Linking Colorimetric Variation with Non-Volatile and Volatile Components of Carob Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Carob Flour Colorimetry
2.3. Carob Flour Concentration in Total Phenolic Compounds and Tannins
2.4. Carob Flour Radical Scavenging Capacity and Antioxidant Potential
2.5. Sugars, Organic Acids, and Protein Concentrations in Carob Flour
2.6. HS-SPME Volatiles Isolation and GC-MS Analysis of Volatile Organic Compounds (VOCs) in Carob Flour
2.6.1. HS-SPME Isolation of VOCs
2.6.2. Analysis of VOCs by Gas Chromatography-Mass Spectrometry
2.7. Statistical Analysis
3. Results and Discussion
3.1. Carob Flour Colorimetry
3.2. Radical Scavenging Capacity and Antioxidant Potential of Carob Flour
3.3. Carob Flour Content in Total Phenolic Compounds and Tannins
3.4. Carob Flour Content in Sugars, Organic Acids, and Protein
3.5. Chromatometric Correlations with Carob Flour Compositional Variables
3.6. Volatile Organic Compounds (VOCs)
| Volatile Compound Name | Molecular Formula | PubChem CID | Odor Description † | Mean ± Std Error (GR) | Mean ± Std Error (NGR) | t-Test | Correlation L (Overall) |
|---|---|---|---|---|---|---|---|
| ACIDS | |||||||
| Propanoic acid, 2-methyl-(isobutyric acid) | C4H8O2 | 6590 | acidic, sour, cheesy, dairy buttery, rancid | 45.98 ± 1.05 *** | 47.33 ± 3.29 *** | ns | −0.021 |
| Hexanoic acid (caproic acid) | C6H12O2 | 8892 | sour, fatty, sweaty, cheesy | 7.40 ± 0.48 *** | 8.23 ± 0.94 *** | ns | −0.529 * |
| Butanoic acid (butyric acid) | C4H8O2 | 264 | sharp, acetic, cheesy, buttery, fruity (product of anaerobic fermentation) | 7.37 ± 0.29 *** | 5.99 ± 0.73 *** | ns | −0.256 |
| Acetic acid | C2H4O2 | 176 | sour | 5.09 ± 0.39 *** | 4.47 ± 0.64 *** | ns | −0.321 |
| Butanoic acid, 3-methyl-(isovaleric acid) | C5H10O2 | 10430 | fruity, sweet, apple, pineapple, tutti, frutti | 2.23 ± 0.16 *** | 2.62 ± 0.19 *** | ns | −0.723 ** |
| Butanoic acid, 2-methyl-(2-methylbutyric acid) | C5H10O2 | 8314 | pungent, acidic, cheesy, roquefort cheese, | 0.18 ± 0.03 ** | 0.07 ± 0.03 *** | * | −0.184 |
| Propanoic acid (propionic acid) | C3H6O2 | 1032 | pungent and unpleasant smell somewhat resembling body odor | 0.13 ± 0.001 ** | 0.11 ± 0.04 *** | ns | −0.063 |
| Pentanoic acid (valeric acid) | C5H10O2 | 7991 | pcidic, sharp, cheesy, sour, milky, tobacco, fruity | 0.04 ± 0.01 *** | 0.03 ± 0.02 *** | ns | −0.081 |
| Total Acids | 68.42 ± 1.61 *** | 68.86 ± 2.77 *** | ns | −0.588 * | |||
| ESTERS | |||||||
| Hexanoic acid, methyl ester | C7H14O2 | 7824 | sweet, fruity, pineapple, waxy, green, banana | 8.09 ± 0.54 *** | 7.92 ± 1.25 *** | ns | 0.300 |
| Propanoic acid, 2-methyl-, methyl ester (methyl isobutyrate) | C5H10O2 | 7749 | fruity, green apple, pear, tart, grape berry, ripe berry, winey, peach | 6.50 ± 0.61 *** | 5.97 ± 1.10 *** | ns | 0.747 ** |
| Butanoic acid, methyl ester | C5H10O2 | 12180 | fruity, apple, sweet, banana, pineapple | 6.22 ± 0.38 *** | 4.64 ± 0.88 *** | ns | 0.643 ** |
| Acetic acid, methyl ester | C3H6O2 | 6584 | ethereal, sweet, fruity | 2.93 ± 0.12 ** | 2.48 ± 0.21 ** | ns | 0.679 ** |
| Octanoic acid, methyl ester | C9H18O2 | 8091 | waxy, green, sweet, orange, aldehydic, vegetable, herbal | 0.66 ± 0.05 *** | 0.92 ± 0.13 *** | * | −0.686 ** |
| Pentanoic acid, methyl ester (methyl valerate) | C6H12O2 | 12206 | sweet, green, fruity, apple, pineapple, nutty | 0.38 ± 0.01 *** | 0.28 ± 0.07 *** | ns | 0.659 ** |
| Propanoic acid, 2-methyl-, 3-methylbutyl ester (isoamyl isobutyrate) | C9H18O2 | 519786 | ethereal, fruity, fruit tropical, fruit pineapple, grape, skin banana | 0.33 ± 0.08 *** | 0.23 ± 0.12 *** | ns | 0.028 |
| Propanoic acid, 2-methyl-, ethyl ester (isobutyric ethyl ester) | C6H12O2 | 7342 | sweet, fruity, ethereal, rummy | 0.29 ± 0.05 *** | 0.22 ± 0.06 *** | ns | 0.633 ** |
| Butanoic acid, 2-methyl-, methyl ester | C6H12O2 | 13357 | ethereal, estery, fruity, tutti, frutti apple, green apple, lily of the valley, powdery, fatty | 0.28 ± 0.03 *** | 0.29 ± 0.06 ** | ns | 0.561 * |
| Propanoic acid, 2-methyl-, 2-methylpropyl ester | C8H16O2 | 7351 | waxy, green, sweet, orange, and aldehydic with vegetative and herbal nuances | 0.20 ± 0.11 *** | 0.37 ± 0.15 *** | ns | −0.253 |
| Propanoic acid, methyl ester (methyl propionate ) | C4H8O2 | 11124 | fresh, rummy, fruity, strawberry, apple | 0.17 ± 0.01 *** | 0.12 ± 0.01 *** | ns | 0.605 * |
| Butanoic acid, ethyl ester | C6H12O2 | 7762 | fruity, green apricot, pear, banana | 0.15 ± 0.02 *** | 0.08 ± 0.02 *** | ns | 0.437 |
| Hexanoic acid, ethyl ester | C8H16O2 | 31265 | sweet, fruity, pineapple, green peach, tropical | 0.15 ± 0.02 *** | 0.14 ± 0.04 *** | ns | 0.058 |
| Butanoic acid, 3-methylbutyl ester | C9H18O2 | 7795 | fruity, green apricot, pear, banana | 0.13 ± 0.07 *** | 0.61 ± 0.25 *** | ns | −0.383 |
| Propanoic acid, 2-methyl-, 2-methylbutyl ester | C9H18O2 | 97883 | fruity, ethereal, tropical, banana | 0.12 ± 0.03 ** | 0.24 ± 0.13 *** | ns | −0.647 ** |
| Ethyl acetate | C4H8O2 | 8857 | ethereal, fruity, sweet, weedy, green/ester | 0.09 ± 0.02 *** | 0.05 ± 0.02 *** | ns | 0.362 |
| Propanoic acid, 2-methyl-, hexyl ester (isobutyric hexyl ester) | C10H20O2 | 16872 | sweet, ethereal, fruity, alcoholic fusel rummy | 0.07 ± 0.02 *** | 0.15 ± 0.06 *** | ns | −0.386 |
| Hexanoic acid, 2-methylpropyl ester | C10H20O2 | 7775 | fruity, apple, sweet, banana, pineapple | 0.04 ± 0.03 *** | 0.22 ± 0.15 *** | ns | −0.821 ** |
| Butanoic acid, 2-methylpropyl ester | C8H16O2 | 10885 | sweet, fruity, pineapple, tutti, frutti, rum, cherry, apple, overripe fruit, bubble gum | 0.02 ± 0.02 *** | 0.05 ± 0.04 *** | ns | −0.589 * |
| Total Esters | 26.81 ± 1.49 *** | 24.99 ± 2.93 *** | ns | 0.550 * | |||
| ALCOHOLS | fusel, alcoholic, whiskey, fruity, banana sweet, green, fruity, apple, pineapple, nutty ethereal, winey mild, ethereal odor a waxy, green, creamy, citrus, orange, cheesy fruity | ||||||
| 2-Heptanol | C7H16O | 10976 | 0.20 ± 0.02 *** | 0.28 ± 0.05 *** | ns | −0.059 | |
| 1-Butanol, 3-methyl-(isoamyl alcochol) | C5H12O | 31260 | 0.08 ± 0.02 *** | 0.11 ± 0.04 *** | ns | −0.207 | |
| 1-Propanol, 2-methyl- | C4H10O | 6560 | 0.02 ± 0.01 *** | 0.06 ± 0.04 *** | ns | −0.732 ** | |
| 2-Propanol, 1-methoxy- | C4H10O2 | 7900 | 0.008 ± 0.01 *** | ND | ns | −0.140 | |
| 2-Nonanol | C9H20O | 12367 | 0.02 ± 0.01 *** | 0.07 ± 0.02 *** | ns | −0.123 | |
| Total Alcohols | 0.32 ± 0.05 *** | 0.51 ± 0.11 *** | ns | −0.355 | |||
| KETONES | fruity, spicy, sweet, herbal, coconut, woody fresh, sweet, green, weedy, earthy, herbal sweet, fruity, ethereal, winey, banana, woody pungent, sweet, creamy, buttery alcoholic, musty, woody buttery, sweet, creamy, pungent, caramellic | ||||||
| 2-Heptanone | C7H14O | 8051 | 2.24 ± 0.21 *** | 3.19 ± 0.60 *** | ns | 0.125 | |
| 2-Nonanone | C9H18O | 13187 | 0.62 ± 0.05 *** | 0.82 ± 0.15 *** | ns | −0.269 | |
| 2-Pentanone | C5H10O | 7895 | 0.44 ± 0.08 *** | 0.46 ± 0.19 *** | ns | 0.659 ** | |
| Acetoin | C4H8O2 | 179 | 0.41 ± 0.05 *** | 0.28 ± 0.04 ** | ns | −0.238 | |
| Acetone | C3H6O | 180 | 0.26 ± 0.03 *** | 0.27 ± 0.06 ** | ns | −0.012 | |
| 2,3-Butanedione | C4H6O2 | 650 | 0.20 ± 0.02 *** | 0.17 ± 0.04 *** | ns | −0.385 | |
| Total Ketones | 4.17 ± 0.31 *** | 5.18 ± 0.92 *** | ns | 0.156 | |||
| ALDEHYDES | ethereal, aldehydic, chocolate, peach, fatty strong, sharp, sweet, bitter almond, cherry fresh, aldehydic, floral, pungent pungent, ethereal, aldehydic, fruity characteristic aromatic odor a | ||||||
| Butanal, 3-methyl- | C5H10O | 11552 | 0.08 ± 0.01 *** | 0.08 ± 0.01 ** | ns | −0.192 | |
| Benzaldehyde | C7H6O | 240 | 0.03 ± 0.02 *** | 0.13 ± 0.09 *** | ns | −0.686 ** | |
| Propanal, 2-methyl-(isobutyraldehyde) | C4H8O | 6561 | 0.02 ± 0.01 *** | 0.04 ± 0.01 *** | ns | −0.200 | |
| Acetaldehyde | C2H4O | 177 | 0.01 ± 0.01 *** | 0.01 ± 0.01 *** | ns | −0.017 | |
| Total Aldehyde | 0.15 ± 0.02 *** | 0.27 ± 0.07 *** | ns | −0.758 ** | |||
| Toluene | C7H8 | 1140 | 0.14 ± 0.04 *** | 0.19 ± 0.06 *** | ns | −0.257 |
| Year | Category | Variable Enter | Standardized Beta | R2 | R2 Change | F Change | Prop. |
|---|---|---|---|---|---|---|---|
| 2018 | GR | Total Phenolics (HCL) | 0.685 | 0.734 | 0.734 | 207.06 | ≥0.001 |
| Glucose | −0.325 | 0.810 | 0.076 | 29.65 | ≥0.001 | ||
| NGR | Total Phenolics (HCL) | 0.547 | 0.592 | 0.592 | 17.39 | 0.0013 | |
| Glucose | −0.424 | 0.722 | 0.130 | 5.14 | 0.0400 | ||
| 2019 | GR | Total Phenolics (HCL) | 0.791 | 0.681 | 0.681 | 168.54 | ≥0.001 |
| Sucrose | 0.205 | 0.742 | 0.061 | 18.62 | ≥0.001 | ||
| Fructose | −0.154 | 0.764 | 0.022 | 7.05 | 0.0090 | ||
| NGR | Total Phenolics (HCL) | 0.606 | 0.534 | 0.534 | 14.91 | 0.0019 | |
| Sucrose | 0.458 | 0.728 | 0.194 | 8.58 | 0.0100 |
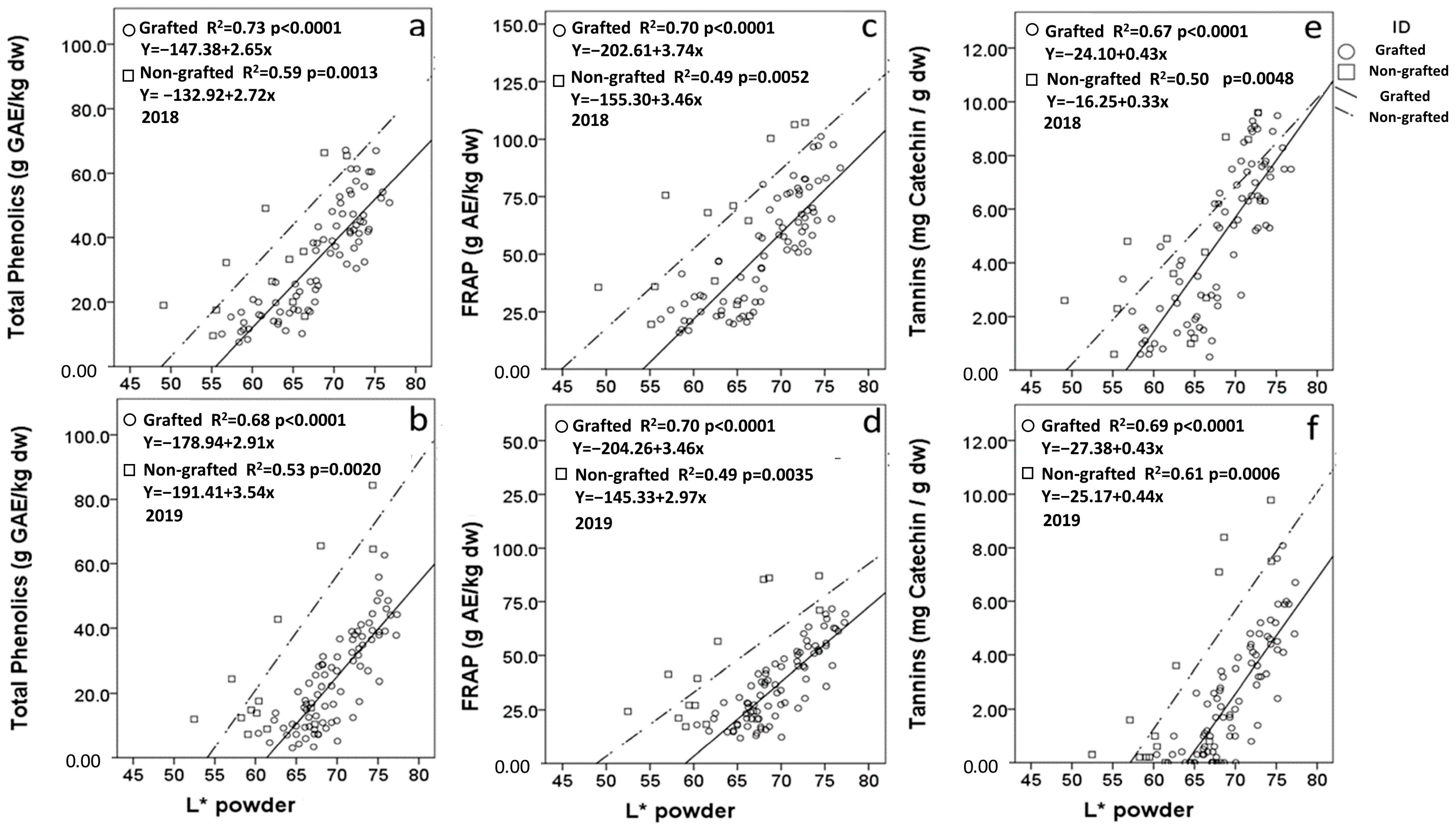
3.7. Regression Analysis of Carob Flour L* and Compositional Variables
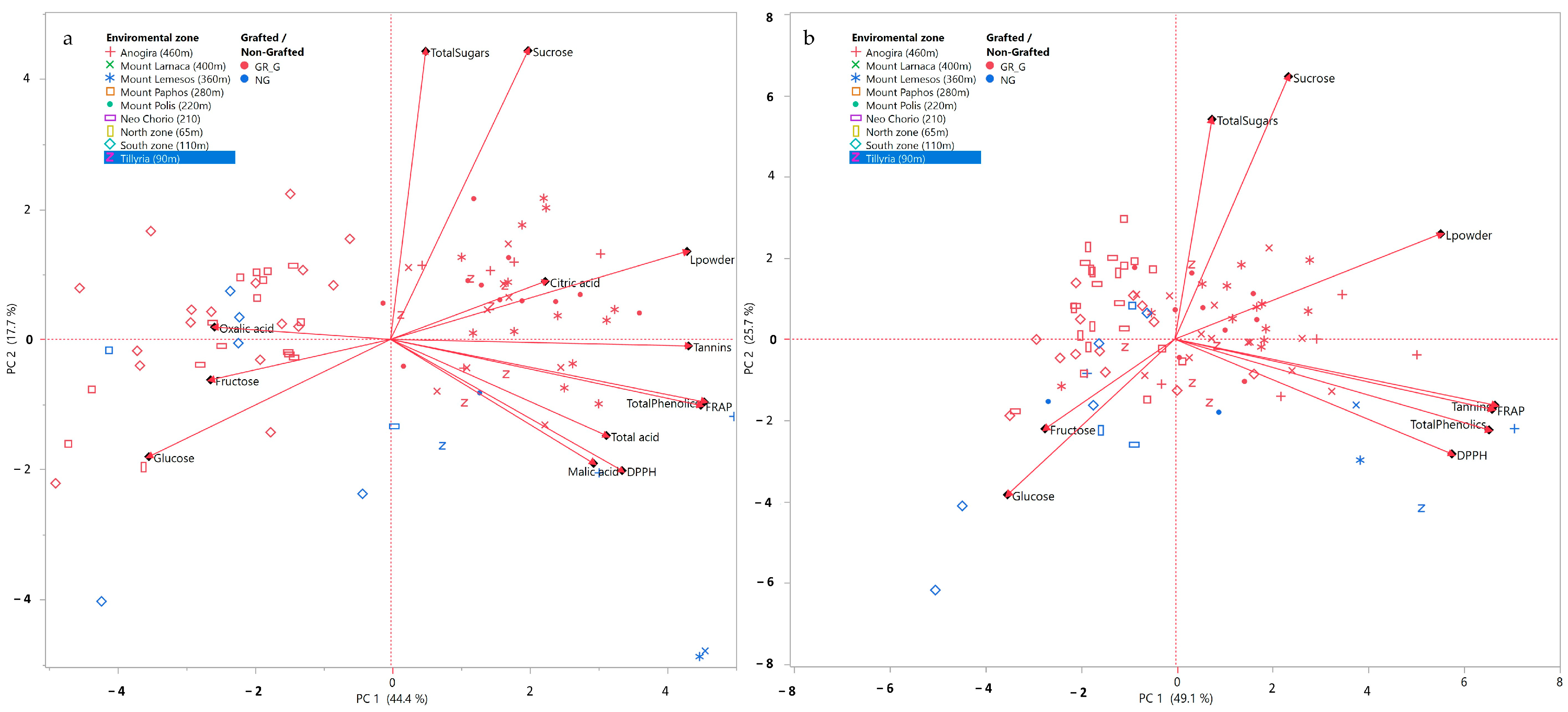
3.8. PCA Analysis of Compositional Variables and Sample Origins
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batlle, I.; Tous, J. Carob tree: Ceratonia siliqua L.-Promoting the conservation and use of underutilized and neglected crops. Bioversity Int. 1997, 17, 92. [Google Scholar]
- Kyratzis, A.C.; Antoniou, C.; Papayiannis, L.C.; Graziani, G.; Rouphael, Y.; Kyriacou, M.C. Pod Morphology, Primary and Secondary Metabolite Profiles in Non-grafted and Grafted Carob Germplasm Are Configured by Agro-Environmental Zone, Genotype, and Growing Season. Front. Plant Sci. 2021, 11, 612376. [Google Scholar] [CrossRef] [PubMed]
- Tous, J.; Romero, A.; Batlle, I. The Carob tree: Botany, horticulture, and genetic resources. In Horticultural Reviews; Wiley: Hoboken, NJ, USA, 2013; Volume 41, pp. 385–456. [Google Scholar]
- Bouzouita, N.; Khaldi, A.; Zgoulli, S.; Chebil, L.; Chekki, R.; Chaabouni, M.; Thonart, P. The analysis of crude and purified locust bean gum: A comparison of samples from different carob tree populations in Tunisia. Food Chem. 2007, 101, 1508–1515. [Google Scholar] [CrossRef]
- Avallone, R.; Plessi, M.; Baraldi, M.; Monzani, A. Determination of Chemical Composition of Carob (Ceratonia siliqua): Protein, Fat, Carbohydrates, and Tannins. J. Food Compos. Anal. 1997, 10, 166–172. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Antoniou, C.; Rouphael, Y.; Graziani, G.; Kyratzis, A. Mapping the Primary and Secondary Metabolomes of Carob (Ceratonia siliqua L.) Fruit and Its Postharvest Antioxidant Potential at Critical Stages of Ripening. Antioxidants 2021, 10, 57. [Google Scholar] [CrossRef]
- Čepo, D.V.; Mornar, A.; Nigović, B.; Kremer, D.; Radanović, D.; Vedrina Dragojević, I. Optimization of roasting conditions as an useful approach for increasing antioxidant activity of carob powder. LWT—Food Sci. Technol. 2014, 58, 578–586. [Google Scholar] [CrossRef]
- Craig, W.J.; Nguyen, T.T. Caffeine and Theobromine Levels in Cocoa and Carob Products. J. Food Sci. 1984, 49, 302–303. [Google Scholar] [CrossRef]
- Benković, M.; Belščak-Cvitanović, A.; Bauman, I.; Komes, D.; Srečec, S. Flow properties and chemical composition of carob (Ceratonia siliqua L.) flours as related to particle size and seed presence. Food Res. Int. 2017, 100, 211–218. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Świeca, M.; Gawlik-Dziki, U. Effect of carob (Ceratonia siliqua L.) flour on the antioxidant potential, nutritional quality, and sensory characteristics of fortified durum wheat pasta. Food Chem. 2016, 194, 637–642. [Google Scholar] [CrossRef]
- Antoniou, C.; Kyratzis, A.C.; Soteriou, G.A.; Rouphael, Y.; Kyriacou, M.C. Configuration of the Volatile Aromatic Profile of Carob Powder Milled From Pods of Genetic Variants Harvested at Progressive Stages of Ripening from High and Low Altitudes. Front. Nutr. 2021, 8, 789169. [Google Scholar] [CrossRef]
- Yousif, A.K.; Alghzawi, H.M. Processing and characterization of carob powder. Food Chem. 2000, 69, 283–287. [Google Scholar] [CrossRef]
- Şahin, H.; Topuz, A.; Pischetsrieder, M.; Özdemir, F. Effect of roasting process on phenolic, antioxidant and browning properties of carob powder. Eur. Food Res. Technol. 2009, 230, 155. [Google Scholar] [CrossRef]
- Eldeeb, G.S.S.; Mosilhey, S.H. Roasting temperature impact on bioactive compounds and PAHs in Carob powder (Ceratonia siliqua L.). J. Food Sci. Technol. 2022, 59, 105–113. [Google Scholar] [CrossRef]
- Berna, A.; Pérez-Gago, M.B.; Guardiola, V.G.; Salazar, D.; Mulet, A. Effect of Temperature on Isobutyric Acid Loss during Roasting of Carob Kibble. J. Agric. Food Chem. 1997, 45, 4084–4087. [Google Scholar] [CrossRef]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.-S.; Nakayama, T. Antioxidant Activity of Polyphenols in Carob Pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Fellows, P.J. Food Processing Technology: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Ioannou, G.D.; Savva, I.K.; Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Phenolic Profile, Antioxidant Activity, and Chemometric Classification of Carob Pulp and Products. Molecules 2023, 28, 2269. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Nguyen, T.T.; Le, V.N.; Nguyen, N.T.; Truong, N.M.; Hoang, M.T.; Pham, T.P.; Bui, Q.M. Towards a Standardized Approach for the Geographical Traceability of Plant Foods Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Principal Component Analysis (PCA). Foods 2023, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G.J.H. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical Factors of Vanillin Assay for Catechins and Proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Sepperer, T.; Hernandez-Ramos, F.; Labidi, J.; Oostingh, G.J.; Bogner, B.; Petutschnigg, A.; Tondi, G. Purification of industrial tannin extract through simple solid-liquid extractions. Ind. Crops Prod. 2019, 139, 111502. [Google Scholar] [CrossRef]
- Antoniou, C.; Kyratzis, A.; Rouphael, Y.; Stylianou, S.; Kyriacou, M.C. Heat- and Ultrasound-Assisted Aqueous Extraction of Soluble Carbohydrates and Phenolics from Carob Kibbles of Variable Size and Source Material. Foods 2020, 9, 1364. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Farag, M.A.; El-Kersh, D.M. Volatiles profiling in Ceratonia siliqua (Carob bean) from Egypt and in response to roasting as analyzed via solid-phase microextraction coupled to chemometrics. J. Adv. Res. 2017, 8, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Krokou, A.; Stylianou, M.; Agapiou, A. Assessing the volatile profile of carob tree (Ceratonia siliqua L.). Environ. Sci. Pollut. Res. 2019, 26, 35365–35374. [Google Scholar] [CrossRef]
- Clewer, A.G.; Scarisbrick, D.H. Practical Statistics and Experimental Design for Plant and Crop Science; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tetik, N.; Turhan, I.; Oziyci, H.R.; Gubbuk, H.; Karhan, M.; Ercisli, S. Physical and chemical characterization of Ceratonia siliqua L. germplasm in Turkey. Sci. Hortic. 2011, 129, 583–589. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Ouatmane, A.; Boulli, A.; Dehbi, F.; Jaouad, A.J.J.o.M.; Science, E. Yield and composition of carob bean gum produced from different Moroccan populations of carob (Ceratonia siliqua L.). J. Mater. Environ. Sci. 2013, 4, 309–314. [Google Scholar]
- Farag, M.A.; El-Kersh, D.M.; Ehrlich, A.; Choucry, M.A.; El-Seedi, H.; Frolov, A.; Wessjohann, L.A. Variation in Ceratonia siliqua pod metabolome in context of its different geographical origin, ripening stage and roasting process. Food Chem. 2019, 283, 675–687. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Rupasinghe, H.V. The role of polyphenols in quality, postharvest handling, and processing of fruits. In Postharvest Biology Technology of Fruits, Vegetables and Flowers; Wiley: Hoboken, NJ, USA, 2008; p. 260e281. [Google Scholar]
- Krokou, A.; Kokkinofta, R.; Stylianou, M.; Agapiou, A. Decoding carob flavor aroma using HS–SPME–GC–MS and chemometrics. Eur. Food Res. Technol. 2020, 246, 1419–1428. [Google Scholar] [CrossRef]
- Racolta, E.; Tofana, M.; Muresan, C.; Socaci, C.; Florin, G.; Vlad, M. Volatile compounds and sensory evaluation of spreadable creams based on roasted sunflower kernels and cocoa or carob powder. J Bull UASVM Food Sci Technol 2014, 71, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Hadi, M.A.M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGorrin, R.J. The Significance of Volatile Sulfur Compounds in Food Flavors. In Volatile Sulfur Compounds in Food; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 3–31. [Google Scholar]

| Grafted (n = 68) | Non-Grafted (n = 13) | t-Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | Mean | Std. Error | Minimum | Maximum | Mean | Std. Error | Minimum | Maximum | Significance |
| L* powder (2018) | 68.05 | 0.61 | 56.24 | 76.78 | 63.54 | 1.95 | 49.10 | 73.54 | ** |
| L* powder (2019) | 69.75 | 0.46 | 60.37 | 77.33 | 63.38 | 1.63 | 52.47 | 74.40 | ** |
| DPPH (g AAE/kg dw) (2018) | 9.23 | 0.39 | 3.19 | 20.87 | 14.26 | 1.45 | 5.38 | 24.14 | ** |
| DPPH (g AAE/kg dw) (2019) | 7.18 | 0.49 | 0.88 | 30.72 | 13.17 | 2.84 | 1.73 | 39.04 | ns |
| FRAP (g AAE/kg dw) (2018) | 51.90 | 2.72 | 15.98 | 101.20 | 64.61 | 9.60 | 19.57 | 130.48 | ns |
| FRAP (g AAE/kg dw) (2019) | 37.04 | 1.89 | 11.88 | 71.63 | 43.14 | 6.90 | 17.19 | 86.98 | ns |
| Total Phenolics (HCL) (g GAE/kg dw) (2018) | 33.39 | 1.89 | 7.55 | 67.17 | 39.99 | 6.88 | 9.57 | 85.35 | ns |
| Total Phenolics (HCL) (g GAE/kg dw) (2019) | 24.36 | 1.61 | 3.02 | 62.67 | 32.92 | 7.90 | 7.30 | 99.18 | ns |
| Tannins (mg Catechin/g dw) (2018) | 4.87 | 0.32 | 0.50 | 9.59 | 4.70 | 0.91 | 0.60 | 10.79 | ns |
| Tannins (mg Catechin/g dw) (2019) | 2.46 | 0.23 | 0.00 | 8.08 | 2.75 | 0.92 | 0.00 | 9.78 | ns |
| Total Phenolics-Acetate (g GAE/kg dw) (2018) | 10.53 | 0.34 | 5.23 | 18.51 | 15.07 | 1.85 | 7.75 | 29.05 | * |
| Fructose (g/100 g dw) (2018) | 10.14 | 0.13 | 8.05 | 13.92 | 9.18 | 0.39 | 6.64 | 11.68 | ** |
| Fructose (g/100 g dw) (2019) | 7.56 | 0.11 | 5.20 | 10.64 | 7.02 | 0.56 | 4.59 | 12.02 | ns |
| Glucose (g/100 g dw) (2018) | 5.16 | 0.13 | 3.14 | 9.52 | 5.45 | 0.41 | 2.37 | 8.54 | ns |
| Glucose (g/100 g dw) (2019) | 3.46 | 0.09 | 1.67 | 6.09 | 4.02 | 0.52 | 1.49 | 9.27 | ns |
| Sucrose (g/100 g dw) (2018) | 35.34 | 0.44 | 23.20 | 43.38 | 29.25 | 1.73 | 12.10 | 35.18 | ** |
| Sucrose (g/100 g dw) (2019) | 30.99 | 0.39 | 23.29 | 38.05 | 24.96 | 1.33 | 12.58 | 32.67 | *** |
| Total Sugars (g/100 g dw) (2018) | 50.64 | 0.32 | 44.33 | 57.01 | 43.88 | 1.52 | 27.85 | 50.94 | ** |
| Total Sugars (g/100 g dw) (2019) | 42.01 | 0.37 | 34.45 | 49.19 | 35.99 | 0.79 | 30.46 | 41.19 | *** |
| F/S (2018) | 0.29 | 0.01 | 0.21 | 0.57 | 0.35 | 0.05 | 0.19 | 0.87 | ns |
| F/S (2019) | 0.25 | 0.01 | 0.16 | 0.44 | 0.32 | 0.05 | 0.15 | 0.96 | ns |
| G/S (2018) | 0.15 | 0.01 | 0.08 | 0.41 | 0.21 | 0.03 | 0.07 | 0.43 | ns |
| G/S (2019) | 0.11 | 0.00 | 0.05 | 0.26 | 0.19 | 0.05 | 0.05 | 0.74 | ns |
| (F + G)/S (2018) | 0.44 | 0.01 | 0.30 | 0.98 | 0.55 | 0.07 | 0.26 | 1.30 | ns |
| (F + G)/S (2019) | 0.36 | 0.01 | 0.22 | 0.70 | 0.51 | 0.10 | 0.20 | 1.69 | ns |
| Malic acid (mg/g dw) (2018) | 5.81 | 0.17 | 1.49 | 9.01 | 6.52 | 0.59 | 3.99 | 10.71 | ns |
| Citric acid (mg/g dw) (2018) | 1.66 | 0.05 | 0.58 | 2.90 | 1.43 | 0.13 | 0.40 | 2.52 | ns |
| Oxalic acid (mg/g dw) (2018) | 0.31 | 0.01 | 0.21 | 0.44 | 0.30 | 0.01 | 0.22 | 0.39 | ns |
| Total acid (mg/g dw) (2018) | 7.78 | 0.19 | 2.57 | 11.25 | 8.25 | 0.64 | 5.45 | 13.45 | ns |
| Protein % (w/w) (2018) | 4.68 | 0.09 | 2.87 | 7.44 | 4.85 | 0.36 | 2.18 | 7.87 | ns |
| Grafted (n = 68) | Non-Grafted (n = 13) | Overall (n = 81) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Trait | Correlation | Sig. | t-Test | Correlation | Sig. | t-Test | Correlation | Sig. | t-Test |
| L* powder | 0.638 | *** | * | 0.694 | ** | ns | 0.693 | *** | * |
| DPPH | 0.539 | *** | *** | 0.771 | ** | ns | 0.685 | *** | ** |
| FRAP | 0.734 | *** | *** | 0.594 | * | * | 0.699 | *** | *** |
| Total Phenolics | 0.734 | *** | *** | 0.550 | ns | ns | 0.663 | *** | *** |
| Tannins | 0.663 | *** | *** | 0.501 | ns | ns | 0.612 | *** | *** |
| Fructose | 0.311 | ** | *** | 0.501 | ns | ** | 0.389 | ** | *** |
| Glucose | 0.303 | * | *** | 0.517 | ns | * | 0.383 | ** | *** |
| Sucrose | 0.369 | ** | *** | 0.712 | ** | ** | 0.586 | *** | *** |
| Total Sugars | 0.320 | ** | *** | 0.376 | ns | ** | 0.517 | *** | *** |
| F/S | 0.337 | ** | *** | 0.662 | * | ns | 0.575 | *** | ** |
| G/S | 0.309 | * | *** | 0.817 | ** | ns | 0.621 | *** | *** |
| (F + G)/S | 0.333 | ** | *** | 0.732 | ** | ns | 0.608 | *** | *** |
| Trait | L* Corr (GR) | L* Corr (NGR) | L* Corr (Overall) |
|---|---|---|---|
| DPPH (2018) | 0.624 ** | 0.450 | 0.369 ** |
| DPPH (2019) | 0.666 ** | 0.639 * | 0.340 ** |
| FRAP (2018) | 0.838 ** | 0.701 ** | 0.696 ** |
| FRAP (2019) | 0.836 ** | 0.703 ** | 0.608 ** |
| Total Phenolics (2018) | 0.857 ** | 0.769 ** | 0.748 ** |
| Total Phenolics (2019) | 0.825 ** | 0.731 ** | 0.567 ** |
| Tannins (2018) | 0.820 ** | 0.705 ** | 0.764 ** |
| Tannins (2019) | 0.833 ** | 0.783 ** | 0.669 ** |
| Fructose (2018) | −0.520 ** | −0.387 | −0.369 ** |
| Fructose (2019) | −0.284 * | −0.496 | −0.243 * |
| Glucose (2018) | −0.688 ** | −0.711 ** | −0.660 ** |
| Glucose (2019) | −0.329 ** | −0.643 ** | −0.452 ** |
| Sucrose (2018) | 0.607 ** | 0.169 | 0.537 ** |
| Sucrose (2019) | 0.331 ** | 0.623 * | 0.557 ** |
| Total Sugars (2018) | 0.329 ** | −0.101 | 0.306 ** |
| Total Sugars (2019) | 0.182 | 0.274 | 0.419 ** |
| F/S (2018) | −0.632 ** | −0.236 | −0.492 ** |
| F/S (2019) | −0.392 ** | −0.576 * | −0.496 ** |
| G/S (2018) | −0.679 ** | −0.512 | −0.645 ** |
| G/S (2019) | −0.396 ** | −0.616 * | −0.525 ** |
| (F + G)/S (2018) | −0.664 ** | −0.347 | −0.571 ** |
| (F + G)/S (2019) | −0.407 ** | −0.596 * | −0.516 ** |
| Malic acid (2018) | 0.457 ** | 0.586 * | 0.440 ** |
| Citric acid (2018) | 0.390 ** | 0.442 | 0.419 ** |
| Oxalic acid (2018) | −0.524 ** | −0.091 | −0.397 ** |
| Total acid (2018) | 0.488 ** | 0.624 * | 0.491 ** |
| Protein (2018) | −0.192 | 0.051 | −0.117 |
| Altitude (2018) | 0.521 ** | 0.673 ** | 0.528 ** |
| Altitude (2019) | 0.547 ** | 0.608 * | 0.472 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antoniou, C.; Kyriacou, M.C.; Kyratzis, A.C.; Rouphael, Y. Linking Colorimetric Variation with Non-Volatile and Volatile Components of Carob Flour. Foods 2023, 12, 2556. https://doi.org/10.3390/foods12132556
Antoniou C, Kyriacou MC, Kyratzis AC, Rouphael Y. Linking Colorimetric Variation with Non-Volatile and Volatile Components of Carob Flour. Foods. 2023; 12(13):2556. https://doi.org/10.3390/foods12132556
Chicago/Turabian StyleAntoniou, Chrystalla, Marios C. Kyriacou, Angelos C. Kyratzis, and Youssef Rouphael. 2023. "Linking Colorimetric Variation with Non-Volatile and Volatile Components of Carob Flour" Foods 12, no. 13: 2556. https://doi.org/10.3390/foods12132556







