Foods 2021, 10, 137. [Google Scholar] [CrossRef]
- Ge, G.; Guo, W.X.; Zheng, J.B.; Zhao, M.M.; Sun, W.Z. Effect of Interaction between Tea Polyphenols with Soymilk Protein on Inactivation of Soybean Trypsin Inhibitor. Food Hydrocolloid 2021, 111, 106177. [Google Scholar] [CrossRef]
- Zhou, J.J.; Li, M.H.; Bai, Q.; de Souza, T.S.P.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Effects of Different Processing Methods on Pulses Phytochemicals: An Overview. Food Rev. Int. 2023. [Google Scholar] [CrossRef]
- Espinosa, D.C.P.; Cortina, J.R.; Carrión, M.H.; Mora, O.O. Drying and Cooking Effects on the Final Quality of Pea Grains (Pisum sativum L.) Varieties. Food Sci. Technol. 2021, 42. [Google Scholar] [CrossRef]
- Yu, F.; Yang, Z.; Tao, Z.C.; Yang, Z.Y. Optimization of Pea Seed Intermittent Drying Assisted with Ultrasound Technology. Int. J. Food Eng. 2020, 16, 10. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Tao, Z.C.; Luo, N.; Yu, F. Ultrasound-Assisted Heat Pump Drying of Pea Seed. Dry. Technol. 2018, 36, 1958–1969. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. Stone Milling Versus Roller Milling: A Systematic Review of the Effects on Wheat Flour Quality, Dough Rheology, and Bread Characteristics. Trends Food Sci. Technol. 2020, 97, 147–155. [Google Scholar] [CrossRef]
- Gu, Z.X.; Jiang, H.Y.; Zha, F.C.; Manthey, F.; Rao, J.J.; Chen, B.C. Toward a Comprehensive Understanding of Ultracentrifugal Milling on the Physicochemical Properties and Aromatic Profile of Yellow Pea Flour. Food Chem. 2021, 345, 128760. [Google Scholar] [CrossRef]
- Schmidt, F.; Blankart, M.; Wanger, J.; Scharfe, M.; Scheuerer, T.; Hinrichs, J. Upscaling of Alkaline Pea Protein Extraction from Dry Milled and Pre-Treated Peas from Laboratory to Pilot Scale: Optimization of Process Parameters for Higher Protein Yields. J. Food Meas. Charact. 2022, 16, 4904–4913. [Google Scholar] [CrossRef]
- Kaiser, A.C.; Barber, N.; Manthey, F.; Hall, C. Physicochemical Properties of Hammer-Milled Yellow Split Pea (Pisum sativum L.). Cereal Chem. 2019, 96, 313–323. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Gidley, M.J.; Sopade, P.A. Dependence of In-Vitro Starch and Protein Digestions on Particle Size of Field Peas (Pisum sativum L.). LWT-Food Sci. Technol. 2015, 63, 541–549. [Google Scholar] [CrossRef]
- Li, H.; Zou, L.; Li, X.Y.; Wu, D.T.; Liu, H.Y.; Li, H.B.; Gan, R.Y. Adzuki Bean (Vigna angularis): Chemical Compositions, Physicochemical Properties, Health Benefits, and Food Applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2335–2362. [Google Scholar] [CrossRef] [PubMed]
- Setia, R.; Dai, Z.X.; Nickerson, M.T.; Sopiwnyk, E.; Malcolmson, L.; Ai, Y.F. Impacts of Short-Term Germination on the Chemical Compositions, Technological Characteristics and Nutritional Quality of Yellow Pea and Faba Bean Flours. Food Res. Int. 2019, 122, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Mu, K.W.; Arntfield, S.D.; Nickerson, M.T. Changes in Levels of Enzyme Inhibitors during Soaking and Cooking for Pulses Available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Hatcher, D.W.; Gawalko, E.J. Effect of Variety and Processing on Nutrients and Certain Anti-Nutrients in Field Peas (Pisum sativum). Food Chem. 2008, 111, 132–138. [Google Scholar] [CrossRef]
- Skalickova, S.; Ridoskova, A.; Slama, P.; Skladanka, J.; Skarpa, P.; Smykalova, I.; Horacek, J.; Dostalova, R.; Horky, P. Effect of Lactic Fermentation and Cooking on Nutrient and Mineral Digestibility of Peas. Front. Nutr. 2022, 9, 838963. [Google Scholar] [CrossRef]
- Stone, A.K.; Waelchli, K.N.; Cabuk, B.; Mcintosh, T.C.; Wanasundara, J.; Arntfield, S.D.; Nickerson, M.T. The Levels of Bioactive Compounds Found in Raw and Cooked Canadian Pulses. Food Sci. Technol. Int. 2021, 27, 528–538. [Google Scholar] [CrossRef]
- Liu, Y.H.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Composition of Phenolic Acids and Antioxidant Properties of Selected Pulses Cooked with Different Heating Conditions. Foods 2020, 9, 908. [Google Scholar] [CrossRef]
- Shin, J.A.; Heo, Y.; Seo, M.; Choi, Y.; Lee, K.T. Effects of Cooking Methods on the Beta-Carotene Levels of Selected Plant Food Materials. Food Sci. Biotechnol. 2016, 25, 955–963. [Google Scholar] [CrossRef]
- Liu, Y.H.; Ragaee, S.; Marcone, M.F.; Abdel-Aal, E.M. Effect of Different Cooking Methods and Heating Solutions on Nutritionally-Important Starch Fractions and Flatus Oligosaccharides in Selected Pulses. Cereal Chem. 2020, 97, 1216–1226. [Google Scholar] [CrossRef]
- Obadi, M.; Xu, B. Review on the Physicochemical Properties, Modifications, and Applications of Starches and its Common Modified Forms Used in Noodle Products. Food Hydrocolloid 2021, 112, 106286. [Google Scholar] [CrossRef]
- He, X.; Dai, T.; Liang, R.; Liu, W.; Cheng, Y.; Liu, C.; Chen, J. A New Partially-Gelatinized Granular Starch Prepared by Industry-Scale Microfluidization Treatment of Pea Starch. Innov. Food Sci. Emerg. Technol. 2023, 86, 103351. [Google Scholar] [CrossRef]
- Shi, M.M.; Gao, Q.Y.; Liu, Y.Q. Changes in the Structure and Digestibility of Wrinkled Pea Starch with Malic Acid Treatment. Polymers 2018, 10, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.H.; Cao, S.P.; Yu, Y.T.; Xu, X.C.; Cao, X.H.; Chen, W.J. Modification in Physicochemical, Structural and Digestive Properties of Pea Starch during Heat-Moisture Process Assisted by Pre- and Post-Treatment of Ultrasound. Food Chem. 2021, 360, 129929. [Google Scholar] [CrossRef] [PubMed]
- Majeed, T.; Majeed, T.; Wani, I.A.; Wani, I.A.; Hamdani, A.M.; Hamdani, A.M.; Bhat, N.A.; Bhat, N.A.; Majeed, T.; Majeed, T.; et al. Effect of Sonication and Gamma-Irradiation on the Properties of Pea (Pisum sativum) and Vetch (Vida villosa) Starches: A Comparative Study. Int. J. Biol. Macromol. 2018, 114, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.T.; Ma, Z.; Yin, X.X.; Hu, X.Z.; Boye, J.I. Structural Characteristics and Physicochemical Properties of Field Pea Starch Modified by Physical, Enzymatic, and Acid Treatments. Food Hydrocolloid 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, Z.W.; Ji, N.; Li, M.; Wang, Y.F.; Xiong, L.; Sun, Q.J. The Effect of Ethanol Solution Annealing on the Physicochemical Properties of Pea and Potato Starches. Food Hydrocolloid 2022, 125, 107428. [Google Scholar] [CrossRef]
- Yan, Y.Z.; Peng, B.X.; Niu, B.; Ji, X.L.; He, Y.; Shi, M.M. Understanding the Structure, Thermal, Pasting, and Rheological Properties of Potato and Pea Starches Affected by Annealing Using Plasma-Activated Water. Front. Nutr. 2022, 9, 842662. [Google Scholar] [CrossRef]
- Arteaga, V.G.; Guardia, M.A.; Muranyi, I.; Eisner, P.; Schweiggert-Weisz, U. Effect of Enzymatic Hydrolysis on Molecular Weight Distribution, Techno- Functional Properties and Sensory Perception of Pea Protein Isolates. Innov. Food Sci. Emerg. Technol. 2020, 65, 102449. [Google Scholar] [CrossRef]
- Vatansever, S.; Ohm, J.B.; Simsek, S.; Hall, C. A Novel Approach: Supercritical Carbon Dioxide Plus Ethanol Extraction to Improve Techno-Functionalities of Pea Protein Isolate. Cereal Chem. 2022, 99, 130–143. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Y.Z.; Xu, L.; Ma, H. An Efficient Ultrasound-Assisted Extraction Method of Pea Protein and its Effect on Protein Functional Properties and Biological Activities. LWT-Food Sci. Technol. 2020, 127, 109348. [Google Scholar] [CrossRef]
- Ozturk, O.K.; Turasan, H. Latest Developments in the Applications of Microfluidization to Modify the Structure of Macromolecules Leading to Improved Physicochemical and Functional Properties. Crit. Rev. Food Sci. Nutr. 2022, 62, 4481–4503. [Google Scholar] [CrossRef]
- He, X.H.; Chen, J.; He, X.M.; Feng, Z.; Li, C.H.; Liu, W.; Dai, T.T.; Liu, C.M. Industry-Scale Microfluidization as a Potential Technique to Improve Solubility and Modify Structure of Pea Protein. Innov. Food Sci. Emerg. Technol. 2021, 67, 102582. [Google Scholar] [CrossRef]
- Oliete, B.; Potin, F.; Cases, E.; Saurel, R. Microfluidization as Homogenization Technique in Pea Globulin-Based Emulsions. Food Bioprocess. Technol. 2019, 12, 877–882. [Google Scholar] [CrossRef]
- Stanisavljevic, N.S.; Ilic, M.; Jovanovic, Z.S.; Cupic, T.; Dabic, D.C.; Natic, M.M.; Tesic, Z.L.; Radovic, S.S. Identification of Seed Coat Phenolic Compounds from Differently Colored Pea Varieties and Characterization of their Antioxidant Activity. Arch. Biol. Sci. 2015, 67, 829–840. [Google Scholar] [CrossRef]
- Stanisavljevic, N.S.; Ilic, M.D.; Matic, I.Z.; Jovanovic, Z.S.; Cupic, T.; Dabic, D.C.; Natic, M.M.; Tesic, Z.L. Identification of Phenolic Compounds from Seed Coats of Differently Colored European Varieties of Pea (Pisum sativum L.) and Characterization of their Antioxidant and In Vitro Anticancer Activities. Nutr. Cancer 2016, 68, 988–1000. [Google Scholar] [CrossRef]
- Hadrich, F.; El Arbi, M.; Boukhris, M.; Sayadi, S.; Cherif, S. Valorization of the Peel of Pea: Pisum sativum by Evaluation of its Antioxidant and Antimicrobial Activities. J. Oleo Sci. 2014, 63, 1177–1183. [Google Scholar] [CrossRef] [Green Version]
- Jalili Safaryan, M.; Ganjloo, A.; Bimakr, M.; Zarringhalami, S. Optimization of Ultrasound-Assisted Extraction, Preliminary Characterization and In Vitro Antioxidant Activity of Polysaccharides from Green Pea Pods. Foods 2016, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Ding, J.; Liang, R.; Yang, Y.Y.; Sun, N.; Lin, S.Y. Optimization of Pea Protein Hydrolysate Preparation and Purification of Antioxidant Peptides Based on an In Silico Analytical Approach. LWT-Food Sci. Technol. 2020, 123, 109126. [Google Scholar] [CrossRef]
- Guo, F.H.; Xiong, H.; Wang, X.Y.; Jiang, L.; Yu, N.X.; Hu, Z.Y.; Sun, Y.; Tsao, R. Phenolics of Green Pea (Pisum sativum L.) Hulls, their Plasma and Urinary Metabolites, Bioavailability, and In Vivo Antioxidant Activities in a Rat Model. J. Agric. Food Chem. 2019, 67, 11955–11968. [Google Scholar] [CrossRef]
- Guo, F.H.; Tsao, R.; Li, C.Y.; Wang, X.Y.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol Content of Green Pea (Pisum sativum L.) Hull Under In Vitro Digestion and Effects of Digestive Products on Anti-Inflammatory Activity and Intestinal Barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef]
- Ndiaye, F.; Vuong, T.; Duarte, J.; Aluko, R.E.; Matar, C. Anti-Oxidant, Anti-Inflammatory and Immunomodulating Properties of an Enzymatic Protein Hydrolysate from Yellow Field Pea Seeds. Eur. J. Nutr. 2012, 51, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bibi, S.; Moraes LF, D.; Lebow, N.; Zhu, M.J. Dietary Green Pea Protects against Dss-Induced Colitis in Mice Challenged with High-Fat Diet. Nutrients 2017, 9, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) Seed Albumin Extracts Show Anti-Inflammatory Effect in the Dss Model of Mouse Colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Baraniak, B. Angiotensin I Converting Enzyme Inhibitory Peptides Obtained after in vitro Hydrolysis of Pea (Pisum sativum Var. Bajka) Globulins. Biomed. Res. Int. 2014, 2014, 438459. [Google Scholar] [CrossRef] [Green Version]
- Liao, W.; Fan, H.B.; Liu, P.; Wu, J.P. Identification of Angiotensin Converting Enzyme 2 (Ace2) Up-Regulating Peptides from Pea Protein Hydrolysate. J. Funct. Food 2019, 60, 103395. [Google Scholar] [CrossRef]
- Wang, X.; Bhullar, K.S.; Fan, H.B.; Liao, W.; Qiao, Y.J.; Su, D.; Wu, J.P. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (Lrw) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells Against Angiotensin Ii Stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef]
- Girgih, A.T.; Nwachukwu, I.D.; Onuh, J.O.; Malomo, S.A.; Aluko, R.E. Antihypertensive Properties of a Pea Protein Hydrolysate during Short-and Long-Term Oral Administration to Spontaneously Hypertensive Rats. J. Food Sci. 2016, 81, H1281–H1287. [Google Scholar] [CrossRef]
- Inagaki, K.; Nishimura, Y.; Iwata, E.; Manabe, S.; Goto, M.; Ogura, Y.; Hotta, H. Hypolipidemic Effect of the Autoclaved Extract Prepared from Pea (Pisum sativum L.) Pods In Vivo and In Vitro. J. Nutr. Sci. Vitam. 2016, 62, 322–329. [Google Scholar] [CrossRef] [Green Version]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic Effect of Dietary Pea Proteins: Impact on Genes Regulating Hepatic Lipid Metabolism. Mol. Nutr. Food Res. 2010, 54, S24–S30. [Google Scholar] [CrossRef]
- Ruiz, R.; Olias, R.; Clemente, A.; Rubio, L.A. A Pea (Pisum sativum L.) Seed Vicilins Hydrolysate Exhibits Ppar Gamma Ligand Activity and Modulates Adipocyte Differentiation in a 3t3-L1 Cell Culture Model. Foods 2020, 9, 793. [Google Scholar] [CrossRef]
- Awosika, T.O.; Aluko, R.E. Inhibition of the in vitro Activities of Alpha-Amylase, Alpha-Glucosidase and Pancreatic Lipase by Yellow Field Pea (Pisum sativum L.) Protein Hydrolysates. Int. J. Food Sci. Technol. 2019, 54, 2021–2034. [Google Scholar] [CrossRef] [Green Version]
- Qin, G.; Xu, W.; Liu, J.; Zhao, L.; Chen, G. Purification, Characterization and Hypoglycemic Activity of Glycoproteins Obtained from Pea (Pisum sativum L.). Food Sci. Hum. Wellness 2021, 10, 297–307. [Google Scholar] [CrossRef]
- Liu, J.P.; Qian, Y.F.; Qin GY, X.; Zhao, L.Y.; Chen, G.T. Antidiabetic Activities of Glycoprotein from Pea (Pisum sativum L.) in Stz-Induced Diabetic Mice. Food Funct. 2021, 12, 5087–5095. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, R.X.; Fang, L.; Qin, X.Y.; Cai, M.Y.; Gu, R.Z.; Lu, J.; Wang, Y.Q. Hypoglycemic Effects and Biochemical Mechanisms of Pea Oligopeptide on High-Fat Diet and Streptozotocin-Induced Diabetic Mice. J. Food Biochem. 2019, 43, e13055. [Google Scholar] [CrossRef]
- Thondre, P.S.; Achebe, I.; Sampson, A.; Maher, T.; Guerin-Deremaux, L.; Lefranc-Millot, C.; Ahlstrom, E.; Lightowler, H. Co-Ingestion of Nutralys® Pea Protein and a High-Carbohydrate Beverage Influences the Glycaemic, Insulinaemic, Glucose-Dependent Insulinotropic Polypeptide (Gip) and Glucagon-Like Peptide-1 (Glp-1) Responses: Preliminary Results of a Randomised Controlled Trial. Eur. J. Nutr. 2021, 60, 3085–3093. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; El-Hack, M.E.A.; Swelum, A.A.; Al-Sultan, S.I.; El-Ghareeb, W.R.; Hussein, E.O.S.; Ba-Awadh, H.A.; Akl, B.A.; Nader, M.M. Enhancing Quality and Safety of Raw Buffalo Meat Using the Bioactive Peptides of Pea and Red Kidney Bean under Refrigeration Conditions. Ital. J. Anim. Sci. 2021, 20, 762–776. [Google Scholar] [CrossRef]
- El-Araby, M.M.; El-Shatoury, E.H.; Soliman, M.M.; Shaaban, H.F. Characterization and Antimicrobial Activity of Lectins Purified from Three Egyptian Leguminous Seeds. Amb. Express 2020, 10, 90. [Google Scholar] [CrossRef]
- Belghith-Fendri, L.; Chaari, F.; Ben Jeddou, K.; Kallel, F.; Bouaziz, F.; Helbert, C.B.; Abdelkefi-Mesrati, L.; Ellouz-Chaabouni, S.; Ghribi-Aydi, D. Identification of Polysaccharides Extracted from Pea Pod By-Products and Evaluation of their Biological and Functional Properties. Int. J. Biol. Macromol. 2018, 116, 947–954. [Google Scholar] [CrossRef]
- Hidayat, M.; Prahastuti, S.; Afifah, E.; Widowati, W.; Yusuf, M.; Hasan, K. The Role of Green Peas Protein Hydrolysate in Tgf/Smad Signaling to Prevent Renal Fibrosis. J. King Saud. Univ. Sci. 2022, 34, 101920. [Google Scholar] [CrossRef]
- Hidayat, M.; Prahastuti, S.; Yusuf, M.; Hasan, K. Nutrition Profile and Potency of Rgd Motif in Protein Hydrolysate of Green Peas as an Antifibrosis in Chronic Kidney Disease. Iran. J. Basic. Med. Sci. 2021, 24, 734–743. [Google Scholar] [CrossRef]
- Kabir, S.R.; Nabi, M.M.; Haque, A.; Zaman, R.U.; Mahmud, Z.H.; Abu Reza, M. Pea Lectin Inhibits Growth of Ehrlich Ascites Carcinoma Cells by Inducing Apoptosis and G2/M Cell Cycle Arrest In Vivo in Mice. Phytomedicine 2013, 20, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Arora, H.; Shang, N.; Bhullar, K.S.; Wu, J.P. Pea Protein-Derived Tripeptide Lrw Shows Osteoblastic Activity on Mc3t3-E1 Cells via the Activation of the Akt/Runx2 Pathway. Food Funct. 2020, 11, 7197–7207. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Huang, Y.Y.; Tang, Z.H.; Wei, D.D.; Mo, J.M. Anti-Fatigue Effects of Pea (Pisum sativum L.) Peptides Prepared by Compound Protease. J. Food Sci. Technol. 2021, 58, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Su, Y.Y.; Yang, Q.; Zhou, T.B. Stem Cells in the Treatment Of Renal Fibrosis: A Review of Preclinical and Clinical Studies of Renal Fibrosis Pathogenesis. Stem Cell Res. Ther. 2021, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Xu, X.; Luo, D.; Lao, F.; Pang, X.; Shen, Q.; Hu, X.; Wu, J. Characterization of Key Aroma Compounds in Raw and Roasted Peas (Pisum sativum L.) by Application of Instrumental and Sensory Techniques. J. Agric. Food Chem. 2020, 68, 2718–2727. [Google Scholar] [CrossRef]
- Trikusuma, M.; Paravisini, L.; Peterson, D.G. Identification of Aroma Compounds in Pea Protein Uht Beverages. Food Chem. 2020, 312, 126082. [Google Scholar] [CrossRef]
- Bi, S.; Lao, F.; Pan, X.; Shen, Q.; Liu, Y.; Wu, J.H. Flavor Formation and Regulation of Peas (Pisum sativum L.) Seed Milk via Enzyme Activity Inhibition and Off-Flavor Compounds Control Release. Food Chem. 2022, 380, 132203. [Google Scholar] [CrossRef]
- Ma, W.Y.; Zhang, C.M.; Kong, X.Z.; Li, X.F.; Chen, Y.M.; Hua, Y.F. Effect of Pea Milk Preparation on the Quality of Non-Dairy Yoghurts. Food Biosci. 2021, 44, 101416. [Google Scholar] [CrossRef]
- Manus, J.; Millette, M.; Dridi, C.; Salmieri, S.; Uscanga BR, A.; Lacroix, M. Protein Quality of a Probiotic Beverage Enriched with Pea and Rice Protein. J. Food Sci. 2021, 86, 3698–3706. [Google Scholar] [CrossRef]
- Yousseef, M.; Lafarge, C.; Valentin, D.; Lubbers, S.; Husson, F. Fermentation of Cow Milk and/or Pea Milk Mixtures by Different Starter Cultures: Physico-Chemical and Sensorial Properties. LWT-Food Sci. Technol. 2016, 69, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.Y.J.; Hua, X.Y.; Henry, C.J. A Novel Approach to Structure Plant-Based Yogurts Using High Pressure Processing. Foods 2020, 9, 10. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Tan, M.V.; Oiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2022, 38, 1064–1102. [Google Scholar] [CrossRef]
- Gan, R.Y.; Lui, W.Y.; Wu, K.; Chan, C.L.; Dai, S.H.; Sui, Z.Q.; Corke, H. Bioactive Compounds and Bioactivities of Germinated Edible Seeds and Sprouts: An Updated Review. Trends Food Sci. Technol. 2017, 59, 1–14. [Google Scholar] [CrossRef]
- Erba, D.; Angelino, D.; Marti, A.; Manini, F.; Faoro, F.; Morreale, F.; Pellegrini, N.; Casiraghi, M.C. Effect of Sprouting on Nutritional Quality of Pulses. Int. J. Food Sci. Nutr. 2019, 70, 30–40. [Google Scholar] [CrossRef]
- Elliott, H.; Woods, P.; Green, B.D.; Nugent, A.P. Can Sprouting Reduce Phytate and Improve the Nutritional Composition and Nutrient Bioaccessibility in Cereals and Legumes? Nutr. Bull. 2022, 47, 138–156. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. A Review on Recent Advances in Cold Plasma Technology for the Food Industry: Current Applications and Future Trends. Trends Food Sci. Technol. 2017, 69, 46–58. [Google Scholar] [CrossRef]
- Svubova, R.; Kyzek, S.; Medvecka, V.; Slovakova, L.; Galova, E.; Zahoranova, A. Novel Insight at the Effect of Cold Atmospheric Pressure Plasma on the Activity of Enzymes Essential for the Germination of Pea (Pisum sativum L. Cv. Prophet) Seeds. Plasma Chem. Plasma Process. 2020, 40, 1221–1240. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, Y.Y.; Zhao, Z.Y.; Liu, W.F.; Chen, Y.Q.; Yang, G.J.; Xia, X.D.; Cao, Y.F. The Application of Slightly Acidic Electrolyzed Water in Pea Sprout Production to Ensure Food Safety, Biological and Nutritional Quality of the Sprout. Food Control. 2019, 104, 83–90. [Google Scholar] [CrossRef]
- Jerse, A.; Kacjan-Marsic, N.; Sircelj, H.; Germ, M.; Kroflic, A.; Stibilj, V. Seed Soaking in I and Se Solutions Increases Concentrations of Both Elements and Changes Morphological and Some Physiological Parameters of Pea Sprouts. Plant. Physiol. Biochem. 2017, 118, 285–294. [Google Scholar] [CrossRef]
- Baczek-Kwinta, R.; Baran, A.; Simlat, M.; Lang, J.; Bieniek, M.; Florek, B. Enrichment of Different Plant Seeds with Zinc and Assessment of Health Risk of Zn-Fortified Sprouts Consumption. Agronomy 2020, 10, 937. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, L.; Duan, X.; Chai, X.; Huang, R.; Kang, Y.; Yang, X. Effects of Exogenous Sucrose and Selenium on Plant Growth, Quality, and Sugar Metabolism of Pea Sprouts. J. Sci. Food Agric. 2022, 102, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Hu, R.J.; Tilley, M.; Siliveru, K.; Li, Y.H. Effect of Pulse Type and Substitution Level on Dough Rheology and Bread Quality of Whole Wheat-Based Composite Flours. Processes 2021, 9, 1687. [Google Scholar] [CrossRef]
- Kotsiou, K.; Sacharidis, D.D.; Matsakidou, A.; Biliaderis, C.G.; Lazaridou, A. Impact of Roasted Yellow Split Pea Flour on Dough Rheology and Quality of Fortified Wheat Breads. Foods 2021, 10, 1832. [Google Scholar] [CrossRef] [PubMed]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Mccarthy, S.; Gallagher, E. Dough Properties and Baking Characteristics of White Bread, as Affected by Addition of Raw, Germinated and Toasted Pea Flour. Innov. Food Sci. Emerg. Technol. 2019, 56, 102189. [Google Scholar] [CrossRef]
- Millar, K.A.; Barry-Ryan, C.; Burke, R.; Hussey, K.; Mccarthy, S.; Gallagher, E. Effect of Pulse Flours on the Physiochemical Characteristics and Sensory Acceptance of Baked Crackers. Int. J. Food Sci. Technol. 2017, 52, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Krause, S.; Debon, S.; Palchen, K.; Jakobi, R.; Rega, B.; Bonazzi, C.; Grauwet, T. In Vitro Digestion of Protein and Starch in Sponge Cakes Formulated with Pea (Pisum sativum L.) Ingredients. Food Funct. 2022, 13, 3206–3219. [Google Scholar] [CrossRef]
- Hanan, E.; Rudra, S.G.; Sagar, V.R.; Sharma, V. Utilizationof Pea Pod Powder for Formulation of Instant Pea Soup Powder. J. Food Process. Preserv. 2020, 44, e14888. [Google Scholar] [CrossRef]
- Polizer-Rocha, Y.J.; Lorenzo, J.M.; Pompeu, D.; Rodrigues, I.; Baldin, J.C.; Pires, M.A.; Freire MT, A.; Barba, F.J.; Trindade, M.A. Physicochemical and Technological Properties of Beef Burger as Influenced by the Addition of Pea Fibre. Int. J. Food Sci. Technol. 2020, 55, 1018–1024. [Google Scholar] [CrossRef]
- Pietrasik, Z.; Sigvaldson, M.; Soladoye, O.P.; Gaudette, N.J. Utilization of Pea Starch and Fibre Fractions for Replacement of Wheat Crumb in Beef Burgers. Meat Sci. 2020, 161, 107974. [Google Scholar] [CrossRef]
- Baugreet, S.; Kerry, J.P.; Botinestean, C.; Allen, P.; Hamill, R.M. Development of Novel Fortified Beef Patties with Added Functional Protein Ingredients for the Elderly. Meat Sci. 2016, 122, 40–47. [Google Scholar] [CrossRef]
- Shoaib, A.; Sahar, A.; Sameen, A.; Saleem, A.; Tahir, A.T. Use of Pea and Rice Protein Isolates as Source of Meat Extenders in the Development of Chicken Nuggets. J. Food Process. Preserv. 2018, 42, e13763. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Eisner, P.; Schweiggert-Weisz, U. Effect of High Moisture Extrusion Cooking on Protein-Protein Interactions of Pea (Pisum sativum L.) Protein Isolates. Int. J. Food Sci. Technol. 2015, 50, 1390–1396. [Google Scholar] [CrossRef]
- Golge, O.; Kilincceker, O.; Koluman, A. Effects of Different Fibers on The Quality of Chicken Meatballs. J. Food Saf. Food Qual. 2018, 69, 177–183. [Google Scholar] [CrossRef]
- Kehlet, U.; Pagter, M.; Aaslyng, M.D.; Raben, A. Meatballs with 3% and 6% Dietary Fibre from Rye Bran or Pea Fibre—Effects on Sensory Quality and Subjective Appetite Sensations. Meat Sci. 2017, 125, 66–75. [Google Scholar] [CrossRef]
- Hadidi, M.; Boostani, S.; Jafari, S.M. Pea Proteins as Emerging Biopolymers for the Emulsification and Encapsulation of Food Bioactives. Food Hydrocolloid 2022, 126, 107474. [Google Scholar] [CrossRef]
- Caballero, S.; Li, Y.O.; Mcclements, D.J.; Davidov-Pardo, G. Hesperetin (Citrus Peel Flavonoid Aglycone) Encapsulation Using Pea Protein-High Methoxyl Pectin Electrostatic Complexes: Complex Optimization and Biological Activity. J. Sci. Food Agric. 2022, 102, 5554–5560. [Google Scholar] [CrossRef]
- Li, J.T.; Zhang, X.G.; Zhao, R.; Lu, Y.C.; Wang, C.; Wang, C.N. Encapsulation of Quercetin in Pea Protein-High Methoxyl Pectin Nanocomplexes: Formation, Stability, Antioxidant Capacity and In Vitro Release Profile. Food Biosci. 2022, 48, 101811. [Google Scholar] [CrossRef]
- Okagu, O.D.; Udenigwe, C.C. Molecular Interactions of Pea Globulin, Albumin and Glutelin with Curcumin: Formation and Gastric Release Mechanisms of Curcumin-Loaded Bio-Nanocomplexes. Food Biophys. 2022, 17, 10–25. [Google Scholar] [CrossRef]
- Zhang, X.G.; Wang, C.; Qi, Z.T.; Zhao, R.; Wang, C.N.; Zhang, T.H. Pea Protein Based Nanocarriers for Lipophilic Polyphenols: Spectroscopic Analysis, Characterization, Chemical Stability, Antioxidant and Molecular Docking. Food Res. Int. 2022, 160, 111713. [Google Scholar] [CrossRef]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-Based Films and Coatings for Food Industry Applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef]
- Cheng, J.J.; Li, Z.Z.; Wang, J.; Zhu, Z.B.; Yi, J.H.; Chen, B.C.; Cui, L.Q. Structural Characteristics of Pea Protein Isolate (PPI) Modified by High-Pressure Homogenization and its Relation to the Packaging Properties of Ppi Edible Film. Food Chem. 2022, 388, 132974. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, V.M.; De Oliveira, A.C.S.; Borges, S.V.; Raguzzoni, J.C.; Dias, M.V.; Costa, A.L.R. Pea Protein Isolate Nanocomposite Films for Packaging Applications: Effect of Starch Nanocrystals on the Structural, Morphological, Thermal, Mechanical and Barrier Properties. Emir. J. Food Agric. 2020, 32, 495–504. [Google Scholar] [CrossRef]
- Li, H.; Shi, H.B.; He, Y.Q.; Fei, X.; Peng, L.C. Preparation and Characterization of Carboxymethyl Cellulose-Based Composite Films Reinforced by Cellulose Nanocrystals Derived from Pea Hull Waste for Food Packaging Applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Salim, M.H.; Abdellaoui, Y.; Benhamou, A.A.; Ablouh, E.H.; El Achaby, M.; Kassab, Z. Influence of Cellulose Nanocrystals from Pea Pod Waste on Mechanical, Thermal, Biodegradability, and Barrier Properties of Chitosan-Based Films. Cellulose 2022, 29, 5117–5135. [Google Scholar] [CrossRef]
- Zhou, X.; Cheng, R.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Kang, L.; Cheng, Z.; Gao, W.; Chen, K. Biodegradable Sandwich-Architectured Films Derived from Pea Starch and Polylactic Acid with Enhanced Shelf-Life for Fruit Preservation. Carbohydr. Polym. 2021, 251, 117117. [Google Scholar] [CrossRef]
- Zhou, X.M.; Yang, R.D.; Wang, B.; Chen, K.F. Development and Characterization of Bilayer Films Based on Pea Starch/Polylactic Acid and Use in the Cherry Tomatoes Packaging. Carbohydr. Polym. 2019, 222, 114912. [Google Scholar] [CrossRef]
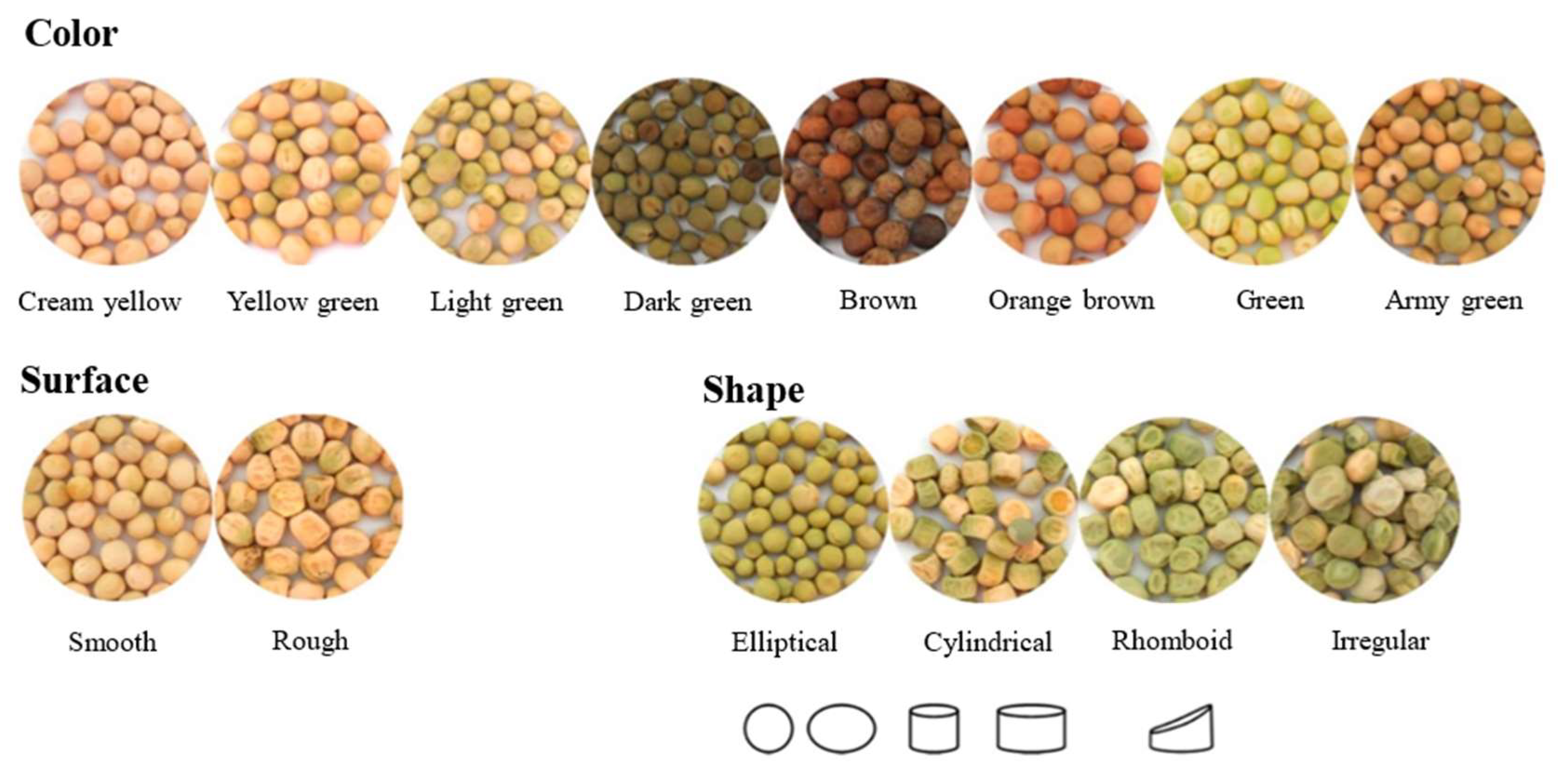

| Sample Types | Experimental Models | Major Results | References |
|---|---|---|---|
| Antioxidant effect | |||
| Seed flour extracted with 95% ethanol | In vitro (DPPH) |
| [74] |
| Seed flour extracted with 80% ethanol | In vitro (ABTS; DPPH; reducing power) In vitro (cell model, OA-induced HepG2 cells) |
| [65] |
| Seed flour extracted with 80% methanol | In vitro (ABTS; DPPH) |
| [82] |
| Seed flour extracted with mixed solution (acetone/water/acetic acid, 70:29.5:0.5, v/v/v) | In vitro (ABTS; FRAP) |
| [68] |
| Seed coat extracted with mixed solution (methanol/water/acetic acid mixture, 80:19:1, v/v/v) | In vitro (DPPH; FRC; FCC) |
| [125] |
| Seed coat extracted with mixed solution (acetone/water/acetic acid mixture, 80:19:1, v/v/v) | In vitro (DPPH; FRC; FCC) |
| [126] |
| Seed coat extracted with water, methanol, and ethyl acetate | In vitro (ABTS; DPPH; FRAP) |
| [127] |
| Red and yellow pea hull in vitro digestion products | In vitro (DPPH; ABTS; H2O2; FRAP) |
| [69] |
| Pea sprout extracted with 80% methanol | In vitro (DPPH; ORAC; CUPRAC) |
| [70] |
| Pea hull extracted with 95% ethanol | In vitro (DPPH; reducing power; FRAP) |
| [128] |
| Peptides derived from pea protein hydrolysate | In vitro (DPPH; OH) |
| [129] |
| Whole seed flour | In vivo (HFD-induced Sprague–Dawley (SD) male rats) |
| [65] |
| Seed coat extracted with water | In vivo (DOX-induced albino male rats) |
| [72] |
| Green pea hull extracted with 80% methanol | In vivo (D-galactose-induced SD female rats) |
| [130] |
| Yellow pea hull extracted with 80% methanol | In vivo (D-galactose-induced SD female rats) |
| [73] |
| Anti-inflammatory effect | |||
| Green pea hull in vitro digestion products | In vitro (LPS-induced Caco-2/Raw264.7 cells coculture) |
| [131] |
| Peptides derived from pea protein hydrolysate | In vitro (LPS/IFN-γ-induced RAW 264.7 cells) |
| [132] |
| Whole seed flour | In vivo (DSS-induced colitis in HFD-fed C57BL/6J female mice) |
| [133] |
| Green pea hull extracted with 80% ethanol | In vivo (DSS-induced colitis in C57BL/6 male mice) |
| [77] |
| Two pea seed albumin extracts (PSE/AF-PSE) | In vivo (DSS-induced colitis in C57BL/6J male mice) |
| [134] |
| Regulation of metabolic syndrome | |||
| Anti-hypertensive activity | |||
| Peptides derived from pea protein hydrolysate | In vitro (ACE inhibition assay) |
| [135] |
| Peptides derived from pea protein hydrolysate | In vitro (A7r5 cells) |
| [136] |
| Tripeptide (Leu-Arg-Trp) | In vitro (A7r5 cells) |
| [137] |
| Peptides derived from pea protein hydrolysate | In vitro (ACE and renin inhibition assays) In vivo (male SHRs) |
| [28] |
| Peptides derived from pea protein hydrolysate | In vitro (ACE and renin inhibition assays) In vivo (male SHRs) |
| [138] |
| Hypolipidemic activity | |||
| Pea pod autoclaved extract (AE) | In vitro (pancreatic lipase inhibition and cholesterol adsorption capacity assay) In vivo (high-sucrose-induced SD male rats) |
| [139] |
| Pea seed flour | In vivo (HFD-induced male SD rats) |
| [65] |
| Pea protein isolate | In vivo (HFD-induced male SD rats) |
| [140] |
| Anti-obesity activity | |||
| Pea protein hydrolysate | In vitro (3T3-L1 preadipocytes subline) |
| [141] |
| Pea flour and dietary fiber | In vivo (HFHSD-induced obese SD male rats) |
| [55] |
| Pea fiber | Clinical trial (12-week single center, double-blind placebo-controlled trial with 53 adults with overweight or obesity) |
| [51] |
| Anti-diabetic effect | |||
| Pea protein hydrolysate | In vitro (α-amylase and α-glucosidase inhibition assays) |
| [142] |
| Purified pea glycoproteins (PGP1, PGP2, and PGP3) | In vitro (α-amylase and α-glucosidase inhibition assays) |
| [143] |
| Purified pea glycoprotein (PGP2) | In vivo (STZ-induced diabetic ICR male mice) |
| [144] |
| Pea oligopeptide | In vivo (HFD and STZ-induced diabetic Kunming male mice) |
| [145] |
| Pea dietary fiber | In vivo (STZ-induced diabetic Balb/c male mice) |
| [50] |
| Pea protein | Clinical trial (a randomised controlled trial with a high-carbohydrate beverage intake in healthy individuals) |
| [146] |
| Antimicrobial effect | |||
| 11S pea globulin (11SGP) | In vitro Bacteria: Bacillus cereus, Listeria monocytogenes, Streptococcus pyogenes, Escherichia coli, Acinetobacter baumannii, and Pseudomonas aeruginosa; Fungi: Alternaria alternate, Aspergillus flavus, Fusarium oxysporum, and Monascus purpureus |
| [147] |
| Pea lectin | In vitro Bacteria: Staphylococcus aureus, Streptococcus mutants, Pseudomonas aeruginosa, and Klebsiella pneumonia Fungi: Candida albicans |
| [148] |
| Pea peel extracted with water, methanol, and ethyl acetate | In vitro Bacteria: Staphylococcus aureus, Salmonella enterica, Escherichia coli, and Pseudomonas aeruginosa Fungi: Aspergillus niger and Candida albicans |
| [127] |
| Pea pod polysaccharide | In vitro Bacteria: Bacillus thuringiensis, B. subtilis, Actinomycete sp., Enterococcus faecalis, Listeria monocytogenes, Micrococcus luteus, Klebsiella pneumonia, Pseudomonas aeruginosa, and Salmonella Typhimirium |
| [149] |
| Anti-renal fibrosis effect | |||
| Peptides derived from pea protein hydrolysate | In vitro (glucose-induced MES13 SV40 cells) |
| [150] |
| Peptides derived from pea protein hydrolysate | In vitro (glucose-induced MES13 SV40 cells) |
| [151] |
| Anti-cancer effect | |||
| Pea seed coat extracted with water | In vitro (cell lines, human colon denocarcinoma LS174, breast carcinoma MDA-MB-453, lung carcinoma A594, and myelogenous leukemia K562) |
| [126] |
| Pea lectin | In vitro (cell line, Ehrlich ascites carcinoma (EAC) cells) In vivo (Ehrlich ascites carcinoma cells in adult Swiss albino mice) |
| [152] |
| Immunomodulatory effect | |||
| Peptides derived from pea protein hydrolysate | In vivo (BALB/c female mice) |
| [132] |
| Anti-osteoporosis effect | |||
| Pea tripeptide (Leu-Arg-Trp) | In vitro (MC3T3-E1 cell) |
| [153] |
| Anti-fatigue effect | |||
| Peptides derived from pea protein hydrolysate | In vivo (Kunming mice) |
| [154] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.-T.; Li, W.-X.; Wan, J.-J.; Hu, Y.-C.; Gan, R.-Y.; Zou, L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods 2023, 12, 2527. https://doi.org/10.3390/foods12132527
Wu D-T, Li W-X, Wan J-J, Hu Y-C, Gan R-Y, Zou L. A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods. 2023; 12(13):2527. https://doi.org/10.3390/foods12132527
Chicago/Turabian StyleWu, Ding-Tao, Wen-Xing Li, Jia-Jia Wan, Yi-Chen Hu, Ren-You Gan, and Liang Zou. 2023. "A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications" Foods 12, no. 13: 2527. https://doi.org/10.3390/foods12132527
APA StyleWu, D.-T., Li, W.-X., Wan, J.-J., Hu, Y.-C., Gan, R.-Y., & Zou, L. (2023). A Comprehensive Review of Pea (Pisum sativum L.): Chemical Composition, Processing, Health Benefits, and Food Applications. Foods, 12(13), 2527. https://doi.org/10.3390/foods12132527








