Non-Thermal Supercritical Carbon Dioxide Processing Retains the Quality Parameters and Improves the Kinetic Stability of an Araticum Beverage Enriched with Inulin-Type Dietary Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Sample Preparation
2.3. Functional Araticum Beverage Formulation
2.4. Non-Thermal SC–CO2 Processing
2.5. pH and Total Soluble Solids (TSS) Analysis
2.6. ζ-Potential Measurement
2.7. Particle Size Distribution
2.8. Kinetic Stability
2.9. Color Parameters
2.10. Determination of Sugars and FOS by HPAEC–PAD
2.11. Statistical Analysis
3. Results and Discussion
3.1. pH
3.2. Total Soluble Solids (TSS)
3.3. ζ-Potential
3.4. Particle Size Distribution
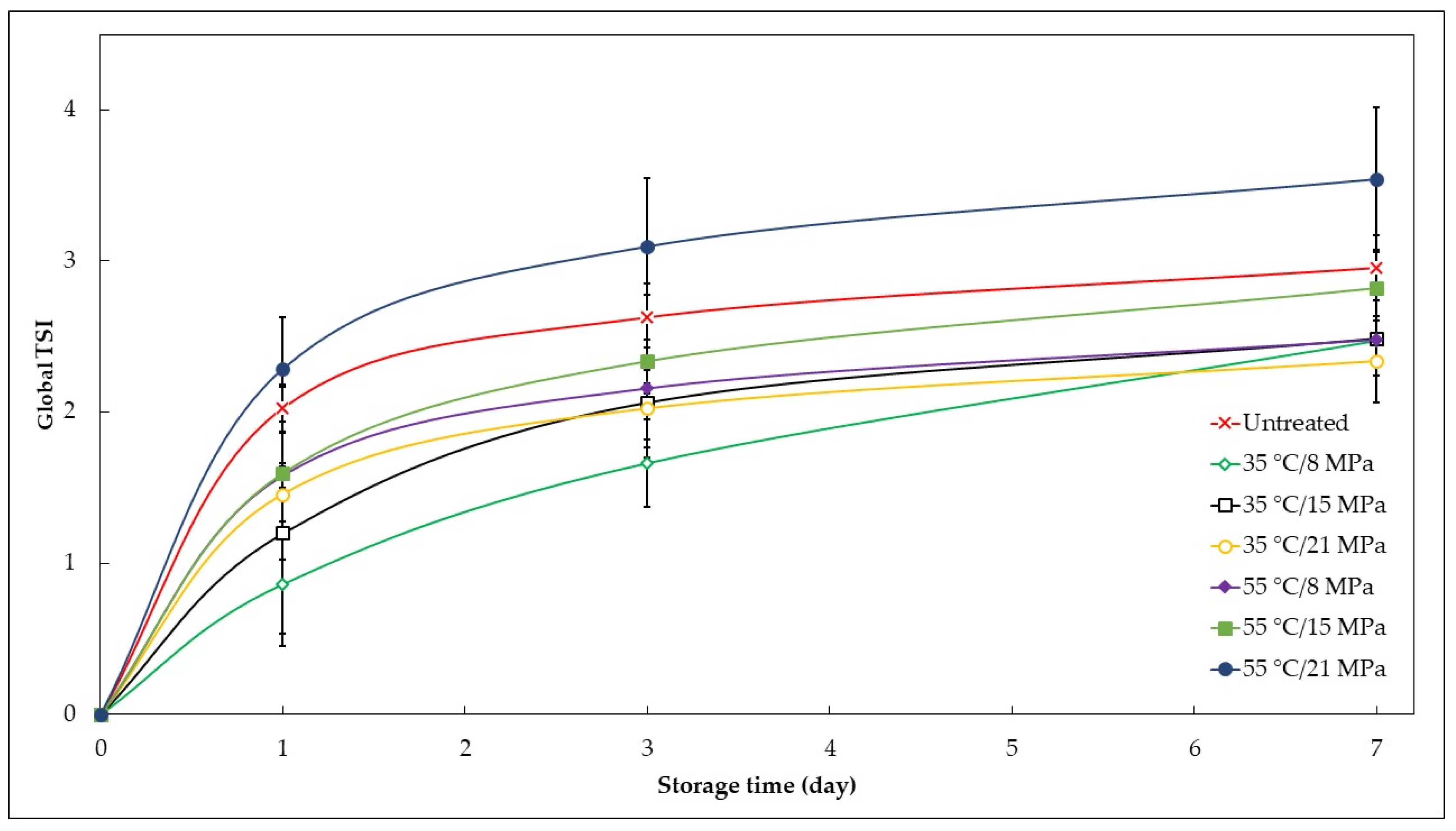
3.5. Kinetic Stability
3.6. Color Parameters
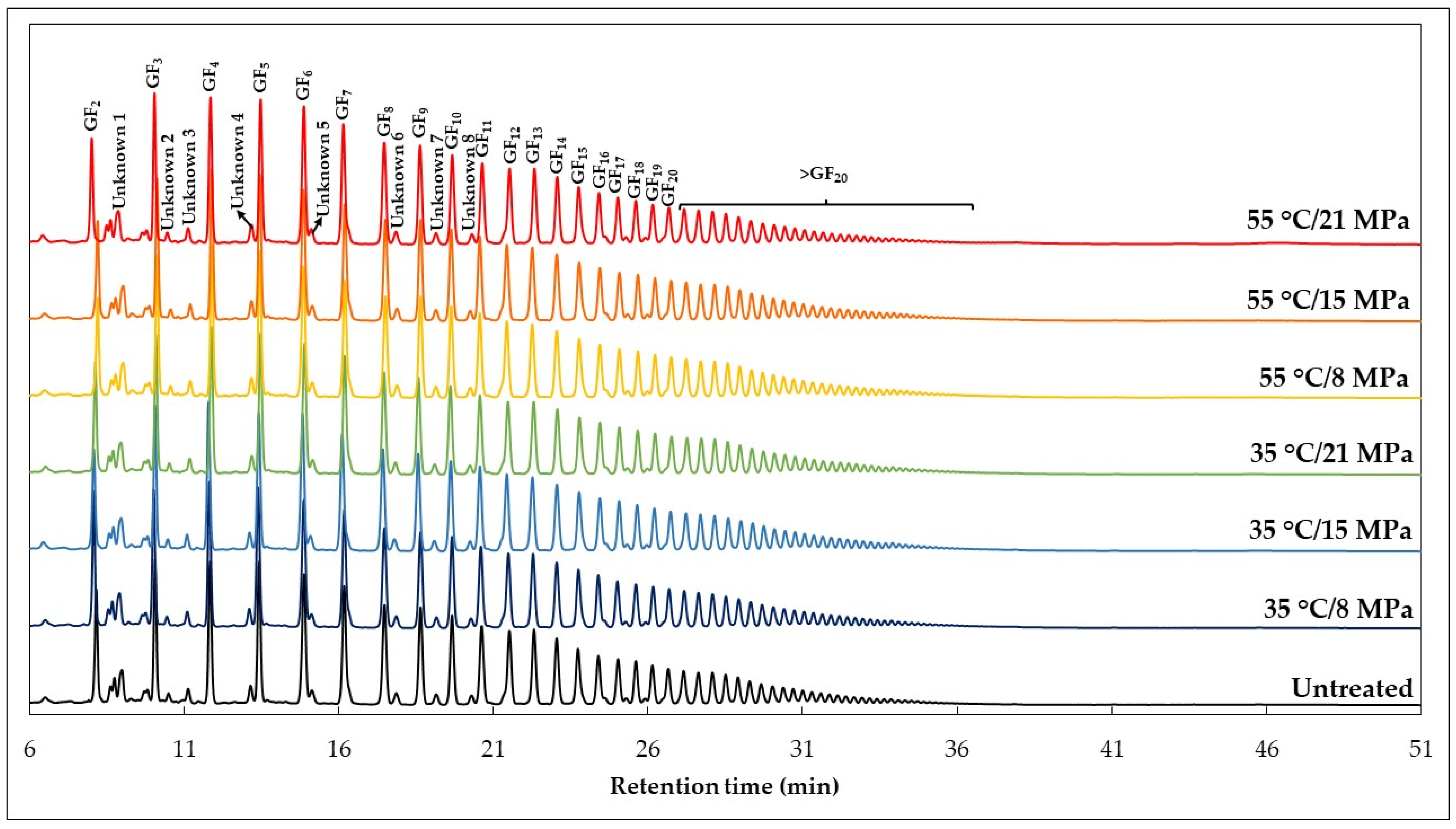
3.7. Sugars and Inulin Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, P.M.P.; Arcanjo, D.D.R.; Peron, A.P. Drug development, Brazilian biodiversity and political choices: Where are we heading? J. Toxicol. Environ. Health Part B 2023, 26, 257–274. [Google Scholar] [CrossRef]
- Convention on Biological Diversity. Brazil-Main Details. Available online: https://www.cbd.int/countries/profile/?country=br (accessed on 18 May 2023).
- SiBBr-Sistema de Informação Sobre a Biodiversidade Brasileira. Available online: https://www.sibbr.gov.br (accessed on 18 May 2023).
- De Souza, V.R.; Pereira, P.A.P.; Queiroz, F.; Borges, S.V.; Deus Souza Carneiro, J. Determination of bioactive compounds, antioxidant activity and chemical composition of Cerrado Brazilian fruits. Food Chem. 2012, 134, 381–386. [Google Scholar] [CrossRef]
- Arruda, H.S.; Borsoi, F.T.; Andrade, A.C.; Pastore, G.M.; Marostica, M.R., Jr. Scientific Advances in the Last Decade on the Recovery, Characterization, and Functionality of Bioactive Compounds from the Araticum Fruit (Annona crassiflora Mart.). Plants 2023, 12, 1536. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pastore, G.M. Araticum (Annona crassiflora Mart.) as a source of nutrients and bioactive compounds for food and non-food purposes: A comprehensive review. Food Res. Int. 2019, 123, 450–480. [Google Scholar] [CrossRef] [PubMed]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Optimization of Extraction Parameters of Total Phenolics from Annona crassiflora Mart. (Araticum) Fruits Using Response Surface Methodology. Food Anal. Methods 2017, 10, 100–110. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Guimarães, A.C.G.; de Gomes, M.S.; Lima, L.M.Z.; Sales, P.F.; da Cunha, M.C.; Rodrigues, L.J.; de Barros, H.E.A.; Pires, C.R.F.; dos Santos, V.F.; Natarelli, C.V.L.; et al. Application of Chemometric Techniques in the Evaluation of Bioactive Compounds and Antioxidant Activity of Fruit From Brazilian Cerrado. J. Food Meas. Charact. 2023, 17, 2095–2106. [Google Scholar] [CrossRef]
- Seixas, F.R.F.; Bassoli, B.K.; Virgolin, L.B.; Garcia, L.C.; Janzantti, N.S. Physicochemical Properties and Effects of Fruit Pulps from the Amazon Biome on Physiological Parameters in Rats. Nutrients 2021, 13, 1484. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.C.C.; Monteiro, O.S.; da Rocha, C.Q.; Longato, G.B.; Smith, R.E.; da Silva, J.K.R.; Maia, J.G.S. Phytochemical Analysis of the Fruit Pulp Extracts from Annona crassiflora Mart. and Evaluation of Their Antioxidant and Antiproliferative Activities. Foods 2022, 11, 2079. [Google Scholar] [CrossRef]
- Lucas dos Santos, E.; Leite, N.; Alves de Araújo, L.C.; Giffoni de Carvalho, J.T.; Souza, K.d.P. Protective effect of Annona crassiflora on oxidative stress and Alzheimer’s models in Caenorhabditis elegans. Free Radic. Biol. Med. 2018, 128, S125. [Google Scholar] [CrossRef]
- Stafussa, A.P.; Maciel, G.M.; Bortolini, D.G.; Maroldi, W.V.; Ribeiro, V.R.; Fachi, M.M.; Pontarolo, R.; Bach, F.; Pedro, A.C.; Haminiuk, C.W.I. Bioactivity and bioaccessibility of phenolic compounds from Brazilian fruit purees. Futur. Foods 2021, 4, 100066. [Google Scholar] [CrossRef]
- Da Silva, J.J.; Cerdeira, C.D.; Chavasco, J.M.; Cintra, A.B.P.; da Silva, C.B.P.; de Mendonça, A.N.; Ishikawa, T.; Boriollo, M.F.G.; Chavasco, J.K. In vitro screening antibacterial activity of Bidens pilosa Linné and Annona crassiflora Mart. against oxacillin resistant Staphylococcus aureus (ORSA) from the aerial environment at the dental clinic. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Valero-Cases, E.; Cerdá-Bernad, D.; Pastor, J.-J.; Frutos, M.-J. Non-Dairy Fermented Beverages as Potential Carriers to Ensure Probiotics, Prebiotics, and Bioactive Compounds Arrival to the Gut and Their Health Benefits. Nutrients 2020, 12, 1666. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Arruda, H.S.; Geraldi, M.V.; Cedran, M.F.; Bicas, J.L.; Marostica Junior, M.R.; Pastore, G.M. Prebiotics and probiotics. In Bioactive Food Components Activity in Mechanistic Approach; Cazarin, C.B.B., Bicas, J.L., Pastore, G.M., Marostica Junior, M.R., Eds.; Academic Press: London, UK, 2022; pp. 55–118. ISBN 9780128225905. [Google Scholar]
- Arruda, H.S.; Silva, E.K.; Pereira, G.A.; Meireles, M.A.A.; Pastore, G.M. Inulin thermal stability in prebiotic carbohydrate-enriched araticum whey beverage. LWT 2020, 128, 109418. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Arruda, H.S.; Geraldi, M.V.; Maróstica Júnior, M.R.; Pastore, G.M. Natural prebiotic carbohydrates, carotenoids and flavonoids as ingredients in food systems. Curr. Opin. Food Sci. 2020, 33, 98–107. [Google Scholar] [CrossRef]
- Mudannayake, D.C.; Jayasena, D.D.; Wimalasiri, K.M.S.; Ranadheera, C.S.; Ajlouni, S. Inulin fructans–food applications and alternative plant sources: A review. Int. J. Food Sci. Technol. 2022, 57, 5764–5780. [Google Scholar] [CrossRef]
- Silva, E.K.; Arruda, H.S.; Eberlin, M.N.; Pastore, G.M.; Meireles, M.A.A. Effects of supercritical carbon dioxide and thermal treatment on the inulin chemical stability and functional properties of prebiotic-enriched apple juice. Food Res. Int. 2019, 125, 108561. [Google Scholar] [CrossRef]
- Silva, E.K.; Bargas, M.A.; Arruda, H.S.; Vardanega, R.; Pastore, G.M.; Meireles, M.A.A. Supercritical CO2 processing of a functional beverage containing apple juice and aqueous extract of Pfaffia glomerata roots: Fructooligosaccharides chemical stability after non-thermal and thermal treatments. Molecules 2020, 25, 3911. [Google Scholar] [CrossRef]
- Bhattacharjee, C.; Saxena, V.K.; Dutta, S. Novel thermal and non-thermal processing of watermelon juice. Trends Food Sci. Technol. 2019, 93, 234–243. [Google Scholar] [CrossRef]
- Li, J.; Zhu, L.; Murtaza, A.; Iqbal, A.; Zhang, J.; Xu, X.; Pan, S.; Hu, W. The effect of high pressure carbon dioxide on the inactivation kinetics and structural alteration of phenylalanine ammonia-lyase from Chinese water chestnut: An investigation using multi-spectroscopy and molecular docking methods. Innov. Food Sci. Emerg. Technol. 2022, 77, 102970. [Google Scholar] [CrossRef]
- Liu, J.; Yuan, S.; Han, D.; Liu, J.; Zhao, L.; Wu, J. Effects of CO2-assisted high-pressure processing on microbiological and physicochemical properties of Chinese spiced beef. Innov. Food Sci. Emerg. Technol. 2023, 84, 103261. [Google Scholar] [CrossRef]
- Prado, J.M.; Dalmolin, I.; Carareto, N.D.D.; Basso, R.C.; Meirelles, A.J.A.; Vladimir Oliveira, J.; Batista, E.A.C.; Meireles, M.A.A. Supercritical fluid extraction of grape seed: Process scale-up, extract chemical composition and economic evaluation. J. Food Eng. 2012, 109, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Silva, E.K.; Alvarenga, V.O.; Bargas, M.A.; Sant’Ana, A.S.; Meireles, M.A.A. Non-thermal microbial inactivation by using supercritical carbon dioxide: Synergic effect of process parameters. J. Supercrit. Fluids 2018, 139, 97–104. [Google Scholar] [CrossRef]
- Silva, E.K.; Guimarães, J.T.; Costa, A.L.R.; Cruz, A.G.; Meireles, M.A.A. Non-thermal processing of inulin-enriched soursop whey beverage using supercritical carbon dioxide technology. J. Supercrit. Fluids 2019, 154, 104635. [Google Scholar] [CrossRef]
- Ordóñez-Santos, L.E.; Martínez-Girón, J.; Arias-Jaramillo, M.E. Effect of ultrasound treatment on visual color, vitamin C, total phenols, and carotenoids content in Cape gooseberry juice. Food Chem. 2017, 233, 96–100. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Miao, J.; Yu, W.; Zou, L.; Zhou, W.; Liu, C.; Liu, W. Effect of Cinnamon Essential Oil Nanoemulsion Combined with Ascorbic Acid on Enzymatic Browning of Cloudy Apple Juice. Food Bioprocess Technol. 2020, 13, 860–870. [Google Scholar] [CrossRef]
- Ramírez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Phytochemical, sensory attributes and aroma stability of dense phase carbon dioxide processed Hibiscus sabdariffa beverage during storage. Food Chem. 2012, 134, 1425–1431. [Google Scholar] [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Martins, C.P.C.; Andrade, L.G.Z.S.; Moraes, J.; Alvarenga, V.O.; Guimarães, J.T.; Esmerino, E.A.; Freitas, M.Q.; et al. Whey-grape juice drink processed by supercritical carbon dioxide technology: Physicochemical characteristics, bioactive compounds and volatile profile. Food Chem. 2018, 239, 697–703. [Google Scholar] [CrossRef]
- Murtaza, A.; Iqbal, A.; Linhu, Z.; Liu, Y.; Xu, X.; Pan, S.; Hu, W. Effect of high-pressure carbon dioxide on the aggregation and conformational changes of polyphenol oxidase from apple (Malus domestica) juice. Innov. Food Sci. Emerg. Technol. 2019, 54, 43–50. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, G.; Cardoso, J.C.; Rubio-Senent, F.; Serrano, A.; Borja, R.; Fernández-Bolaños, J.; Fermoso, F.G. Thermally-treated strawberry extrudate: A rich source of antioxidant phenols and sugars. Innov. Food Sci. Emerg. Technol. 2019, 51, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Pereira, G.A.; Silva, E.K.; Peixoto Araujo, N.M.; Arruda, H.S.; Meireles, M.A.A.; Pastore, G.M. Obtaining a novel mucilage from mutamba seeds exploring different high-intensity ultrasound process conditions. Ultrason. Sonochemistry 2019, 55, 332–340. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Beltrán, S.; Melgosa, R.; Solaesa, A.G.; Ruiz, M.O. Evaluation of HPCD batch treatments on enzyme inactivation kinetics and selected quality characteristics of cloudy juice from Golden delicious apples. J. Food Eng. 2018, 221, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Amaral, G.V.; Silva, E.K.; Costa, A.L.R.; Alvarenga, V.O.; Cavalcanti, R.N.; Esmerino, E.A.; Guimarães, J.T.; Freitas, M.Q.; Sant’Ana, A.S.; Cunha, R.L.; et al. Whey-grape juice drink processed by supercritical carbon dioxide technology: Physical properties and sensory acceptance. LWT 2018, 92, 80–86. [Google Scholar] [CrossRef]
- Pereira, G.A.; Silva, E.K.; Peixoto Araujo, N.M.; Arruda, H.S.; Meireles, M.A.A.; Pastore, G.M. Mutamba seed mucilage as a novel emulsifier: Stabilization mechanisms, kinetic stability and volatile compounds retention. Food Hydrocoll. 2019, 97, 105190. [Google Scholar] [CrossRef]
- Strieder, M.M.; Arruda, H.S.; Pastore, G.M.; Silva, E.K. Inulin-type dietary fiber stability after combined thermal, mechanical, and chemical stresses related to ultrasound processing of prebiotic apple beverage. Food Hydrocoll. 2023, 139, 108489. [Google Scholar] [CrossRef]
- Guimarães, J.T.; Silva, E.K.; Alvarenga, V.O.; Costa, A.L.R.; Cunha, R.L.; Sant’Ana, A.S.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Physicochemical changes and microbial inactivation after high-intensity ultrasound processing of prebiotic whey beverage applying different ultrasonic power levels. Ultrason. Sonochemistry 2018, 44, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, J.T.; Silva, E.K.; Costa, A.L.R.; Cunha, R.L.; Freitas, M.Q.; Meireles, M.A.A.; Cruz, A.G. Manufacturing a prebiotic whey beverage exploring the influence of degree of inulin polymerization. Food Hydrocoll. 2018, 77, 787–795. [Google Scholar] [CrossRef]
- Monteiro, S.H.M.C.; Silva, E.K.; Alvarenga, V.O.; Moraes, J.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Effects of ultrasound energy density on the non-thermal pasteurization of chocolate milk beverage. Ultrason. Sonochemistry 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Schiassi, M.C.E.V.; de Souza, V.R.; Lago, A.M.T.; Campos, L.G.; Queiroz, F. Fruits from the Brazilian Cerrado region: Physico-chemical characterization, bioactive compounds, antioxidant activities, and sensory evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Nisha, P. Advances and prospects in the food applications of pectin hydrogels. Crit. Rev. Food Sci. Nutr. 2022, 62, 4393–4417. [Google Scholar] [CrossRef]
- Tarone, A.G.; Silva, E.K.; Betim Cazarin, C.B.; Marostica Junior, M.R. Inulin/fructooligosaccharides/pectin-based structured systems: Promising encapsulating matrices of polyphenols recovered from jabuticaba peel. Food Hydrocoll. 2021, 111, 106387. [Google Scholar] [CrossRef]
- Valencia-Flores, D.C.; Hernández-Herrero, M.; Guamis, B.; Ferragut, V. Comparing the Effects of Ultra-High-Pressure Homogenization and Conventional Thermal Treatments on the Microbiological, Physical, and Chemical Quality of Almond Beverages. J. Food Sci. 2013, 78, E199–E205. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, J.; Bi, J.; Cao, F.; Ding, Y.; Peng, J. Effects of high pressure homogenization on physical stability and carotenoid degradation kinetics of carrot beverage during storage. J. Food Eng. 2019, 263, 63–69. [Google Scholar] [CrossRef]
- Glibowski, P.; Wasko, A. Effect of thermochemical treatment on the structure of inulin and its gelling properties. Int. J. Food Sci. Technol. 2008, 43, 2075–2082. [Google Scholar] [CrossRef]
- Beccard, S.; Bernard, J.; Wouters, R.; Gehrich, K.; Zielbauer, B.; Mezger, M.; Vilgis, T.A. Alteration of the structural properties of inulin gels. Food Hydrocoll. 2019, 89, 302–310. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, J.; Pi, F.; Zhang, T.; Ai, C.; Yu, S. Rheological characterization of RG-I chicory root pectin extracted by hot alkali and chelators. Int. J. Biol. Macromol. 2020, 164, 759–770. [Google Scholar] [CrossRef]
- Popescu, L.; Ceșco, T.; Gurev, A.; Ghendov-Mosanu, A.; Sturza, R.; Tarna, R. Impact of Apple Pomace Powder on the Bioactivity, and the Sensory and Textural Characteristics of Yogurt. Foods 2022, 11, 3565. [Google Scholar] [CrossRef]
- De Cardoso, L.M.; da Oliveira, D.S.; de Bedetti, S.F.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Araticum (Annona crassiflora Mart.) from the Brazilian Cerrado: Chemical composition and bioactive compounds. Fruits 2013, 68, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Rodrigues, M.M.; Plaza, M.L.; Azeredo, A.; Balaban, M.O.; Marshall, M.R. Physicochemical and Phytochemical Properties of Cold and Hot Water Extraction from Hibiscus sabdariffa. J. Food Sci. 2011, 76, C428–C435. [Google Scholar] [CrossRef]
- Schwartz, S.J.; Cooperstone, J.L.; Cichon, M.J.; von Elbe, J.H.; Giusti, M.M. Colorants. In Fennema’s Food Chemistry; Damodaran, S., Parkin, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 681–752. ISBN 9781315372914. [Google Scholar]
- Scudino, H.; Silva, E.K.; Gomes, A.; Guimarães, J.T.; Cunha, R.L.; Sant’Ana, A.S.; Meireles, M.A.A.; Cruz, A.G. Ultrasound stabilization of raw milk: Microbial and enzymatic inactivation, physicochemical properties and kinetic stability. Ultrason. Sonochem. 2020, 67, 105185. [Google Scholar] [CrossRef] [PubMed]
- Fustier, P.; St-Germain, F.; Lamarche, F.; Mondor, M. Non-enzymatic browning and ascorbic acid degradation of orange juice subjected to electroreduction and electro-oxidation treatments. Innov. Food Sci. Emerg. Technol. 2011, 12, 491–498. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; Pastore, G.M. Oligosaccharide profile in Brazilian Cerrado fruit araticum (Annona crassiflora Mart.). LWT 2017, 76, 278–283. [Google Scholar] [CrossRef]
- Cappelletti, M.; Ferrentino, G.; Endrizzi, I.; Aprea, E.; Betta, E.; Corollaro, M.L.; Charles, M.; Gasperi, F.; Spilimbergo, S. High Pressure Carbon Dioxide pasteurization of coconut water: A sport drink with high nutritional and sensory quality. J. Food Eng. 2015, 145, 73–81. [Google Scholar] [CrossRef]
- Logtenberg, M.J.; Akkerman, R.; An, R.; Hermes, G.D.A.; Haan, B.J.; Faas, M.M.; Zoetendal, E.G.; Schols, H.A.; Vos, P. Fermentation of Chicory Fructo-Oligosaccharides and Native Inulin by Infant Fecal Microbiota Attenuates Pro-Inflammatory Responses in Immature Dendritic Cells in an Infant-Age-Dependent and Fructan-Specific Way. Mol. Nutr. Food Res. 2020, 64, 2000068. [Google Scholar] [CrossRef]
- Klewicki, R. The stability of gal-polyols and oligosaccharides during pasteurization at a low pH. LWT 2007, 40, 1259–1265. [Google Scholar] [CrossRef]
- Vega, R.; Zuniga-Hansen, M.E. The effect of processing conditions on the stability of fructooligosaccharides in acidic food products. Food Chem. 2015, 173, 784–789. [Google Scholar] [CrossRef]


| Parameter | SC–CO2 Treatments | ||||||
|---|---|---|---|---|---|---|---|
| 35 °C | 55 °C | ||||||
| Untreated | 8 MPa | 15 MPa | 21 MPa | 8 MPa | 15 MPa | 21 MPa | |
| pH | 4.5 ± 0.1 | 4.43 ± 0.01 | 4.46 ± 0.01 | 4.52 ± 0.01 | 4.54 ± 0.02 | 4.51 ± 0.01 | 4.5 ± 0.1 |
| TSS (°Brix) | 7.4 ± 0.1 | 7.4 ± 0.1 | 7.1 ± 0.1 | 7.3 ± 0.2 | 7.4 ± 0.1 | 7.5 ± 0.2 | 7.4 ± 0.1 |
| ζ-potential (mV) | −36 ± 1 | −35.1 ± 0.4 | −32 ± 3 | −35 ± 2 | −36 ± 2 | −34 ± 1 | −35 ± 1 |
| D4,3 (µm) | 143 ± 6 | 121 ± 7 | 148 ± 6 | 136 ± 4 | 105 ± 5 | 114 ± 2 | 106 ± 3 |
| Global TSI (day 7) | 3.0 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.3 | 2.3 ± 0.3 | 2.48 ± 0.03 | 2.8 ± 0.4 | 3.5 ± 0.5 |
| L* | 55.6 ± 0.3 | 55 ± 1 | 54.9 ± 0.4 | 55.2 ± 0.3 | 54.6 ± 0.5 | 54.8 ± 0.4 | 53 ± 1 |
| C* | 20.6 ± 0.3 | 21 ± 1 | 21.4 ± 0.2 | 21.2 ± 0.1 | 20.66 ± 0.03 | 21.3 ± 0.1 | 20.5 ± 0.4 |
| h | 81 ± 1 | 81 ± 1 | 80.2 ± 0.1 | 80.7 ± 0.1 | 80.2 ± 0.4 | 79.7 ± 0.2 | 80.0 ± 0.3 |
| a* | 3.3 ± 0.3 | 3.4 ± 0.6 | 3.64 ± 0.01 | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.8 ± 0.1 | 3.53 ± 0.02 |
| b* | 20.5 ± 0.1 | 21 ± 1 | 21.1 ± 0.2 | 20.97 ± 0.02 | 20.4 ± 0.1 | 21.0 ± 0.1 | 20.2 ± 0.4 |
| Color index | 2.8 ± 0.1 | 2.9 ± 0.2 | 2.98 ± 0.02 | 2.92 ± 0.04 | 2.9 ± 0.1 | 3.0 ± 0.1 | 3.0 ± 0.1 |
| Yellow index | 53 ± 1 | 54 ± 3 | 54.9 ± 0.2 | 54.3 ± 0.3 | 53.3 ± 0.3 | 55 ± 1 | 54.0 ± 0.1 |
| Browning index | 49 ± 1 | 50 ± 4 | 51.7 ± 0.2 | 50.6 ± 0.4 | 50 ± 1 | 52 ± 1 | 50.64 ± 0.03 |
| ΔE* | - | 1.7 ± 0.3 | 1.4 ± 0.1 | 0.7 ± 0.3 | 1.3 ± 0.6 | 1.6 ± 0.4 | 2 ± 1 |
| Sugar | r.t. (min) | SC–CO2 Treatments | ||||||
|---|---|---|---|---|---|---|---|---|
| 35 °C | 55 °C | |||||||
| Untreated | 8 MPa | 15 MPa | 21 MPa | 8 MPa | 15 MPa | 21 MPa | ||
| Glucose | 4.42 | 13.6 ± 0.4 | 14.1 ± 0.5 | 14.2 ± 0.2 | 14.0 ± 0.1 | 13.8 ± 0.3 | 14.4 ± 0.1 | 14.1 ± 0.3 |
| Fructose | 4.96 | 16.2 ± 0.5 | 17 ± 1 | 16.9 ± 0.1 | 16.4 ± 0.3 | 16.4 ± 0.4 | 17.0 ± 0.1 | 17.0 ± 0.3 |
| GF2 | 8.14 | 0.67 ± 0.02 | 0.68 ± 0.03 | 0.64 ± 0.01 | 0.65 ± 0.03 | 0.6 ± 0.1 | 0.64 ± 0.04 | 0.65 ± 0.04 |
| GF3 | 10.07 | 0.79 ± 0.02 | 0.78 ± 0.01 | 0.78 ± <0.01 | 0.77 ± 0.01 | 0.78 ± <0.01 | 0.77 ± 0.02 | 0.79 ± 0.01 |
| GF4 | 11.84 | 1.05 ± <0.01 | 1.05 ± 0.01 | 1.06 ± <0.01 | 1.04 ± 0.01 | 1.06 ± <0.01 | 1.05 ± 0.03 | 1.06 ± 0.02 |
| FOS | r.t. (min) | SC–CO2 Treatments | ||||||
|---|---|---|---|---|---|---|---|---|
| 35 °C | 55 °C | |||||||
| Untreated | 8 MPa | 15 MPa | 21 MPa | 8 MPa | 15 MPa | 21 MPa | ||
| GF2 | 8.14 | 15.9 ± 0.4 | 16 ± 1 | 15.2 ± 0.1 | 15 ± 1 | 15.2 ± 0.5 | 15.0 ± 0.1 | 15 ± 1 |
| Uk 1 | 8.96 | 5.34 ± 0.03 | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.4 ± 0.1 | 5.5 ± 0.2 | 5.5 ± 0.1 | 5.5 ± 0.2 |
| GF3 | 10.07 | 18.6 ± 0.4 | 18.2 ± 0.1 | 18.4 ± 0.1 | 18.1 ± 0.1 | 18.3 ± 0.1 | 18.1 ± 0.4 | 18.5 ± 0.4 |
| Uk 2 | 10.49 | 1.1 ± 0.1 | 1.10 ± 0.02 | 1.10 ± 0.03 | 1.09 ± 0.03 | 1.12 ± 0.02 | 1.08 ± 0.03 | 1.1 ± 0.1 |
| Uk 3 | 11.14 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.1 |
| GF4 | 11.84 | 21.6 ± 0.1 | 21.6 ± 0.2 | 21.9± 0.1 | 21.5 ± 0.2 | 21.8 ± 0.1 | 22 ± 1 | 21.9 ± 0.4 |
| Uk 4 | 13.14 | 2.37 ± 0.02 | 2.39 ± 0.02 | 2.41 ± 0.02 | 2.38 ± 0.03 | 2.45 ± 0.02 | 2.4 ± 0.1 | 2.5 ± 0.1 |
| GF5 | 13.42 | 20.8 ± 0.1 | 20.8 ± 0.1 | 21.1 ± 0.1 | 20.7 ± 0.1 | 21.0 ± 0.1 | 20.9 ± 0.4 | 21.1 ± 0.3 |
| GF6 | 14.86 | 21.9 ± 0.4 | 21.9 ± 0.3 | 22.3 ± 0.1 | 21.8 ± 0.2 | 22.2 ± 0.3 | 22.0 ± 0.5 | 22.3 ± 0.3 |
| Uk 5 | 15.12 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.6 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.2 | 1.6 ± 0.1 | 1.5 ± 0.1 |
| GF7 | 16.17 | 22.1 ± 0.2 | 21.9 ± 0.2 | 22.3 ± 0.1 | 21.9 ± 0.3 | 22.3 ± 0.1 | 22.2 ± 0.4 | 22.5 ± 0.4 |
| GF8 | 17.49 | 20.0 ± 0.1 | 19.9 ± 0.2 | 19.1 ± 0.1 | 19.8 ± 0.1 | 19.1 ± 0.1 | 20.0 ± 0.4 | 19.2 ± 0.3 |
| Uk 6 | 17.87 | 2.05 ± 0.02 | 2.05 ± 0.02 | 2.09 ± 0.01 | 2.05 ± 0.01 | 2.15 ± 0.04 | 2.1 ± 0.1 | 2.2 ± 0.1 |
| GF9 | 18.64 | 18.2 ± 0.2 | 18.1 ± 0.2 | 18.4 ± 0.1 | 18.1 ± 0.2 | 18.3 ± 0.1 | 18.2 ± 0.4 | 18.3 ± 0.2 |
| Uk 7 | 19.16 | 2.01 ± 0.02 | 2.01 ± 0.03 | 2.05 ± 0.01 | 2.01 ± <0.01 | 2.11 ± 0.03 | 2.07 ± 0.04 | 2.1 ± 0.1 |
| GF10 | 19.68 | 16.9 ± 0.2 | 16.9 ± 0.2 | 17.1 ± 0.1 | 16.8 ± 0.2 | 17.1 ± 0.1 | 17.0 ± 0.4 | 17.1 ± 0.1 |
| Uk 8 | 20.31 | 1.42 ± 0.03 | 1.39 ± 0.02 | 1.44 ± 0.01 | 1.40 ± 0.04 | 1.46 ± 0.02 | 1.46 ± 0.04 | 1.5 ± 0.1 |
| GF11 | 20.63 | 15.0 ± 0.3 | 14.9 ± 0.2 | 15.2 ± 0.3 | 14.9 ± 0.4 | 15.2 ± 0.1 | 15.2 ± 0.4 | 15.1 ± 0.1 |
| GF12 | 21.50 | 15.9 ± 0.3 | 15.9 ± 0.2 | 16.1 ± 0.2 | 15.9 ± 0.2 | 16.2 ± 0.2 | 16.1 ± 0.4 | 16.1 ± 0.1 |
| GF13 | 22.31 | 14.6 ± 0.3 | 14.7 ± 0.2 | 14.8 ± 0.3 | 14.7 ± 0.2 | 15.0 ± 0.1 | 14.9 ± 0.4 | 14.8 ± 0.2 |
| GF14 | 23.08 | 13.1 ± 0.3 | 13.2 ± 0.2 | 13.3 ± 0.3 | 13.2 ± 0.2 | 13.5 ± 0.2 | 13.4 ± 0.4 | 13.3 ± 0.1 |
| GF15 | 23.78 | 12.1 ± 0.3 | 12.2 ± 0.2 | 12.3 ± 0.3 | 12.3 ± 0.2 | 12.5 ± 0.2 | 12.4 ± 0.3 | 12.2 ± 0.1 |
| GF16 | 24.44 | 10.4 ± 0.4 | 10.6 ± 0.1 | 10.5 ± 0.3 | 10 ± 1 | 10.8 ± 0.1 | 10.8 ± 0.3 | 10.6 ± 0.1 |
| GF17 | 25.06 | 8.6 ± 0.3 | 8.4 ± 0.1 | 8.4 ± 0.3 | 8.5 ± 0.1 | 8.5 ± 0.1 | 8.5 ± 0.3 | 8.3 ± 0.2 |
| GF18 | 25.64 | 7.7 ± 0.3 | 7.9 ± 0.1 | 7.9 ± 0.3 | 7.9 ± 0.1 | 8.0 ± 0.2 | 8.0 ± 0.2 | 7.7 ± 0.2 |
| GF19 | 26.19 | 6.8 ± 0.3 | 7.0 ± 0.1 | 7.0 ± 0.3 | 7.0 ± 0.1 | 7.1 ± 0.2 | 7.1 ± 0.2 | 6.8 ± 0.2 |
| GF20 | 26.71 | 7.5 ± 0.3 | 7.7 ± 0.1 | 7.7 ± 0.3 | 7.7 ± 0.1 | 7.8 ± 0.2 | 7.7 ± 0.2 | 7.6 ± 0.2 |
| GF21 | 27.20 | 7.2 ± 0.4 | 7.3 ± 0.1 | 7.3 ± 0.3 | 7.4 ± 0.1 | 7.5 ± 0.2 | 7.5 ± 0.2 | 7.2 ± 0.2 |
| GF22 | 27.67 | 6.5 ± 0.3 | 6.7 ± 0.1 | 6.7 ± 0.3 | 6.8 ± 0.1 | 6.8 ± 0.2 | 6.9 ± 0.2 | 6.6 ± 0.2 |
| GF23 | 28.11 | 6.1 ± 0.3 | 6.3 ± 0.1 | 6.3 ± 0.3 | 6.3 ± 0.1 | 6.4 ± 0.2 | 6.4 ± 0.2 | 6.2 ± 0.2 |
| GF24 | 28.54 | 5.3 ± 0.3 | 5.5 ± 0.1 | 5.5 ± 0.3 | 5.5 ± 0.1 | 5.6 ± 0.2 | 5.6 ± 0.2 | 5.4 ± 0.2 |
| GF25 | 28.95 | 4.9 ± 0.3 | 5.1 ± 0.1 | 5.1 ± 0.3 | 5.2 ± 0.1 | 5.2 ± 0.1 | 5.2 ± 0.2 | 4.9 ± 0.2 |
| GF26 | 29.33 | 4.5 ± 0.3 | 4.7 ± 0.1 | 4.7 ± 0.3 | 4.7 ± 0.1 | 4.8 ± 0.2 | 4.8 ± 0.2 | 4.6 ± 0.2 |
| GF27 | 29.70 | 3.9 ± 0.3 | 4.1 ± 0.1 | 4.0 ± 0.2 | 4.1 ± 0.1 | 4.1 ± 0.1 | 4.2 ± 0.2 | 3.9 ± 0.2 |
| GF28 | 30.05 | 3.3 ± 0.2 | 3.4 ± 0.1 | 3.4 ± 0.2 | 3.4 ± 0.1 | 3.4 ± 0.1 | 3.5 ± 0.1 | 3.3 ± 0.2 |
| GF29 | 30.39 | 2.6 ± 0.2 | 2.8 ± 0.1 | 2.7 ± 0.2 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.8 ± 0.1 | 2.6 ± 0.2 |
| GF30 | 30.72 | 2.1 ± 0.2 | 2.27 ± 0.03 | 2.2 ± 0.2 | 2.30 ± 0.04 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.1 ± 0.2 |
| GF31 | 31.03 | 1.8 ± 0.2 | 1.94 ± 0.03 | 1.9 ± 0.2 | 1.95 ± 0.02 | 1.9 ± 0.1 | 2.0 ± 0.1 | 1.8 ± 0.2 |
| GF32 | 31.34 | 1.7 ± 0.2 | 1.77 ± 0.02 | 1.7 ± 0.2 | 1.79 ± 0.01 | 1.8 ± 0.1 | 1.8 ± 0.1 | 1.6 ± 0.2 |
| GF33 | 31.63 | 1.5 ± 0.2 | 1.65 ± 0.03 | 1.6 ± 0.2 | 1.67 ± 0.01 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.5 ± 0.2 |
| GF34 | 31.91 | 1.4 ± 0.2 | 1.53 ± 0.01 | 1.5 ± 0.2 | 1.55 ± 0.01 | 1.5 ± 0.1 | 1.6 ± 0.1 | 1.4 ± 0.1 |
| GF35 | 32.19 | 1.3 ± 0.2 | 1.40 ± 0.02 | 1.4 ± 0.1 | 1.42 ± 0.01 | 1.4 ± 0.1 | 1.5 ± 0.1 | 1.3 ± 0.1 |
| GF36 | 32.45 | 1.2 ± 0.2 | 1.27 ± 0.02 | 1.2 ± 0.1 | 1.29 ± 0.01 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 |
| GF37 | 32.71 | 1.0 ± 0.2 | 1.14 ± 0.01 | 1.1 ± 0.1 | 1.15 ± 0.01 | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.0 ± 0.1 |
| GF38 | 32.96 | 0.9 ± 0.2 | 1.00 ± 0.01 | 1.0 ± 0.1 | 1.02 ± <0.01 | 1.0 ± 0.1 | 1.05 ± 0.04 | 0.9 ± 0.1 |
| GF39 | 33.20 | 0.8 ± 0.1 | 0.87 ± 0.01 | 0.9 ± 0.1 | 0.89 ± <0.01 | 0.9 ± 0.1 | 0.91 ± 0.04 | 0.8 ± 0.1 |
| GF40 | 33.44 | 0.7 ± 0.1 | 0.75 ± 0.01 | 0.7 ± 0.1 | 0.77 ± <0.01 | 0.8 ± 0.1 | 0.79 ± 0.03 | 0.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arruda, H.S.; Silva, E.K.; Pastore, G.M.; Marostica Junior, M.R. Non-Thermal Supercritical Carbon Dioxide Processing Retains the Quality Parameters and Improves the Kinetic Stability of an Araticum Beverage Enriched with Inulin-Type Dietary Fibers. Foods 2023, 12, 2595. https://doi.org/10.3390/foods12132595
Arruda HS, Silva EK, Pastore GM, Marostica Junior MR. Non-Thermal Supercritical Carbon Dioxide Processing Retains the Quality Parameters and Improves the Kinetic Stability of an Araticum Beverage Enriched with Inulin-Type Dietary Fibers. Foods. 2023; 12(13):2595. https://doi.org/10.3390/foods12132595
Chicago/Turabian StyleArruda, Henrique Silvano, Eric Keven Silva, Glaucia Maria Pastore, and Mario Roberto Marostica Junior. 2023. "Non-Thermal Supercritical Carbon Dioxide Processing Retains the Quality Parameters and Improves the Kinetic Stability of an Araticum Beverage Enriched with Inulin-Type Dietary Fibers" Foods 12, no. 13: 2595. https://doi.org/10.3390/foods12132595










