Extracts of Dunkelfelder Grape Seeds and Peel Increase the Metabolic Rate and Reduce Fat Deposition in Mice Maintained on a High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sample Preparation
2.2. Phenolic Compound Analyses
2.2.1. Spectrophotometric Characterization
2.2.2. High-Performance Liquid Chromatography (HPLC) Analyses of the GSE Phenolic Profiles
2.2.3. HPLC Analyses of the GPE Anthocyanin Profiles
2.3. Animals and Diets
2.4. Energy Intake and Food Efficiency Ratio
2.5. Metabolic Chamber Analyses
2.6. Serum Profile Analysis
2.7. Histology Analysis
2.8. RNA Isolation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.9. Statistical Analysis
3. Results
3.1. Phenolic Compounds in GSE and GPE
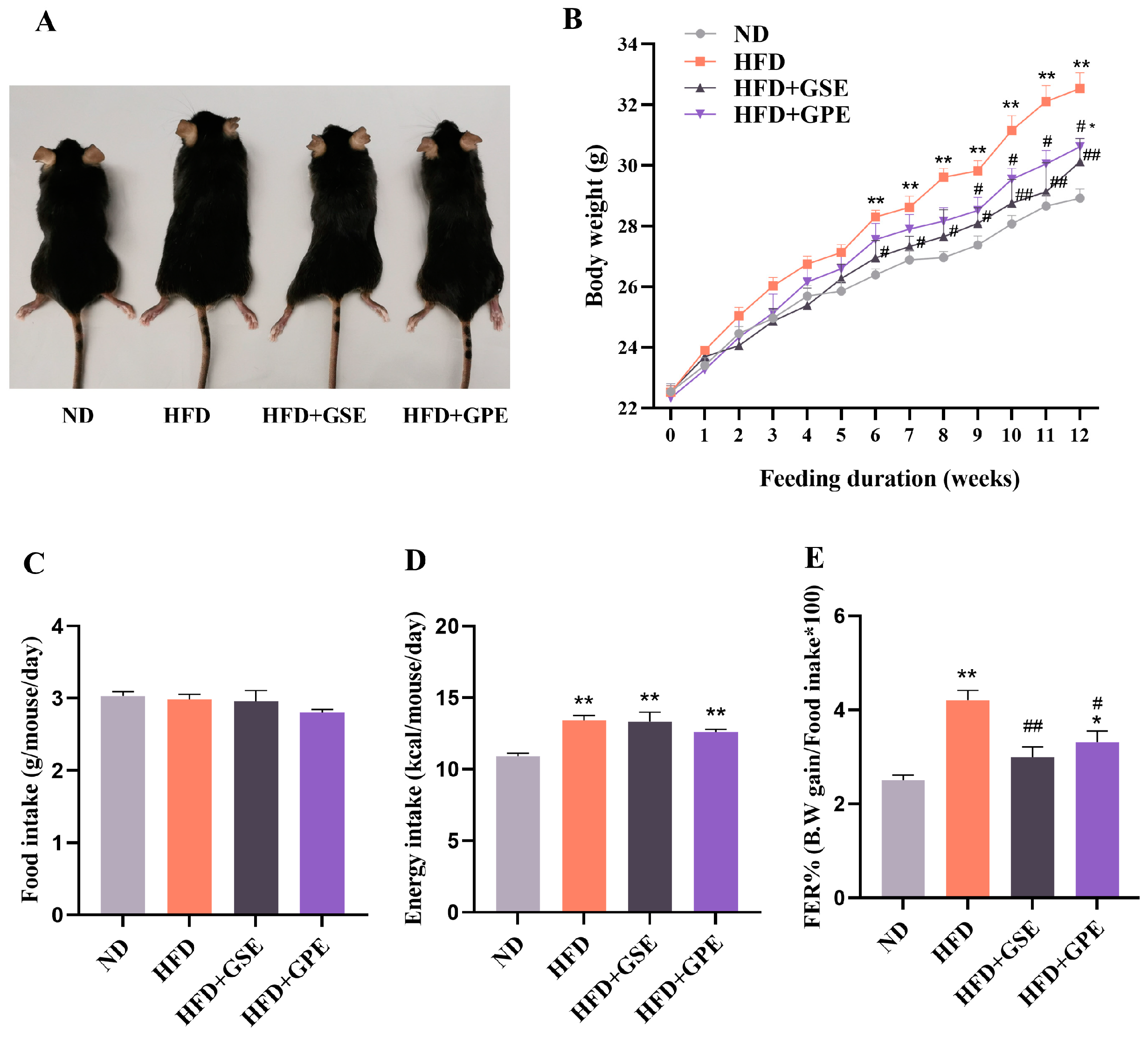
3.2. GSE and GPE Reduced Body Weight and Normalized Blood Constituents in HFD-Fed Mice
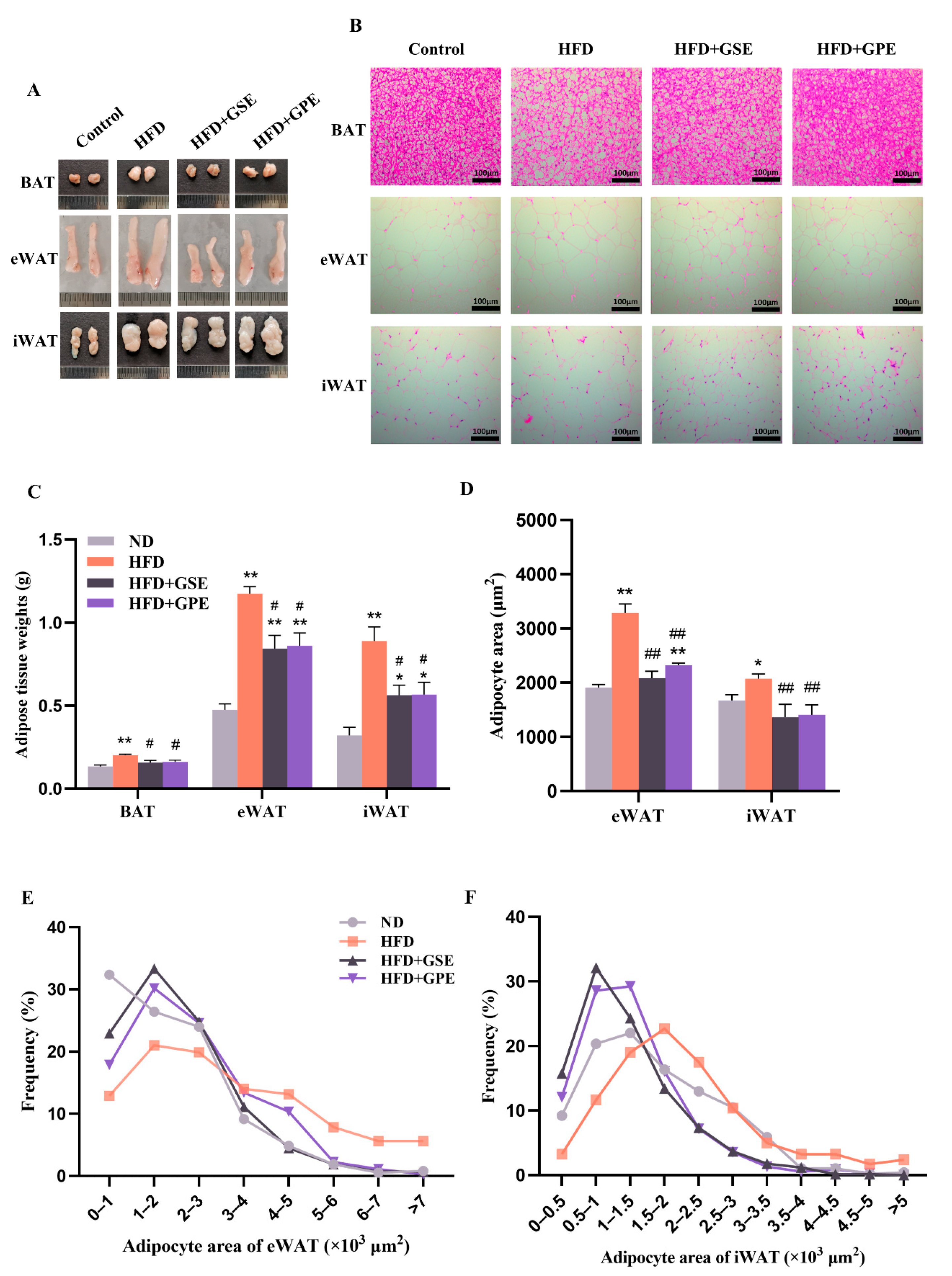
3.3. GSE and GPE Reduced Adipose Tissue Weight and Adipocyte Size in HFD-Fed Mice
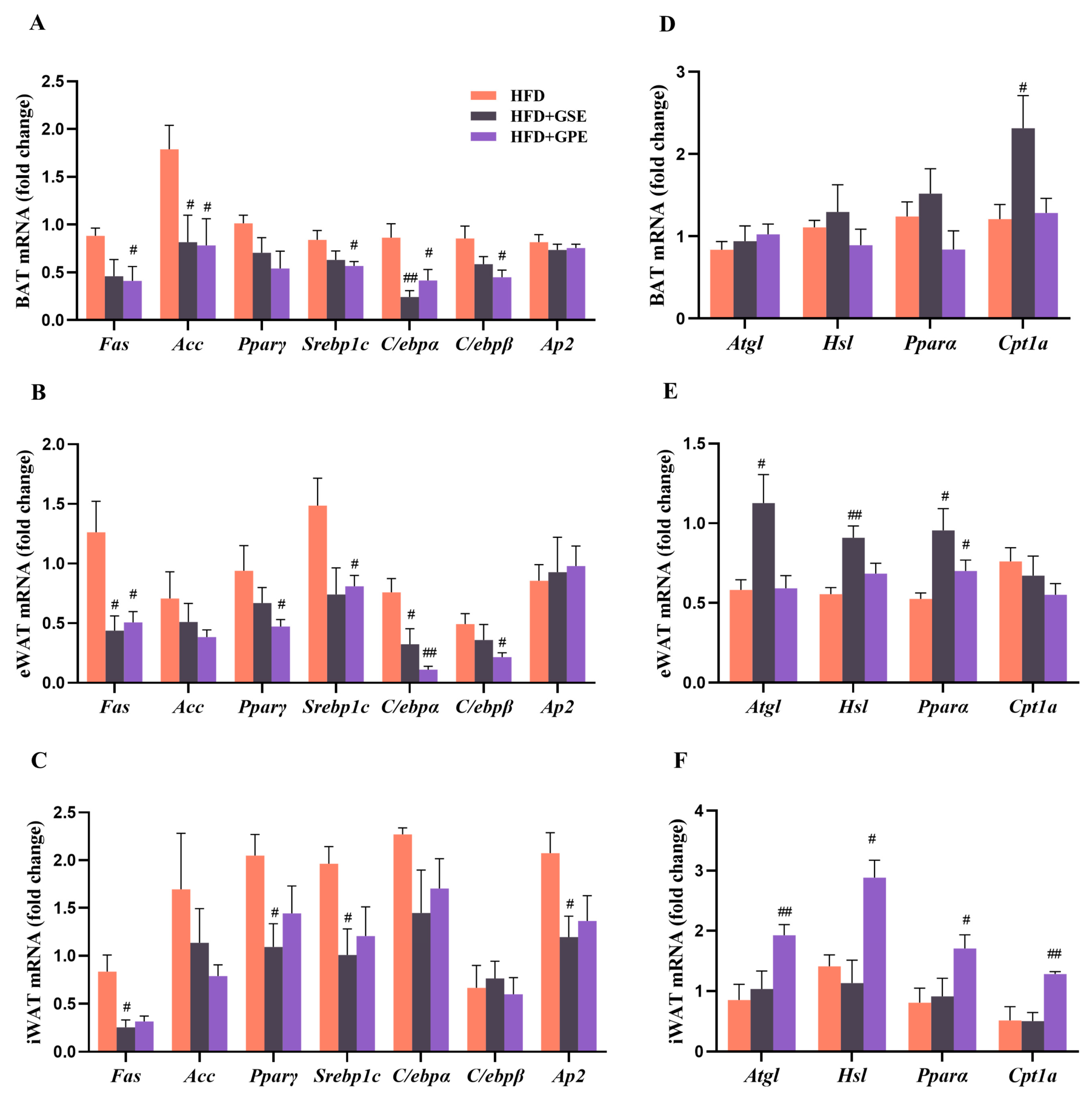
3.4. Effects of GSE and GPE on the Expression Levels of the Genes Regulating Lipid Metabolism in the Adipose Tissues of HFD-Fed Mice
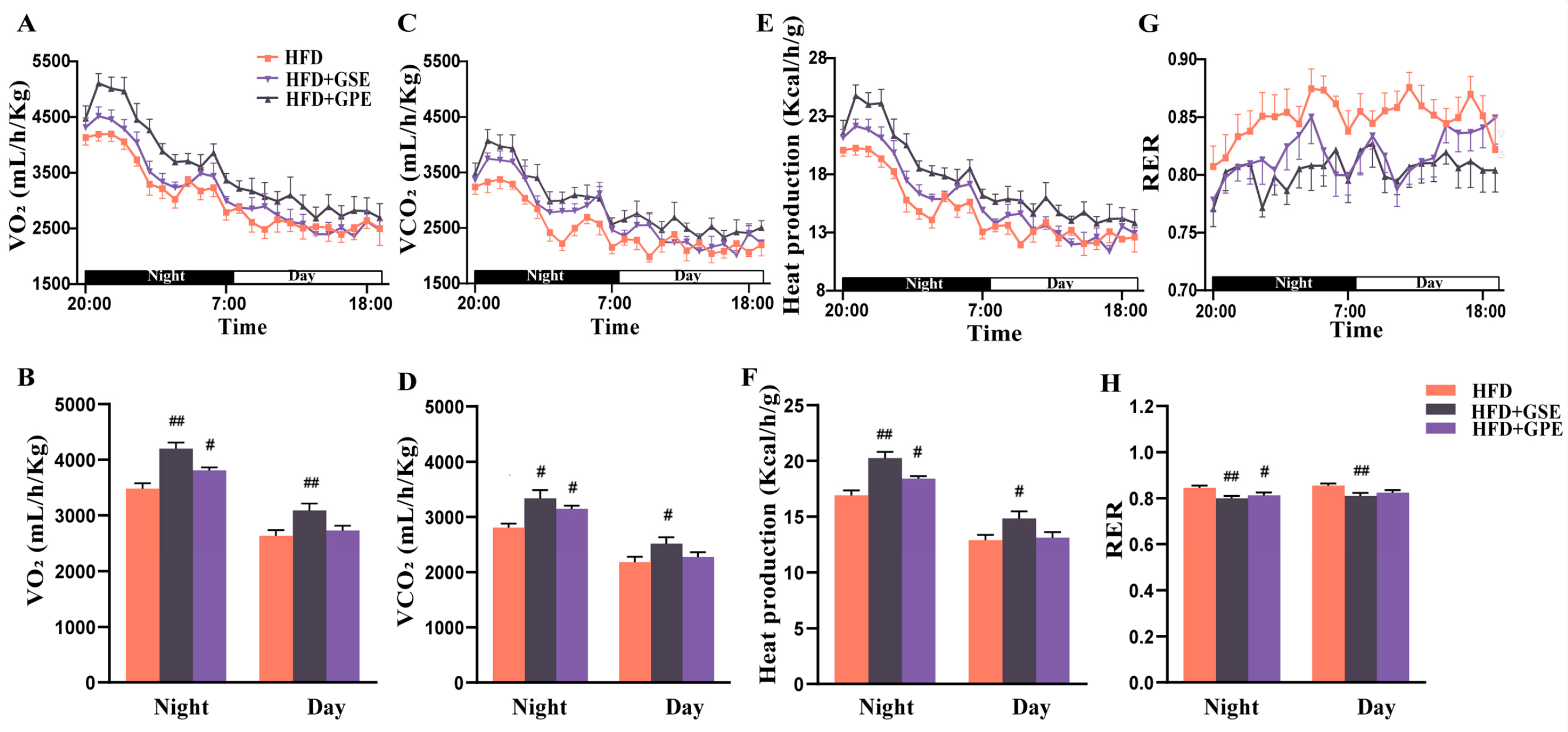
3.5. GSE and GPE Improved Energy Expenditure in HFD-FED mice
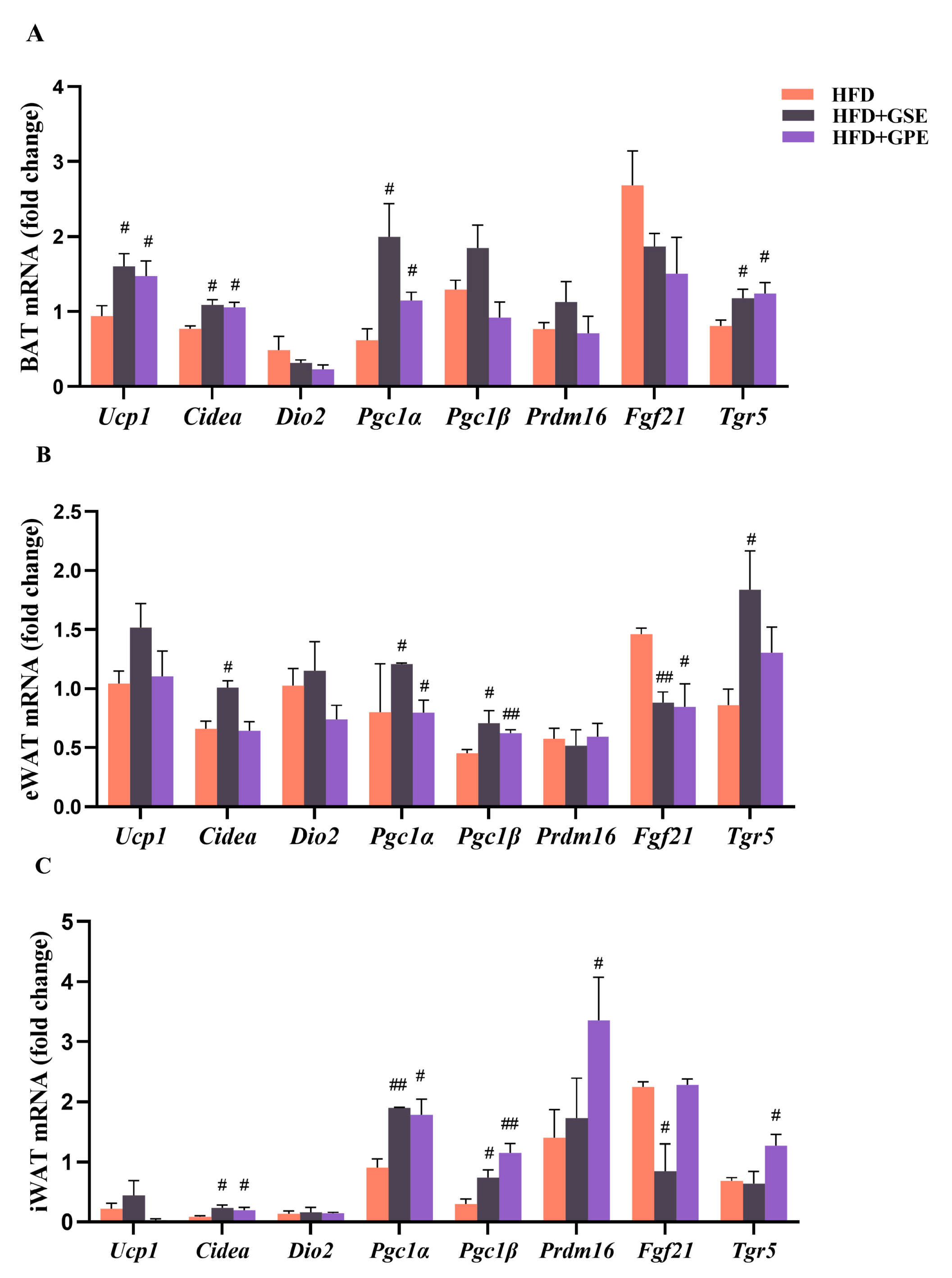
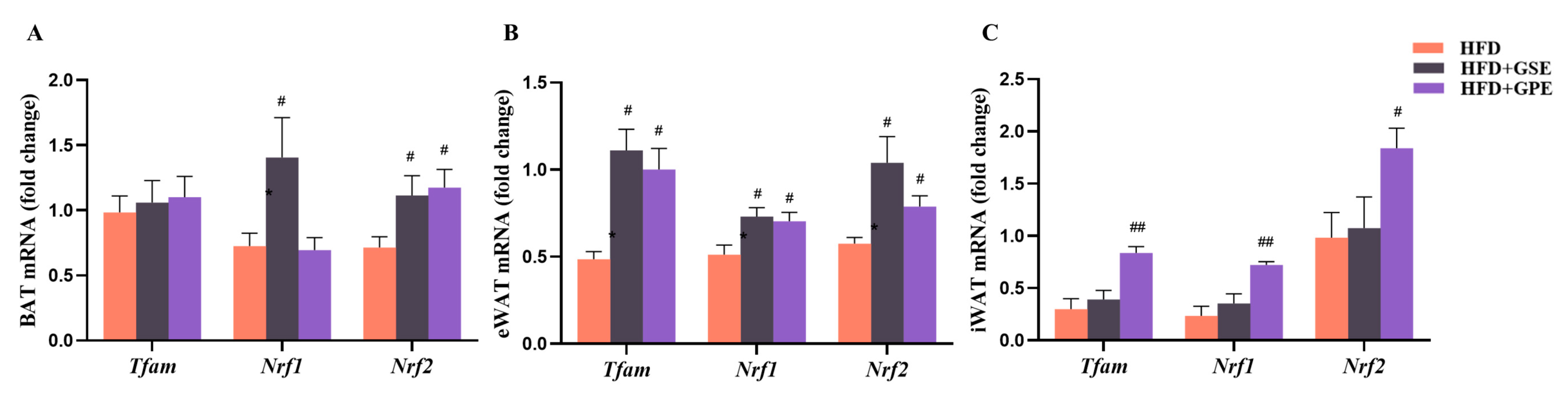
3.6. Effects of GSE and GPE on Expression Levels of Genes Regulating Thermogenesis and Mitochondrial Biogenesis in Adipose Tissues of HFD-Fed Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef]
- Ye, J.; Keller, J.N. Regulation of energy metabolism by inflammation: A feedback response in obesity and calorie restriction. Aging 2010, 2, 361–368. [Google Scholar] [CrossRef]
- Engin, A. The definition and prevalence of obesity and metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar]
- Ren, H.; Guo, Y.; Wang, D.; Kang, X.; Yuan, G. Association of normal-weight central obesity with hypertension: A cross-sectional study from the China health and nutrition survey. BMC Cardiovasc. Disord. 2023, 23, 120. [Google Scholar] [CrossRef]
- Piché, M.E.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution ofobesity and body fat distribution to cardiovascular disease: An update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef]
- King, R.J.; Ajjan, R.A. Vascular risk in obesity: Facts, misconceptions and the unknown. Diabetes Vasc. Dis. Res. 2017, 14, 2–13. [Google Scholar] [CrossRef]
- Wesling, M.; D’Souza, J.J. Diabetes: How to manage overweight and obesity in type 2 diabetes mellitus. Drugs Context 2022, 11, 2021-11-7. [Google Scholar] [CrossRef]
- Feldmann, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef]
- Lasar, D.; Julius, A.; Fromme, T.; Klingenspor, M. Browning attenuates murine white adipose tissue expansion during postnatal development. Biochim. Biophys. Acta 2013, 1831, 960–968. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, H.; Li, C. Research progress of ontogeny and thermogenesis of brown adipose tissue. Chin. J. Anim. Nutr. 2021, 33, 5416–5423. [Google Scholar]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Machado, N.F.; Dominguez-Perles, R. Addressing facts and gaps in the phenolics chemistry of winery by-products. Molecules 2017, 22, 286. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bansode, R.R.; Smith, I.N.; Hurley, S.L. Impact of grape pomace consumption on the blood lipid profile and liver genes associated with lipid metabolism of young rats. Food Funct. 2017, 8, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Akaberi, M.; Hosseinzadeh, H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother. Res. 2016, 30, 540–556. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and human health: The role of bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Mahanna, M.; Millan-Linares, M.C.; Grao-Cruces, E.; Claro, C.; Toscano, R.; Rodriguez-Martin, N.M.; Naranjo, M.C.; la Paz, S.M.-D. Resveratrol-enriched grape seed oil (Vitis vinifera L.) protects from white fat dysfunction in obese mice. J. Funct. Foods 2019, 62, 103546. [Google Scholar] [CrossRef]
- Lanzi, C.R.; Perdicaro, D.J.; Antoniolli, A.; Fontana, A.R.; Miatello, R.M.; Bottini, R.; Prieto, M.A.V. Grape pomace and grape pomace extract improve insulin signaling in high-fat-fructose fed rat-induced metabolic syndrome. Food Funct. 2016, 7, 1544–1553. [Google Scholar] [CrossRef]
- Pascual-Serrano, A.; Arola-Arnal, A.; Suárez-García, S.; Bravo, F.I.; Suárez, M.; Arola, L.; Bladé, C. Grape seed proanthocyanidin supplementation reduces adipocyte size and increases adipocyte number in obese rats. Int. J. Obes. 2017, 41, 1246–1255. [Google Scholar] [CrossRef]
- Kim, H.; Bartley, G.E.; Arvik, T.; Lipson, R.; Nah, S.-Y.; Seo, K.; Yokoyama, W. Dietary supplementation of chardonnay grape seed flour reduces plasma cholesterol concentration, hepatic steatosis, and abdominal fat content in high-fat diet-induced obese hamsters. J. Agric. Food Chem. 2014, 62, 1919–1925. [Google Scholar] [CrossRef]
- Daniel, T.; Ben-Shachar, M.; Drori, E.; Hamad, S.; Permyakova, A.; Ben-Cnaan, E.; Tam, J.; Kerem, Z.; Rosenzweig, T. Grape pomace reduces the severity of non-alcoholic hepatic steatosis and the development of steatohepatitis by improving insulin sensitivity and reducing ectopic fat deposition in mice. J. Nutr. Biochem. 2021, 98, 108867. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, C.L.; Ren, Y.M.; Fang, Y.F.; Yang, X.Y.; Zhang, S.J. Extraction and anti-ultraviolet activity of proanthocyanidins from grape seed. Food Sci. 2014, 35, 69–73. [Google Scholar]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenols with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Sarneckis, C.; Dambergs, R.; Jones, P.; Mercurio, M.; Herderich, M.; Smith, P. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Peinado, J.; de Lerma, N.L.; Moreno, J.; Peinado, R. Antioxidant activity of different phenolics fractions isolated in must from Pedro Ximenez grapes at different stages of the off-vine drying process. Food Chem. 2009, 114, 1050–1055. [Google Scholar] [CrossRef]
- Li, Y.G.; Tanner, G.; Larkin, P. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Meng, J.F.; Fang, Y.L.; Qin, M.Y.; Zhuang, X.F.; Zhang, Z.W. Varietal differences among the phenolic profiles and antioxidant properties of four cultivars of spine grape (Vitis davidii Foex) in Chongyi County (China). Food Chem. 2012, 134, 2049–2056. [Google Scholar] [CrossRef]
- Yue, X.F.; Jing, S.S.; Ni, X.F.; Zhang, K.K.; Fang, Y.L.; Zhang, Z.W.; Ju, Y.L. Anthocyanin and phenolic acids contents influence the color stability and antioxidant capacity of wine treated with mannoprotein. Front. Nutr. 2021, 8, 691784. [Google Scholar] [CrossRef]
- Yue, W.; Han, F. Effects of monoglucoside and diglucoside anthocyanins from Yan 73 (Vitis vinifera L.) and spine grape (Vitis davidii Foex) skin on intestinal microbiota in vitro. Food Chem. X 2022, 16, 100501. [Google Scholar] [CrossRef]
- Bentivegna, S.S.; Whitney, K.M. Subchronic 3-month oral toxicity study of grape seed and grape skin extracts. Food Chem. Toxicol. 2002, 40, 1731–1743. [Google Scholar] [CrossRef]
- Yang, C.; Han, Y.; Tian, X.; Sajid, M.; Mehmood, S.; Wang, H.; Li, H. Phenolic composition of grape pomace and its metabolism. Crit. Rev. Food Sci. Nutr. 2022, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activ ity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2013, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape pomace as a source of phenolic compounds and diverse bioactive properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Duan, X.M. Effects of Thyroid Hormone Metabolism Related microRNAs on Different Propensities to Obesity. Master’s Thesis, Jiangnan University, Wuxi, China, 2015. [Google Scholar]
- Din, M.U.; Saari, T.; Raiko, J.; Kudomi, N.; Maurer, S.F.; Lahesmaa, M.; Fromme, T.; Amri, E.Z.; Klingenspor, M.; Solin, O.; et al. Postprandial oxidative metabolism of human brown fat indicates thermogenesis. Cell Metab. 2018, 28, 207–216. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Trajkovski, M.; Lodish, H. MicroRNA networks regulate development of brown adipocytes. Trends Endocrinol. Metab. 2013, 24, 442–450. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape extract activates brown adipose tissue through pathway involving the regulation of gut microbiota and bile acid. Mol. Nutr. Food Res. 2020, 64, e2000149. [Google Scholar] [CrossRef]
- Emiliano, A.F.; de Cavalho, L.C.R.M.; Cordeiro, V.D.S.C.; da Costa, C.A.; de Oliveira, P.B.R.; Queiroz, E.F.; Moreira, D.D.C.; Boaventura, G.T.; de Moura, R.S.; Resende, A.C. Metabolic disorders and oxidative stress programming in offspring of rats fed a high-fat diet during lactation: Effects of a vinifera grape skin (ACH09) extract. J. Cardiovasc. Pharmacol. 2011, 58, 319–328. [Google Scholar] [CrossRef]
- Bays, H.E.; González-Campoy, J.M.; Bray, G.A.; Kitabchi, A.E.; Bergman, D.A.; Schorr, A.B.; Rodbard, H.W.; Henry, R.R. Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert. Rev. Cardiovasc. Ther. 2008, 6, 343–368. [Google Scholar] [CrossRef]
- Collins, S. Beta-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. 2011, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.F. Regulation of PGC-1α and PGC-1β Expression and Its Relation to Mitochondrial Development. Ph.D. Thesis, Northwest A&F University, Xianyang, China, 2007. [Google Scholar]
- Dempersmier, J.; Sambeat, A.; Gulyaeva, O.; Paul, S.M.; Hudak, C.S.; Raposo, H.F.; Kwan, H.-Y.; Kang, C.; Wong, R.H.; Sul, H.S. Cold-inducible Zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol. Cell 2015, 57, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Li, T.; Ren, D.; Yang, X. Grape seed proantho-cyanidins reduced the overweight of C57BL/6J mice through modulating adipose thermogenesis and gut microbiota. Food Funct. 2021, 12, 8467–8477. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape seed proanthocyanidin extract ameliorates inflammation and adiposity by modulating gut microbiota in high-fat diet mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Lu, Y.; Bo, Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0162089. [Google Scholar] [CrossRef]
- Park, S.; Choi, M.; Lee, M. Effects of Anthocyanin Supplementation on Reduction of Obesity Criteria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef]
- Gavrieli, A.; Mantzoros, C.S. Novel molecules regulating energy homeostasis: Physiology and regulation by macronutrient intake and weight loss. Endocrinol. Metab. 2016, 31, 361–372. [Google Scholar] [CrossRef]
- Fisher, F.M.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes. Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Al-Amrani, A.; AbdelKarim, M.; Alzabin, M.; Alzoghaibi, M. Low expression of brown and beige fat genes in subcutaneous tissues in obese patients. Arch. Med. Sci. 2019, 15, 1113–1122. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef]
- Mei, W.; Xiong, W.; Zhao, Y. Research progress of mammalian mitochondrial transcriptional regulatory factor. Biotechnology 2022, 32, 506–512. [Google Scholar]
- Wu, Z.; Puigserver, P.; Andersson, U.; Zhang, C.; Adelmant, G.; Mootha, V.; Troy, A.; Cinti, S.; Lowell, B.; Scarpulla, R.C.; et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 1999, 98, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, W.Z.; Shu, Q.L.; Yao, P.C.; Peng, S.H. Progress of nuclear respiratory factors. Chin. Bull. Life Sci. 2012, 24, 456–462. [Google Scholar]
| TPC (GAE mg/g) | TANC (CE mg/g) | TFOC (RE mg/g) | TFAC (CE mg/g) | TAC (C3GE mg/g) | |
|---|---|---|---|---|---|
| GSE | 656.40 ± 8.07 | 680.62 ± 3.82 | 271.20 ± 0.85 | 147.64 ± 1.78 | - |
| GPE | 173.70 ± 6.22 | 109.89 ± 1.66 | 105.53 ± 0.55 | 14.76 ± 0.54 | 75.46 ± 1.88 |
| Phenolic Profiles of GSE (μg/mg) | Anthocyanin Profiles of GPE (μg/mg) | ||
|---|---|---|---|
| Flavan-3-ols | Malvidin-3-glucoside | 30.02 ± 0.01 | |
| Epicatechin | 49.31 ± 0.03 | Malvidin-3-coumaroyl-glucoside | 28.50 ± 0.02 |
| Catechin | 46.80 ± 0.02 | Malvidin-3-acetyl-glucoside | 6.43 ± 0.00 |
| Gallocatechin | 0.25 ± 0.01 | Cyanidin-3-glucoside | 2.90 ± 0.01 |
| Epigallocatechin | 0.12 ± 0.00 | Peonidin-3-acetyl-glucoside | 1.94 ± 0.01 |
| Procyanidin B1 | 19.41 ± 0.02 | Peonidin-3-coumaroyl-glucoside | 1.86 ± 0.00 |
| Procyanidin C1 | 16.81 ± 0.01 | Delphinidin-3-glucoside | 1.60 ± 0.01 |
| Procyanidin B2 | 6.02 ± 0.01 | Peonidin-3-glucoside | 1.45 ± 0.00 |
| Phenolic acids and Flavonols | Petunidin-3-glucoside | 0.64 ± 0.00 | |
| Gallic acid | 8.47 ± 0.02 | Total | 77.66 ± 0.09 |
| Epicatechin-3-gallate | 4.98 ± 0.01 | ||
| Quercetin-glucoside | 0.12 ± 0.01 | ||
| Total | 152.64 ± 0.07 | ||
| Sum of flavan-3-ols | 138.71 ± 0.05 (91%) | ||
| ND | HFD | HFD + GSE | HFD + GPE | |
|---|---|---|---|---|
| Triglyceride (mmol/L) | 0.71 ± 0.13 b | 1.03 ± 0.20 a | 0.53 ± 0.09 b | 0.66 ± 0.15 b |
| Total cholesterol (mmol/L) | 2.43 ± 0.21 c | 4.10 ± 0.65 a | 3.49 ± 0.30 b | 3.21 ± 0.19 b |
| HDL cholesterol (mmol/L) | 3.24 ± 0.40 c | 3.95 ± 0.15 b | 4.57 ± 0.32 a | 4.57 ± 0.33 a |
| LDL cholesterol (mmol/L) | 0.16 ± 0.04 b | 0.50 ± 0.16 a | 0.43 ± 0.16 a | 0.41 ± 0.12 a |
| Fasting serum glucose (mmol/L) | 5.28 ± 0.61 b | 6.92 ± 0.65 a | 5.85 ± 0.64 b | 6.04 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Tian, X.; Han, Y.; Shi, X.; Wang, H.; Li, H. Extracts of Dunkelfelder Grape Seeds and Peel Increase the Metabolic Rate and Reduce Fat Deposition in Mice Maintained on a High-Fat Diet. Foods 2023, 12, 3251. https://doi.org/10.3390/foods12173251
Yang C, Tian X, Han Y, Shi X, Wang H, Li H. Extracts of Dunkelfelder Grape Seeds and Peel Increase the Metabolic Rate and Reduce Fat Deposition in Mice Maintained on a High-Fat Diet. Foods. 2023; 12(17):3251. https://doi.org/10.3390/foods12173251
Chicago/Turabian StyleYang, Chenlu, Xuelin Tian, Yulei Han, Xueqing Shi, Hua Wang, and Hua Li. 2023. "Extracts of Dunkelfelder Grape Seeds and Peel Increase the Metabolic Rate and Reduce Fat Deposition in Mice Maintained on a High-Fat Diet" Foods 12, no. 17: 3251. https://doi.org/10.3390/foods12173251
APA StyleYang, C., Tian, X., Han, Y., Shi, X., Wang, H., & Li, H. (2023). Extracts of Dunkelfelder Grape Seeds and Peel Increase the Metabolic Rate and Reduce Fat Deposition in Mice Maintained on a High-Fat Diet. Foods, 12(17), 3251. https://doi.org/10.3390/foods12173251






