Inhibitory Effects of Chlorogenic Acid Containing Green Coffee Bean Extract on Lipopolysaccharide-Induced Inflammatory Responses and Progression of Colon Cancer Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Extraction
2.2. Chemical Analysis
2.2.1. Total Phenolic Acid Content
2.2.2. Total Flavonoid Content
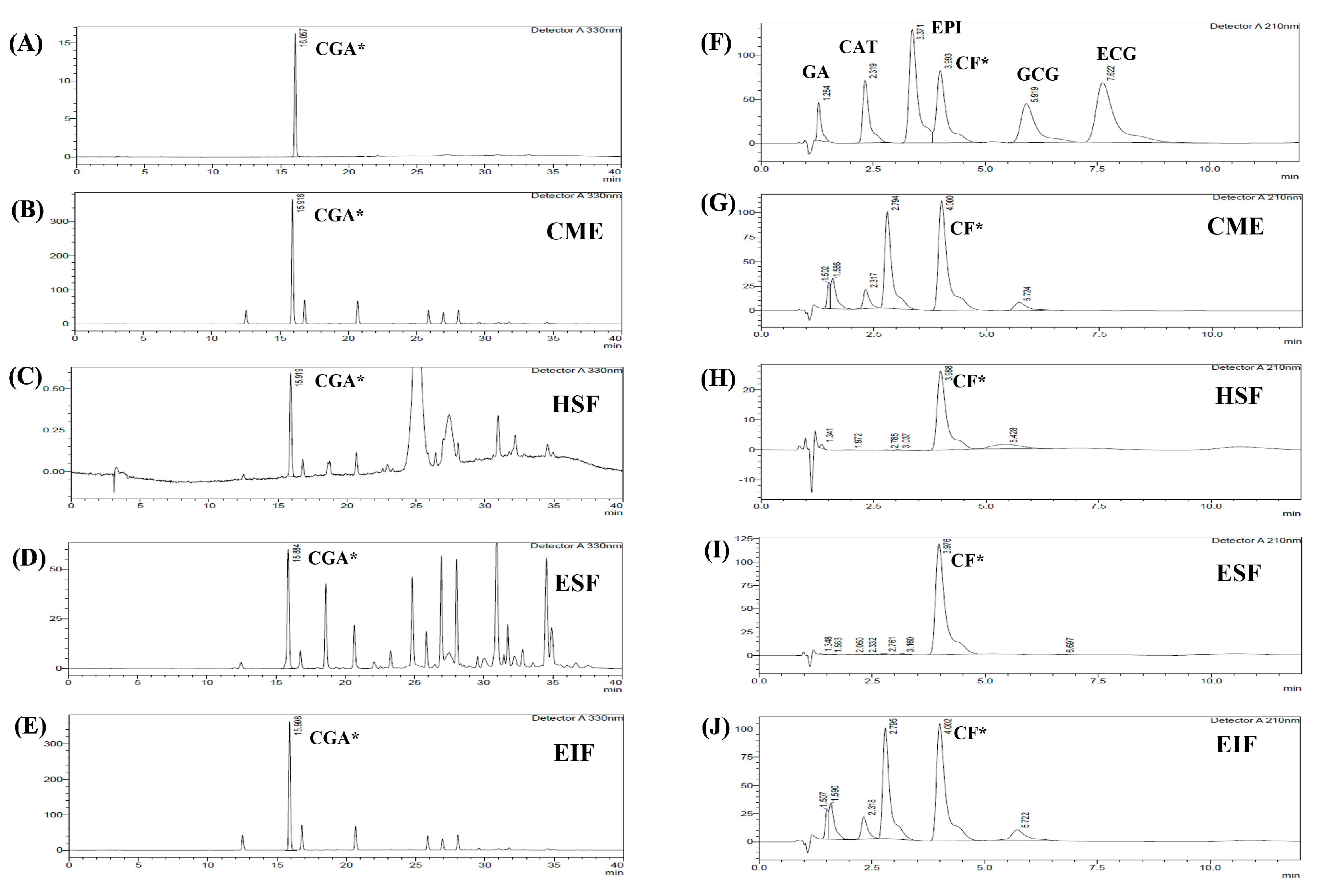
2.2.3. High Performance Liquid Chromatography
2.3. Antioxidant Assays
2.3.1. DPPH Scavenging Assay
2.3.2. Iron Chelation Assay
2.4. Cell Culture
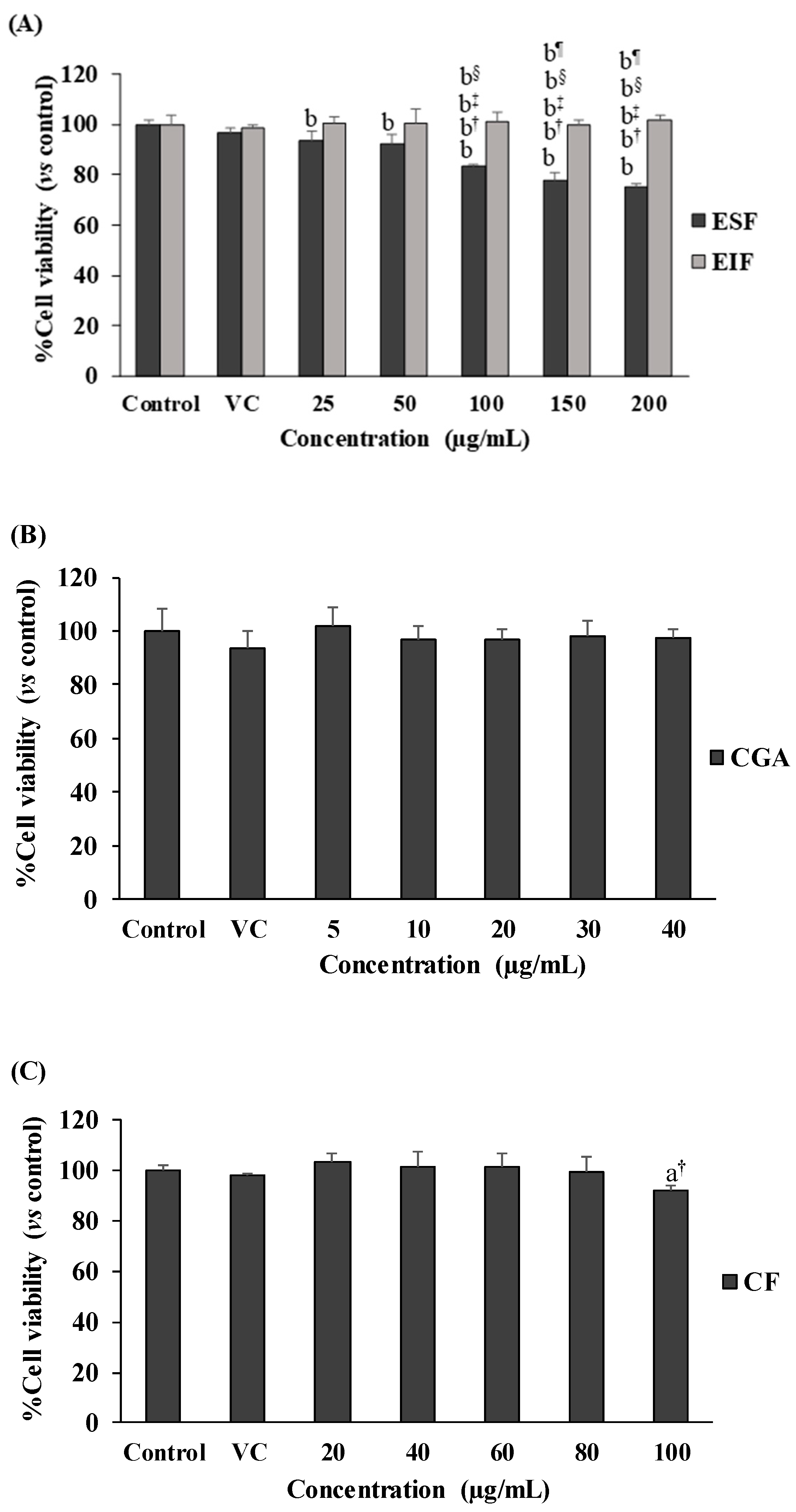
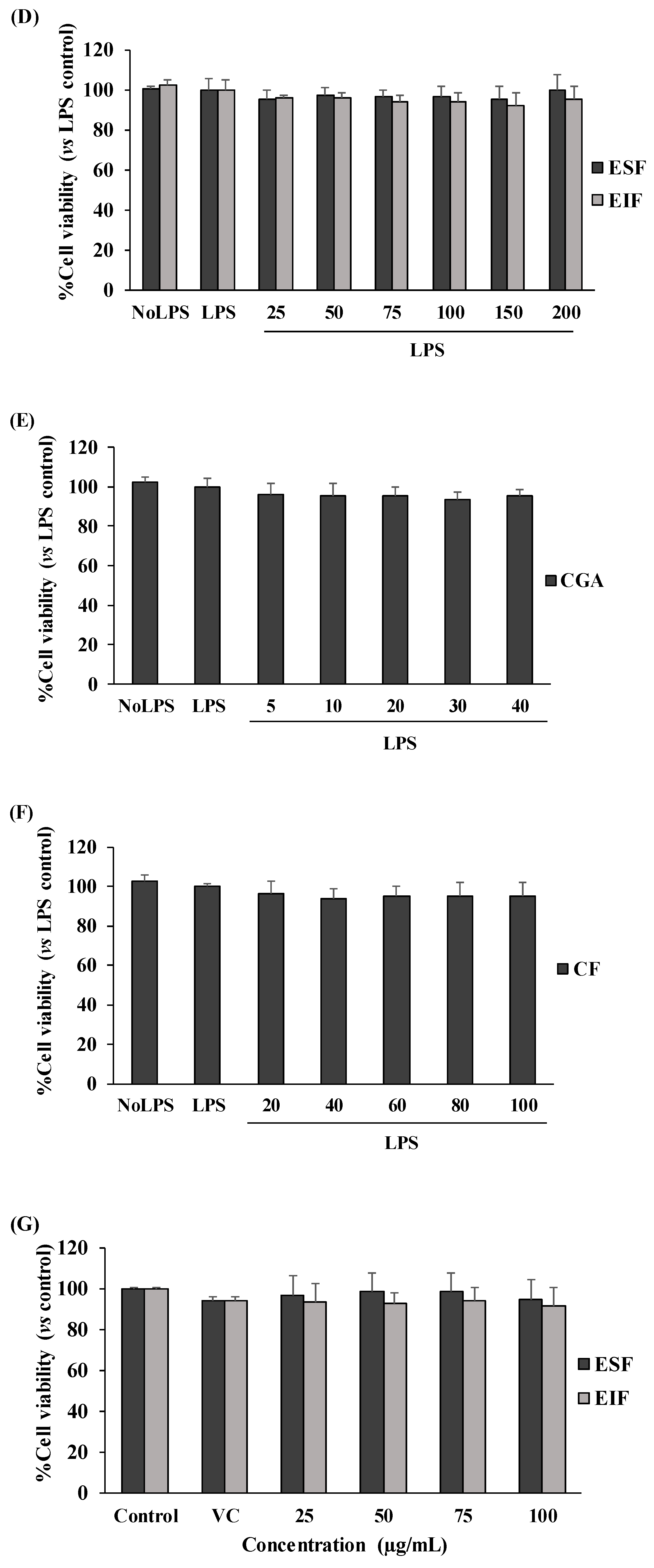
2.5. Cell Viability Assay
2.6. Quantitation of Secretory Proteins
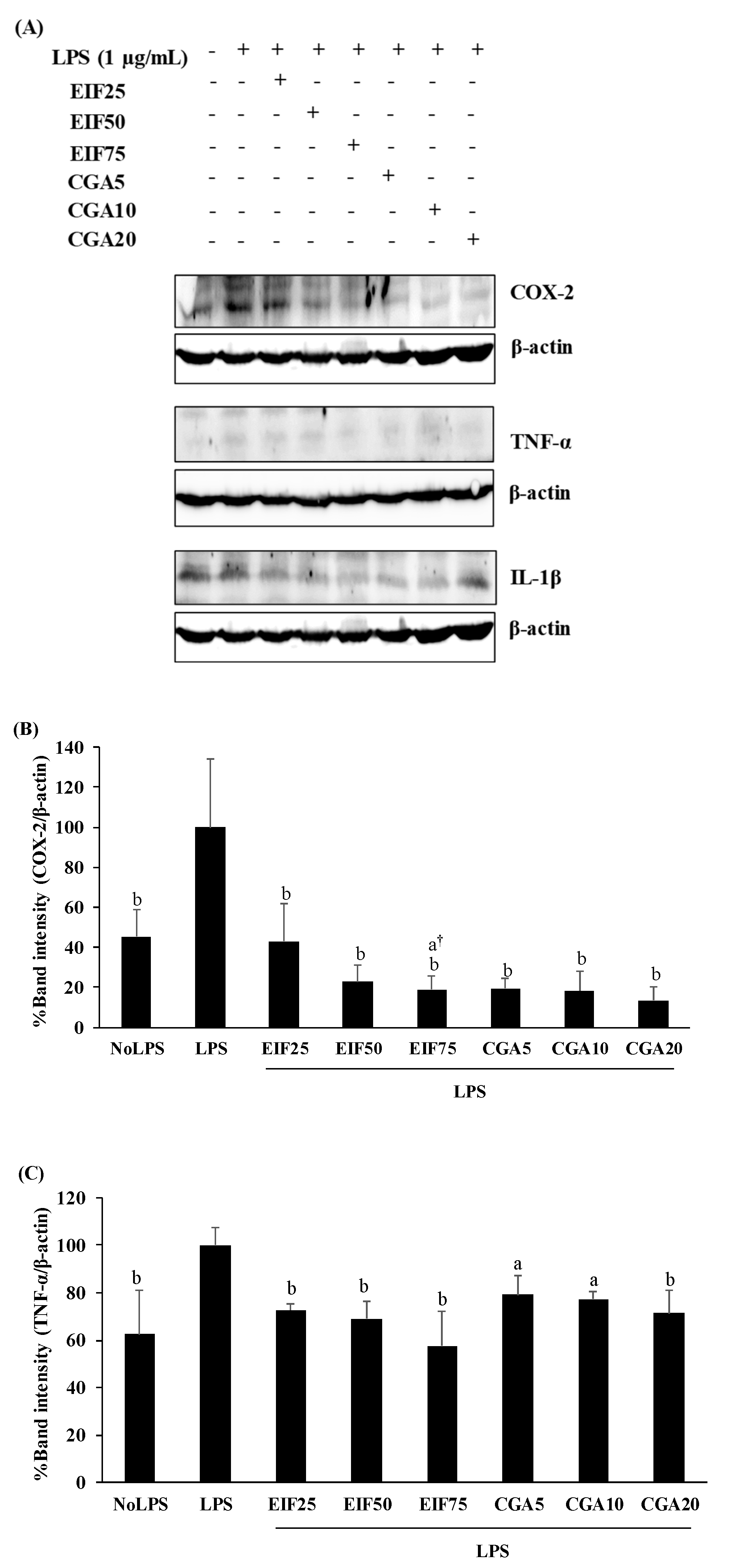
2.7. Immunodetection of Intracellular Proteins
2.8. Cell Adhesion Assay
2.9. Cell Migration Assay
2.9.1. Wound Healing Assay
2.9.2. Transwell Migration Assay
2.10. Cell Invasion Assay
2.11. Statistical Analysis
3. Results
3.1. Chemical Contents and Antioxidant Activities
3.2. Effect of EIF, ESF, CGA, and CF on Colon Cancer Cell Viability under w/wo LPS Stimulation
3.3. Effect of EIF, ESF, CGA, and CF on TLR4 Expression of LPS—Induced Colon Cancer Cells
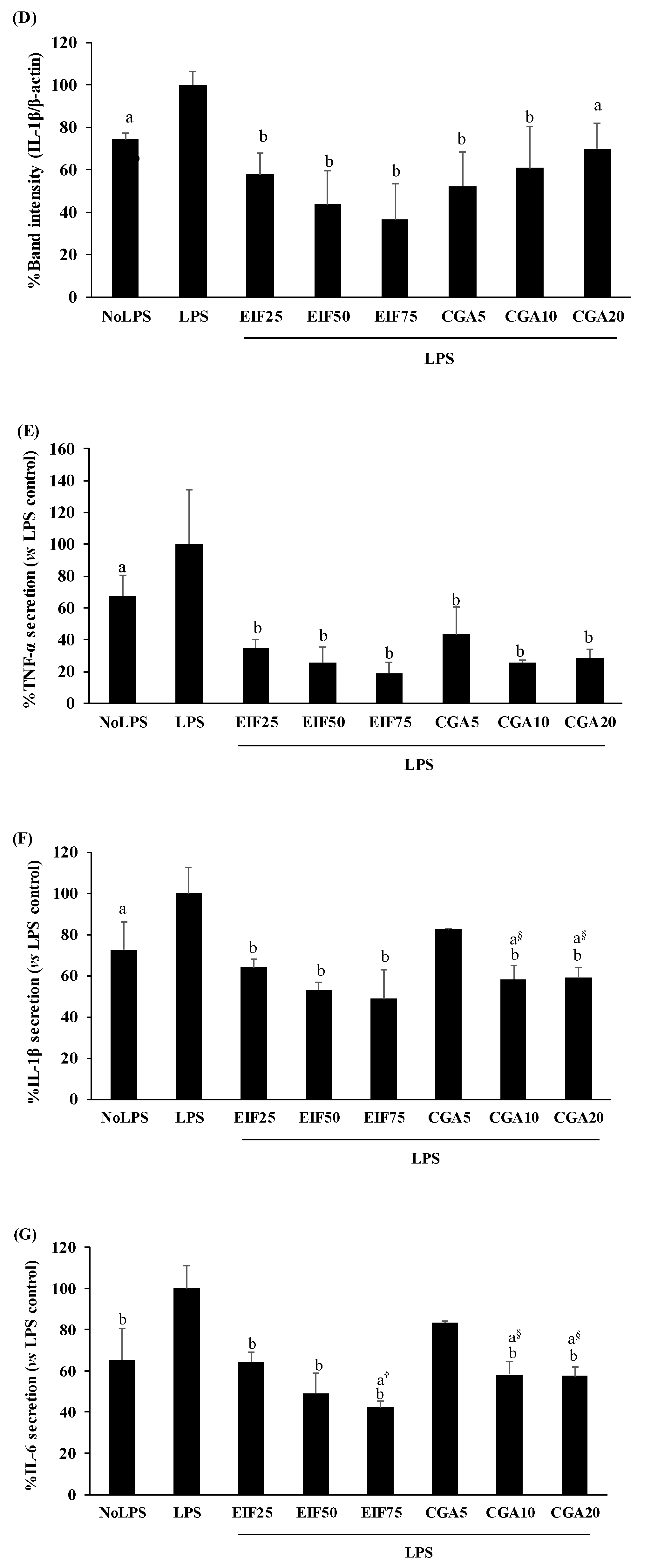
3.4. Effect of EIF and CGA on the Expression and Secretion of Inflammatory Mediators of LPS-Induced Colon Cancer Cells
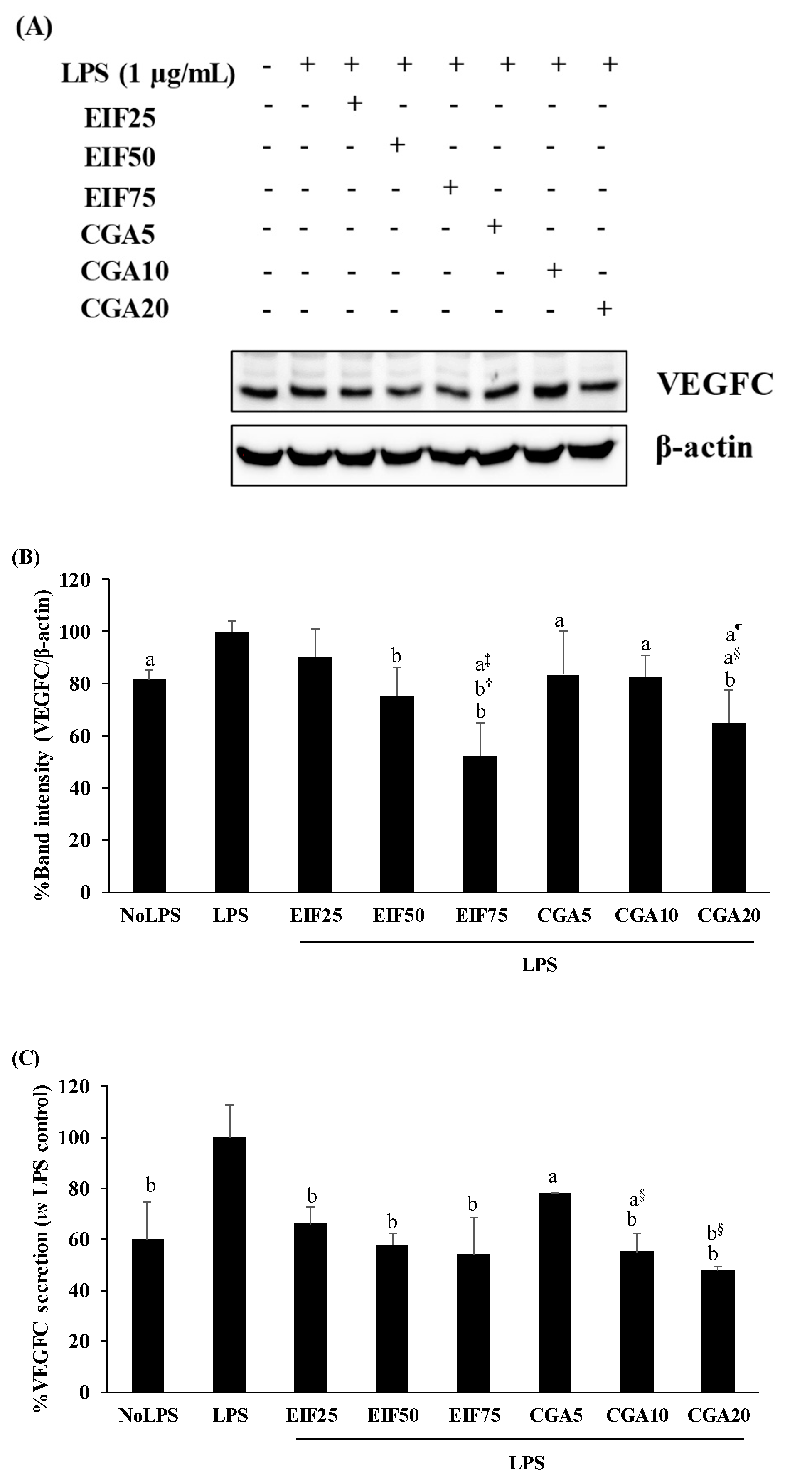
3.5. Effect of EIF and CGA on the Expression and Secretion of VEGFC of LPS-Induced Colon Cancer Cells
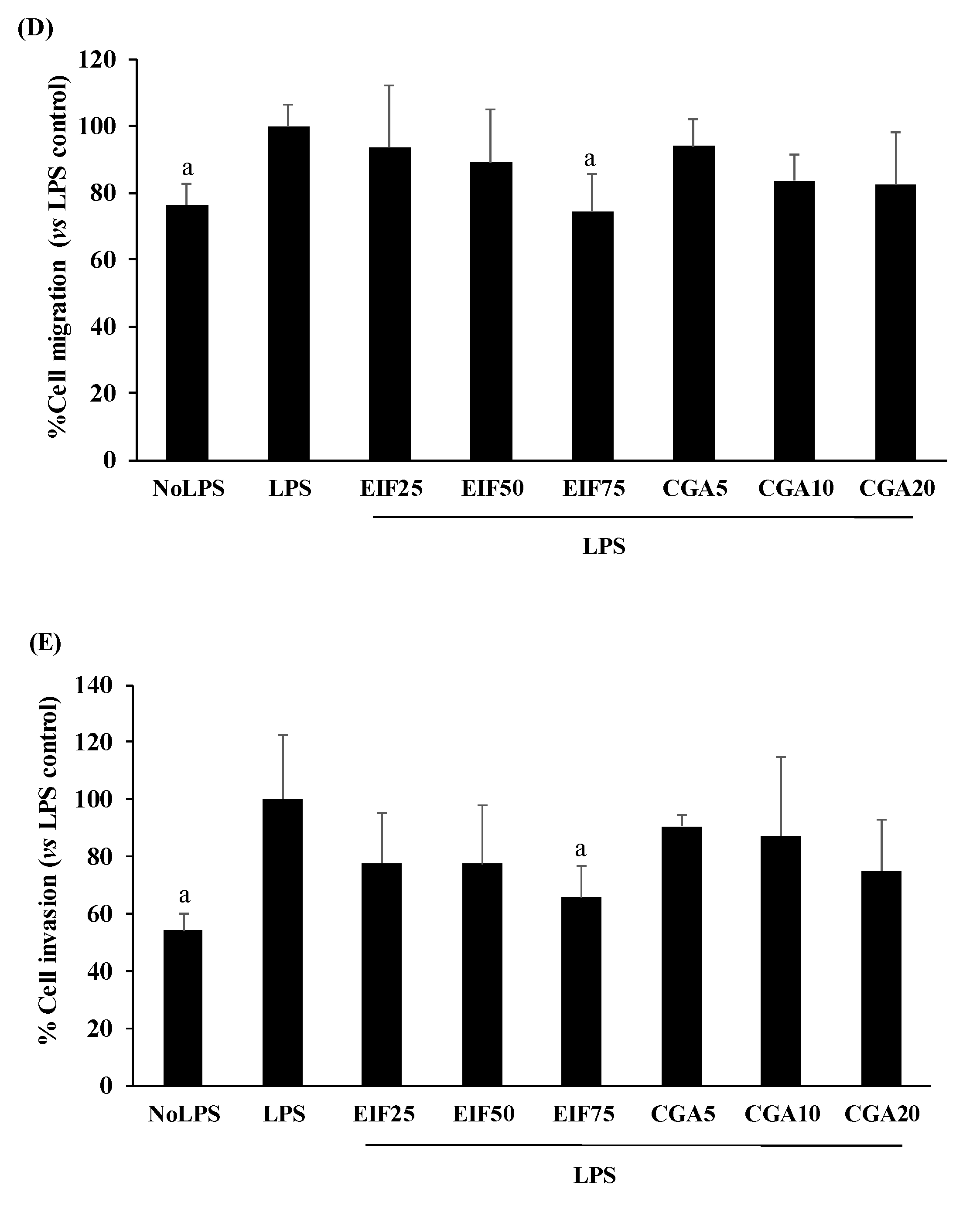
3.6. Effect of EIF and CGA on Adhesion, Migration, and Invasion Ability of LPS-Induced Colon Cancer Cells
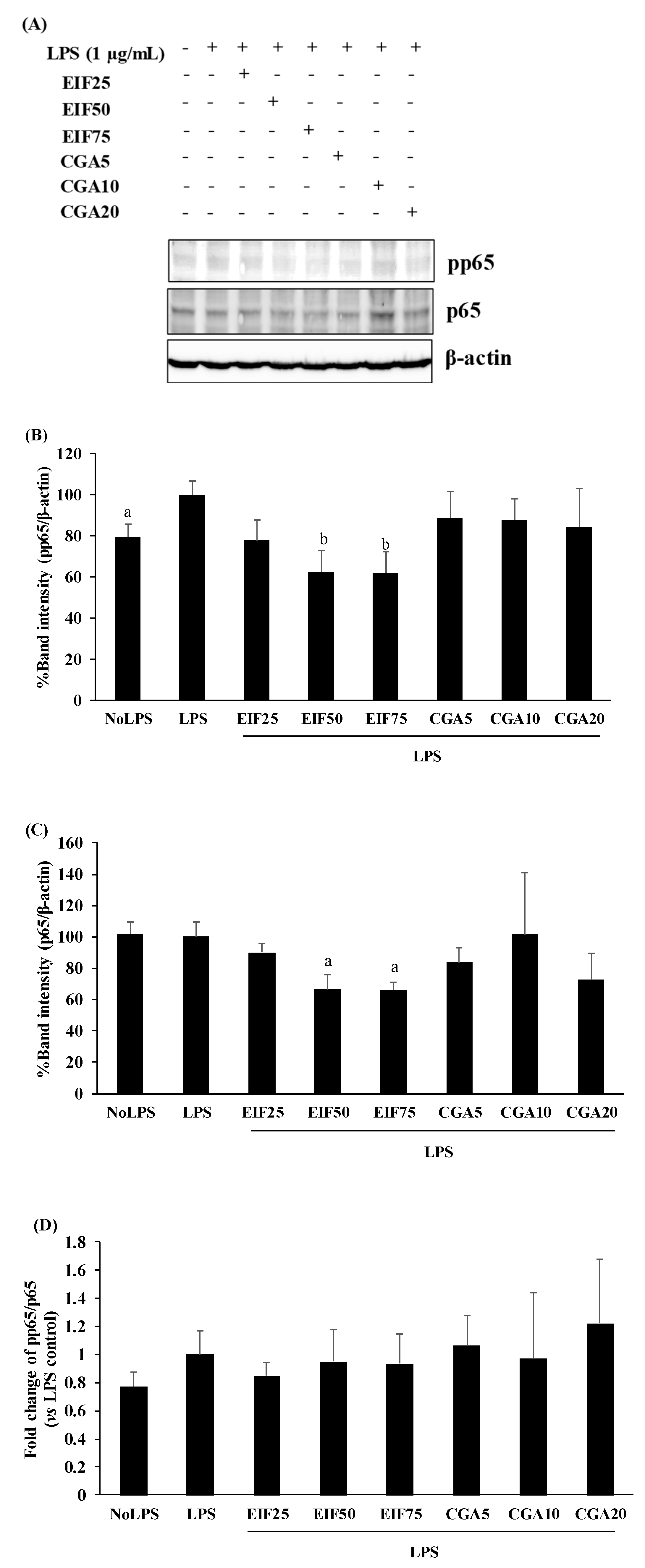
3.7. Effect of EIF and CGA on NF-κB Activation of LPS-Induced Colon Cancer Cells
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McSorley, S.T.; Watt, D.G.; Horgan, P.G.; McMillan, D.C. Postoperative Systemic Inflammatory Response, Complication Severity, and Survival Following Surgery for Colorectal Cancer. Ann. Surg. Oncol. 2016, 23, 2832–2840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artinyan, A.; Orcutt, S.T.; Anaya, D.A.; Richardson, P.; Chen, G.J.; Berger, D.H. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann. Surg. 2015, 261, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Sun, J. Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin. Med. J. 2022, 135, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Seely, K.D.; Morgan, A.D.; Hagenstein, L.D.; Florey, G.M.; Small, J.M. Bacterial Involvement in Progression and Metastasis of Colorectal Neoplasia. Cancers 2022, 14, 1019. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, X.; Jiang, M.; Bi, Y.; Xu, J.; Han, M. Lipopolysaccharide induces inflammation and facilitates lung metastasis in a breast cancer model via the prostaglandin E2-EP2 pathway. Mol. Med. Rep. 2015, 11, 4454–4462. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; von Ehrlich-Treuenstatt, V.; Schardey, J.; Wirth, U.; Zimmermann, P.; Andrassy, J.; Bazhin, A.V.; Werner, J.; Kuhn, F. Gut Barrier Dysfunction and Bacterial Lipopolysaccharides in Colorectal Cancer. J. Gastrointest. Surg. 2023. [Google Scholar] [CrossRef]
- Guo, J.; Liao, M.; Wang, J. TLR4 signaling in the development of colitis-associated cancer and its possible interplay with microRNA-155. Cell Commun. Signal. 2021, 19, 90. [Google Scholar] [CrossRef]
- Tan, Y.; Zou, K.F.; Qian, W.; Chen, S.; Hou, X.H. Expression and implication of toll-like receptors TLR2, TLR4 and TLR9 in colonic mucosa of patients with ulcerative colitis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2014, 34, 785–790. [Google Scholar] [CrossRef]
- Yesudhas, D.; Gosu, V.; Anwar, M.A.; Choi, S. Multiple roles of toll-like receptor 4 in colorectal cancer. Front. Immunol. 2014, 5, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Y.; Qin, F.; Chen, J.; Meng, J.; Wei, L.; Wu, C.; Zhang, Q.; Wei, D.; Chen, X.; Wu, H.; et al. sTLR4/MD-2 complex inhibits colorectal cancer in vitro and in vivo by targeting LPS. Oncotarget 2016, 7, 52032–52044. [Google Scholar] [CrossRef] [Green Version]
- Mishra, V.; Pathak, C. Human Toll-Like Receptor 4 (hTLR4): Structural and functional dynamics in cancer. Int. J. Biol. Macromol. 2019, 122, 425–451. [Google Scholar] [CrossRef]
- Zhu, G.; Huang, Q.; Huang, Y.; Zheng, W.; Hua, J.; Yang, S.; Zhuang, J.; Wang, J.; Ye, J. Lipopolysaccharide increases the release of VEGF-C that enhances cell motility and promotes lymphangiogenesis and lymphatic metastasis through the TLR4- NF-kappaB/JNK pathways in colorectal cancer. Oncotarget 2016, 7, 73711–73724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajamanickam, V.; Yan, T.; Xu, S.; Hui, J.; Xu, X.; Ren, L.; Liu, Z.; Liang, G.; Wang, O.; Wang, Y. Selective targeting of the TLR4 co-receptor, MD2, prevents colon cancer growth and lung metastasis. Int. J. Biol. Sci. 2020, 16, 1288–1302. [Google Scholar] [CrossRef]
- Khromova, N.; Kopnin, P.; Rybko, V.; Kopnin, B.P. Downregulation of VEGF-C expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene 2012, 31, 1389–1397. [Google Scholar] [CrossRef] [Green Version]
- Tacconi, C.; Correale, C.; Gandelli, A.; Spinelli, A.; Dejana, E.; D’Alessio, S.; Danese, S. Vascular endothelial growth factor C disrupts the endothelial lymphatic barrier to promote colorectal cancer invasion. Gastroenterology 2015, 148, 1438–1451.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Ouyang, Y.; Lu, N.; Li, N. The NF-kappaB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front. Immunol. 2020, 11, 1387. [Google Scholar] [CrossRef]
- Friis, S.; Riis, A.H.; Erichsen, R.; Baron, J.A.; Sorensen, H.T. Low-Dose Aspirin or Nonsteroidal Anti-inflammatory Drug Use and Colorectal Cancer Risk: A Population-Based, Case-Control Study. Ann. Intern. Med. 2015, 163, 347–355. [Google Scholar] [CrossRef]
- Veettil, S.K.; Nathisuwan, S.; Ching, S.M.; Jinatongthai, P.; Lim, K.G.; Kew, S.T.; Chaiyakunapruk, N. Efficacy and safety of celecoxib on the incidence of recurrent colorectal adenomas: A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Panyathep, A.; Punturee, K.; Chewonarin, T. Gamma-Oryzanol-Rich Fraction from Purple Rice Extract Attenuates Lipopolysaccharide-Stimulated Inflammatory Responses, Migration and VEGFA Production in SW480 Cells via Modulation of TLR4 and NF-kappaB Pathways. Nutr. Cancer 2022, 74, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Yepes, Y.; Uribe, D.; Röthlisberger, S. A review of the chemopreventive effects of the main bioactive compounds in coffee in colorectal cancer. J. Appl. Pharm. Sci. 2021, 11, 046–054. [Google Scholar] [CrossRef]
- Canci, L.A.; Benassi, M.T.; Canan, C.; Kalschne, D.L.; Colla, E. Antimicrobial potential of aqueous coffee extracts against pathogens and Lactobacillus species: A food matrix application. Food Biosci. 2022, 47, 101756. [Google Scholar] [CrossRef]
- Bharath, N.; Sowmya, N.K.; Mehta, D.S. Determination of antibacterial activity of green coffee bean extract on periodontogenic bacteria like Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum and Aggregatibacter actinomycetemcomitans: An in vitro study. Contemp. Clin. Dent. 2015, 6, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.L.; de Paula, J.; Mellinger Silva, C.; Cruz, A.; Lemos Miguel, M.A.; Farah, A. Effects of regular and decaffeinated roasted coffee (Coffea arabica and Coffea canephora) extracts and bioactive compounds on in vitro probiotic bacterial growth. Food Funct. 2020, 11, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Murai, T.; Matsuda, S. The Chemopreventive Effects of Chlorogenic Acids, Phenolic Compounds in Coffee, against Inflammation, Cancer, and Neurological Diseases. Molecules 2023, 28, 2381. [Google Scholar] [CrossRef]
- Panyathep, A.; Chewonarin, T.; Taneyhill, K.; Vinitketkumnuen, U. Antioxidant and anti-matrix metalloproteinases activities of dried longan (Euphoria longana) seed extract. ScienceAsia 2013, 39, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Panyathep, A.; Chewonarin, T. Inhibitory effect of a gamma-oryzanol-rich fraction from purple rice extract on lipopolysaccharide-induced metastasis in human colon cancer cells. J. Food Biochem. 2020, 44, e13487. [Google Scholar] [CrossRef]
- Panyathep, A.; Punturee, K.; Chewonarin, T. Effect of gamma oryzanol-rich fraction from purple rice extract against lipopolysaccharide-induced vascular endothelial growth factor C production of human colon cancer cells and angiogenesis of human umbilical vein endothelial cells. Agric. Nat. Resour. 2022, 56, 557–568. [Google Scholar] [CrossRef]
- Ramalakshmi, K.; Raghavan, B. Caffeine in coffee: Its removal. Why and how? Crit. Rev. Food Sci. Nutr. 1999, 39, 441–456. [Google Scholar] [CrossRef]
- Pietsch, A. Decaffeination-Process and Quality. In The Craft and Science of Coffee; Elsevier: Amsterdam, The Netherlands; Boston, MA, USA, 2017; pp. 225–243. [Google Scholar]
- Rakhesh, M.; Cate, M.; Vijay, R.; Shrikant, A.; Shanjana, A. A TLR4-interacting peptide inhibits lipopolysaccharide-stimulated inflammatory responses, migration and invasion of colon cancer SW480 cells. Oncoimmunology 2012, 1, 1495–1506. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, D.P.; Bhatt, L.; Woolley, J.F.; Gough, D.R.; Wang, J.H.; Cotter, T.G.; Redmond, H.P. TLR-4 signalling accelerates colon cancer cell adhesion via NF-kappaB mediated transcriptional up-regulation of Nox-1. PLoS ONE 2012, 7, e44176. [Google Scholar] [CrossRef] [Green Version]
- Martins, S.F.; Garcia, E.A.; Luz, M.A.; Pardal, F.; Rodrigues, M.; Filho, A.L. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genom. Proteom. 2013, 10, 55–67. [Google Scholar]
- Ye, J.; Wu, X.; Wu, D.; Wu, P.; Ni, C.; Zhang, Z.; Chen, Z.; Qiu, F.; Xu, J.; Huang, J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS ONE 2013, 8, e60687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, T.; Shayan, R.; Fox, S.; Achen, M.G.; Stacker, S.A. The connection between lymphangiogenic signalling and prostaglandin biology: A missing link in the metastatic pathway. Oncotarget 2012, 3, 893–906. [Google Scholar] [CrossRef] [Green Version]
- Soumaoro, L.T.; Uetake, H.; Takagi, Y.; Iida, S.; Higuchi, T.; Yasuno, M.; Enomoto, M.; Sugihara, K. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis. Colon Rectum 2006, 49, 392–398. [Google Scholar] [CrossRef]
- Liu, H.; Yang, Y.; Xiao, J.; Lv, Y.; Liu, Y.; Yang, H.; Zhao, L. COX-2-mediated regulation of VEGF-C in association with lymphangiogenesis and lymph node metastasis in lung cancer. Anat. Rec. 2010, 293, 1838–1846. [Google Scholar] [CrossRef]




| Green Coffee Bean Extract (GBE) | % Yield (vs. 50 g of Dry GBE) |
|---|---|
| Crude methanol extract (CME) | 23.92 |
| Hexane soluble fraction (HSF) | 0.08 |
| Ethyl acetate soluble fraction (ESF) | 0.18 |
| Ethyl acetate insoluble fraction (EIF) | 17.74 |
| GBE | TPC (mg GAE/g Dry Weight) | TFC (mg CAE/g Dry Weight) | DPPH Scavenging (SC50) | Iron Chelation (EC50) |
|---|---|---|---|---|
| CME | 358.88 ± 17.09 | 232.58 ± 6.83 | - | - |
| HSF | 13.48 ± 2.26 | 24.57 ± 1.77 | - | - |
| ESF | 298.72 ± 7.82 | 172.61 ± 5.16 | 35.73 ± 2.17 | 146.00 ± 16.52 |
| EIF | 303.02 ± 1.87 | 201.25 ± 4.93 | 41.24 ± 5.81 | 106.67 ± 2.89 |
| GBE | Content (mg/100 g Extract) | ||||||
|---|---|---|---|---|---|---|---|
| Chlorogenic Acid (CGA)* | Gallic Acid (GA) | Catechin Gallate (CAT) | Epicatechin (EPI) | Caffeine (CF)* | Gallocatechin Gallate (GCG) | Epicatechin Gallate (ECG) | |
| CME | 17,768.36 ± 14.29 | UD | 132.60 ± 0.38 | UD | 7033.88 ± 60.34 | 93.25 ± 1.61 | UD |
| HSF | 41.10 ± 0.32 | 2.15 ± 0.21 | UD | UD | 240.69 ± 0.91 | UD | UD |
| ESF | 2845.18 ± 9.31 | 98.02 ± 4.62 | UD | UD | 61,043.30 ± 486.96 | UD | UD |
| EIF | 17,683.78 ± 19.51 | UD | 133.35 ± 0.64 | UD | 6589.86 ± 10.18 | 105.99 ± 2.69 | UD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panyathep, A.; Punturee, K.; Chewonarin, T. Inhibitory Effects of Chlorogenic Acid Containing Green Coffee Bean Extract on Lipopolysaccharide-Induced Inflammatory Responses and Progression of Colon Cancer Cell Line. Foods 2023, 12, 2648. https://doi.org/10.3390/foods12142648
Panyathep A, Punturee K, Chewonarin T. Inhibitory Effects of Chlorogenic Acid Containing Green Coffee Bean Extract on Lipopolysaccharide-Induced Inflammatory Responses and Progression of Colon Cancer Cell Line. Foods. 2023; 12(14):2648. https://doi.org/10.3390/foods12142648
Chicago/Turabian StylePanyathep, Atita, Khanittha Punturee, and Teera Chewonarin. 2023. "Inhibitory Effects of Chlorogenic Acid Containing Green Coffee Bean Extract on Lipopolysaccharide-Induced Inflammatory Responses and Progression of Colon Cancer Cell Line" Foods 12, no. 14: 2648. https://doi.org/10.3390/foods12142648
APA StylePanyathep, A., Punturee, K., & Chewonarin, T. (2023). Inhibitory Effects of Chlorogenic Acid Containing Green Coffee Bean Extract on Lipopolysaccharide-Induced Inflammatory Responses and Progression of Colon Cancer Cell Line. Foods, 12(14), 2648. https://doi.org/10.3390/foods12142648






