Quantification and Distribution of Thiols in Fermented Grains of Sauce-Aroma Baijiu Production Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards, Chemicals, and Materials
2.2. Fermented Grains of Baijiu
2.3. Derivatizing Reagent
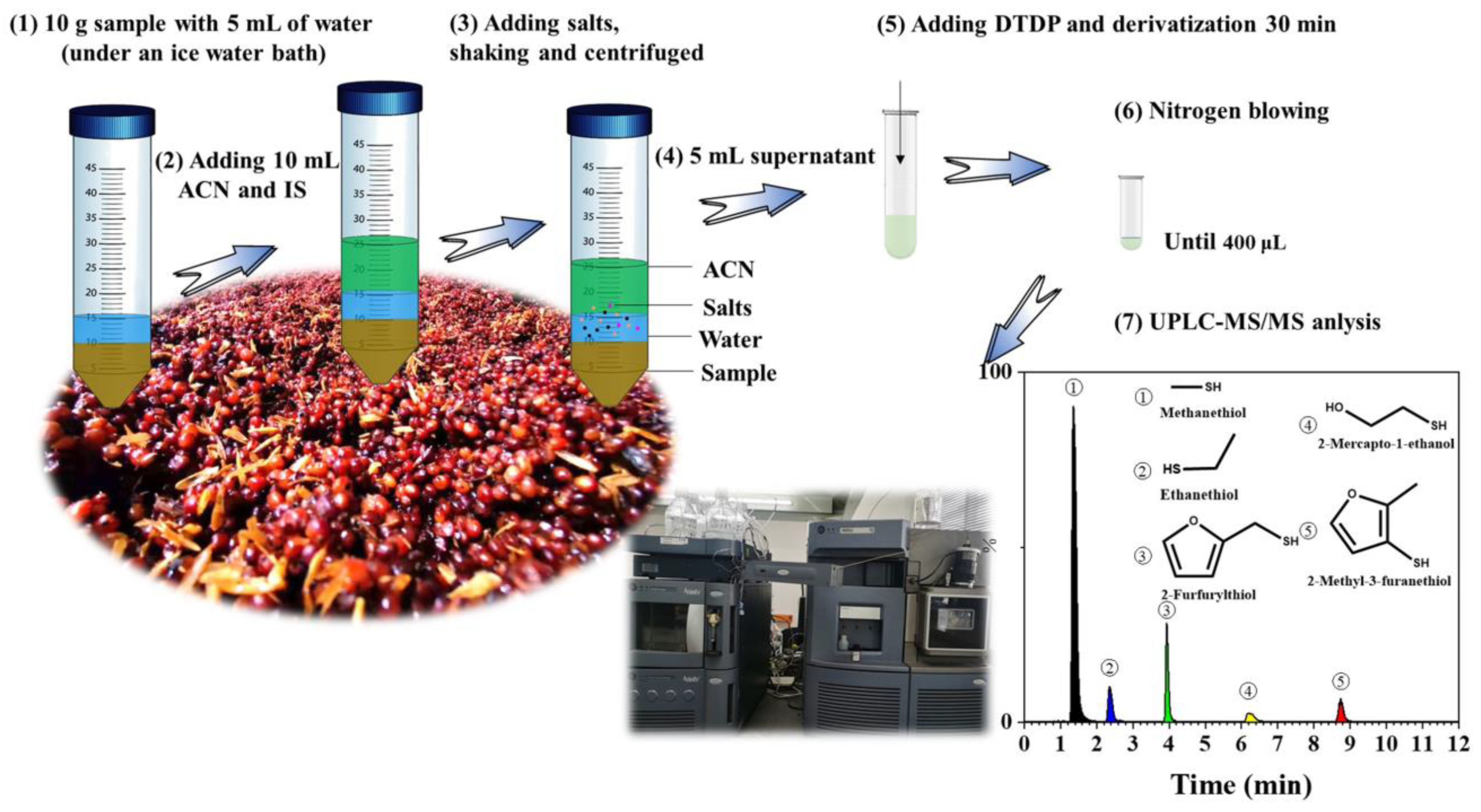
2.4. Optimization of the QuEChERS Method
2.5. Preparation of Sample for Analysis
2.6. UPLC-MS/MS Analysis
2.7. Statistical Analyses
3. Results and Discussion
3.1. Optimization of the Extraction Solvent
3.2. Selection of Clean-Up QuEChERS Extracts
3.3. Validation of the Method
3.4. Measurement of Fermented Grains of Baijiu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Sha, S.; Qian, M.; Xu, Y. Characterization of Volatile Sulfur Compounds in Moutai Liquors by Headspace Solid-Phase Microextraction Gas Chromatography-Pulsed Flame Photometric Detection and Odor Activity Value. J. Food Sci. 2017, 82, 2816–2822. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M.C. Sensitive quantification of sulfur compounds in wine by headspace solid-phase microextraction technique. J. Chromatogr. A 2005, 1080, 177–185. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of organic sulfur compounds in wine aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Ferreira, A.C.S.; Rodrigues, P.; Hogg, T.; De Pinho, P.G. Influence of some technological parameters on the formation of dimethyl sulfide, 2-mercaptoethanol, methionol, and dimethyl sulfone in port wines. J. Agric. Food Chem. 2003, 51, 727–732. [Google Scholar] [CrossRef]
- Jimenez-Lorenzo, R.; Bloem, A.; Farines, V.; Sablayrolles, J.-M.; Camarasa, C. How to modulate the formation of negative volatile sulfur compounds during wine fermentation? Fems Yeast Res. 2021, 21, foab038. [Google Scholar] [CrossRef]
- Yan, Y.; Lu, J.; Nie, Y.; Li, C.; Chen, S.; Xu, Y. Characterization of volatile thiols in Chinese liquor (Baijiu) by ultraperformance liquid chromatography-mass spectrometry and ultraperformance liquid chromatography-quadrupole-time-of-flight mass spectrometry. Front. Nutr. 2022, 9, 1022600. [Google Scholar] [CrossRef]
- Zhu, J.; Niu, Y.; Xiao, Z. Characterization of important sulfur and nitrogen compounds in Lang baijiu by application of gas chromatography-olfactometry, flame photometric detection, nitrogen phosphorus detector and odor activity value. Food Res. Int. 2020, 131, 109001. [Google Scholar] [CrossRef]
- Song, X.; Zhu, L.; Wang, X.; Zheng, F.; Zhao, M.; Liu, Y.; Li, H.; Zhang, F.; Zhang, Y.; Chen, F. Characterization of key aroma-active sulfur-containing compounds in Chinese Laobaigan Baijiu by gas chromatography-olfactometry and comprehensive two-dimensional gas chromatography coupled with sulfur chemiluminescence detection. Food Chem. 2019, 297, 124959. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Z.; Sun, B. Low quantity but critical contribution to flavor: Review of the current understanding of volatile sulfur-containing compounds in baijiu. J. Food Compos. Anal. 2021, 103, 104079. [Google Scholar] [CrossRef]
- Song, X.; Zhu, L.; Jing, S.; Li, Q.; Ji, J.; Zheng, F.; Zhao, Q.; Sun, J.; Chen, F.; Zhao, M.; et al. Insights into the role of 2-methyl-3-furanthiol and 2-furfurylthiol as markers for the differentiation of Chinese light, strong, and soy sauce aroma types of Baijiu. J. Agric. Food Chem. 2020, 68, 7946–7954. [Google Scholar] [CrossRef]
- Wang, L.; Fan, S.; Yan, Y.; Yang, L.; Chen, S.; Xu, Y. Characterization of potent odorants causing a pickle-like off-odor in Moutai-aroma type Baijiu by comparative aroma extract dilution analysis, quantitative measurements, aroma addition, and omission studies. J. Agric. Food Chem. 2020, 68, 1666–1677. [Google Scholar] [CrossRef]
- Yang, L.; He, J.; Luo, C.; Zhang, C. Analysis of volatile flavor compounds from defective product of sauce-flavor Baijiu by GC*GC-TOF-MS. China Brew. 2019, 38, 67–72. [Google Scholar] [CrossRef]
- Kawano, Y.; Suzuki, K.; Ohtsu, I. Development of quantitative analytical method for volatile thiol compound with LC-ESI-MS as nonvolatile derivative by integrating a thiol-specific derivatization. Biosci. Biotechnol. Biochem. 2021, 85, 1932–1936. [Google Scholar] [CrossRef]
- Slaghenaufi, D.; Tonidandel, L.; Moser, S.; Villegas, T.R.; Larcher, R. Rapid Analysis of 27 Volatile Sulfur Compounds in Wine by Headspace Solid-Phase Microextraction Gas Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2017, 10, 3706–3715. [Google Scholar] [CrossRef]
- Davis, P.M.; Qian, M.C. Effect of Wine Matrix Composition on the Quantification of Volatile Sulfur Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography-Pulsed Flame Photometric Detection. Molecules 2019, 24, 3320. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.H. Performance characterization of the GC/PFPD for H2S, CH3SH, DMS, and DMDS in air. Atmos. Environ. 2005, 39, 2235–2242. [Google Scholar] [CrossRef]
- Choi, Y.G.; Ko, D.H.; Kim, H.J.; Chung, T.H. Determination of nanogram level of VSCs using GC-SCD and an adsorption tube. Water Sci. Technol. 2004, 49, 329–334. [Google Scholar] [CrossRef]
- Herbst-Johnstone, M.; Piano, F.; Duhamel, N.; Barker, D.; Fedrizzi, B. Ethyl propiolate derivatisation for the analysis of varietal thiols in wine. J. Chromatogr. A 2013, 1312, 104–110. [Google Scholar] [CrossRef]
- Mateo-Vivaracho, L.; Cacho, J.; Ferreira, V. Quantitative determination of wine polyfunctional mercaptans at nanogram per liter level by gas chromatography-negative ion mass spectrometric analysis of their pentafluorobenzyl derivatives. J. Chromatogr. A 2007, 1146, 242–250. [Google Scholar] [CrossRef]
- Capone, D.L.; Ristic, R.; Pardon, K.H.; Jeffery, D.W. Simple Quantitative Determination of Potent Thiols at Ultratrace Levels in Wine by Derivatization and High-Performance Liquid Chromatography-Tandem Mass Spectrometry (HPLC-MS/MS) Analysis. Anal. Chem. 2015, 87, 1226–1231. [Google Scholar] [CrossRef]
- Niu, J.; Yang, S.; Shen, Y.; Cheng, W.; Li, H.; Sun, J.; Huang, M.; Sun, B. What Are the Main Factors That Affect the Flavor of Sauce-Aroma Baijiu. Foods 2022, 11, 3534. [Google Scholar] [CrossRef]
- Tonidandel, L.; Larcher, R.; Barbero, A.; Jelley, R.E.; Fedrizzi, B. A single run liquid chromatography-tandem mass spectrometry method for the analysis of varietal thiols and their precursors in wine. J. Chromatogr. A 2021, 1658, 462603. [Google Scholar] [CrossRef]
- Riener, C.K.; Kada, G.; Gruber, H.J. Quick measurement of protein sulfhydryls with Ellman’s reagent and with 4,4’-dithiodipyridine. Anal. Bioanal. Chem. 2002, 373, 266–276. [Google Scholar] [CrossRef]
- Santana-Mayor, A.; Socas-Rodriguez, B.; Herrera-Herrera, A.V.; Angel Rodriguez-Delgado, M. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. Trac-Trends Anal. Chem. 2019, 116, 214–235. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, S.; Xu, Y. Determination of non-glucosidic cyanogen in Chinese liquor-fermentation ingredients using QuEChERS sample preparation and spectrophotometric method. Food Control 2022, 140, 109101. [Google Scholar] [CrossRef]
- Cerny, C.; Guntz-Dubini, R. Formation of cysteine-S-conjugates in the Maillard reaction of cysteine and xylose. Food Chem. 2013, 141, 1078–1086. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, W.; Lao, F.; Mi, R.; Liao, X.; Luo, D.; Wu, J. Isolation and identification of putative precursors of the volatile sulfur compounds and their inhibition methods in heat-sterilized melon juices. Food Chem. 2021, 343, 128459. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Q.; Wang, P.; Lin, J.; Huang, L.; Xu, Y. Synergistic effect in core microbiota associated with sulfur metabolism in spontaneous Chinese liquor fermentation. Appl. Environ. Microbiol. 2017, 83, e01475-17. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Yang, C.; Song, H. Key meat flavour compounds formation mechanism in a glutathione-xylose Maillard reaction. Food Chem. 2012, 131, 280–285. [Google Scholar] [CrossRef]
- Thomas, C.; Mercier, F.; Tournayre, P.; Martin, J.-L.; Berdague, J.-L. Identification and origin of odorous sulfur compounds in cooked ham. Food Chem. 2014, 155, 207–213. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Hui, T.; Fang, F.; Zhang, D. New insight into the formation mechanism of 2-furfurylthiol in the glucose-cysteine reaction with ribose. Food Res. Int. 2021, 143, 110295. [Google Scholar] [CrossRef]
- Kinzurik, M.I.; Deed, R.C.; Herbst-Johnstone, M.; Slaghenaufi, D.; Guzzon, R.; Gardner, R.C.; Larcher, R.; Fedrizzi, B. Addition of volatile sulfur compounds to yeast at the early stages of fermentation reveals distinct biological and chemical pathways for aroma formation. Food Microbiol. 2020, 89, 103435. [Google Scholar] [CrossRef]
- Lu, X.; Fan, C.; He, W.; Deng, J.; Yin, H. Sulfur-containing amino acid methionine as the precursor of volatile organic sulfur compounds in algea-induced black bloom. J. Environ. Sci. 2013, 25, 33–43. [Google Scholar] [CrossRef]
- Boatright, W.L.; Lu, G.P. A ≥ 10000-MW soybean protein fraction that inhibits the formation of methanethiol and hydrogen sulfide in aqueous slurries of isolated soy proteins with added L-cysteine. J. Food Sci. 2006, 71, C185–C189. [Google Scholar] [CrossRef]
- Mottram, D.S.; Nobrega, I.C.C. Formation of sulfur aroma compounds in reaction mixtures containing cysteine and three different forms of ribose. J. Agric. Food Chem. 2002, 50, 4080–4086. [Google Scholar] [CrossRef]
- Hofmann, T.; Schieberle, P. Quantitative model studies on the effectiveness of different precursor systems in the formation of the intense food odorants 2-furfurylthiol and 2-methyl-3-furanthiol. J. Agric. Food Chem. 1998, 46, 235–241. [Google Scholar] [CrossRef]
- Cerny, C. Origin of carbons in sulfur-containing aroma compounds from the Maillard reaction of xylose, cysteine and thiamine. Lwt-Food Sci. Technol. 2007, 40, 1309–1315. [Google Scholar] [CrossRef]
- Tang, W.; Jiang, D.; Yuan, P.; Ho, C.-T. Flavor chemistry of 2-methyl-3-furanthiol, an intense meaty aroma compound. J. Sulfur Chem. 2013, 34, 38–47. [Google Scholar] [CrossRef]
- Gao, P.; Xia, W.; Li, X.; Liu, S. Optimization of the Maillard reaction of xylose with cysteine for modulating aroma compound formation in fermented tilapia fish head hydrolysate using response surface methodology. Food Chem. 2020, 331, 127353. [Google Scholar] [CrossRef]
- Cerny, C.; Davidek, T. α-Mercaptoketone formation during the Maillard reaction of cysteine and 1-C-13 ribose. J. Agric. Food Chem. 2004, 52, 958–961. [Google Scholar] [CrossRef]






| No. | Compounds | Slope | Intercept | Linear Range (μg/kg) | R2 | LOD (μg/kg) | LOQ (μg/kg) |
|---|---|---|---|---|---|---|---|
| 1 | Methanethiol | 2.5499 | 0.9861 | 10.15–1299.77 | 0.9907 | 0.26 | 0.86 |
| 2 | Ethanethiol | 0.6538 | 0.0186 | 0.27–34.24 | 0.9916 | 0.017 | 0.058 |
| 3 | 2-Mercapto-1-ethanol | 0.4181 | 0.00007 | 0.06–32.42 | 0.9932 | 0.004 | 0.015 |
| 4 | 2-Furfurylthiol | 3.8213 | 0.0079 | 0.45–28.79 | 0.9935 | 0.034 | 0.11 |
| 5 | 2-Methyl-3-furanthiol | 3.272 | 0.0016 | 0.17–21.34 | 0.9900 | 0.019 | 0.063 |
| Compounds | Spiked Level (μg/L) | Recovery (%) | Precision (RSD, %) | |
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| Methanethiol | 5 | 74.33 | 0.63 | 4.28 |
| 50 | 81.81 | 2.88 | 7.24 | |
| 500 | 75.26 | 3.01 | 1.96 | |
| Ethanethiol | 0.3 | 71.72 | 4.66 | 3.43 |
| 3 | 78.04 | 1.52 | 3.89 | |
| 30 | 104.72 | 1.40 | 2.85 | |
| 2-Mercapto-1-ethanol | 0.3 | 85.17 | 2.02 | 3.67 |
| 3 | 74.53 | 6.04 | 9.44 | |
| 30 | 72.40 | 7.72 | 6.67 | |
| 2-Furfurylthiol | 0.2 | 82.86 | 4.51 | 6.22 |
| 2 | 100.45 | 1.42 | 6.62 | |
| 20 | 102.73 | 5.60 | 3.31 | |
| 2-Methyl-3-furanthiol | 0.1 | 81.12 | 5.07 | 4.76 |
| 1 | 72.55 | 4.23 | 3.49 | |
| 10 | 77.79 | 2.48 | 8.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, D.; Li, P.; Gong, R.; Sun, Y.; Chen, X.; Wei, H.; Xu, Y. Quantification and Distribution of Thiols in Fermented Grains of Sauce-Aroma Baijiu Production Process. Foods 2023, 12, 2658. https://doi.org/10.3390/foods12142658
Xiang D, Li P, Gong R, Sun Y, Chen X, Wei H, Xu Y. Quantification and Distribution of Thiols in Fermented Grains of Sauce-Aroma Baijiu Production Process. Foods. 2023; 12(14):2658. https://doi.org/10.3390/foods12142658
Chicago/Turabian StyleXiang, Danhua, Peiqi Li, Rong Gong, Yanbin Sun, Xiangmei Chen, Heli Wei, and Yan Xu. 2023. "Quantification and Distribution of Thiols in Fermented Grains of Sauce-Aroma Baijiu Production Process" Foods 12, no. 14: 2658. https://doi.org/10.3390/foods12142658





