Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Heating Procedure
2.3. Chemical Characteristics of Fresh and Heat-Treated Oil Blends
2.4. Nutritional Quality Indicators of Oils
2.5. LF NMR Studies
2.6. Oxidative Stability of the Blends Measured by DSC
2.7. Statistical Analysis
3. Results and Discussion
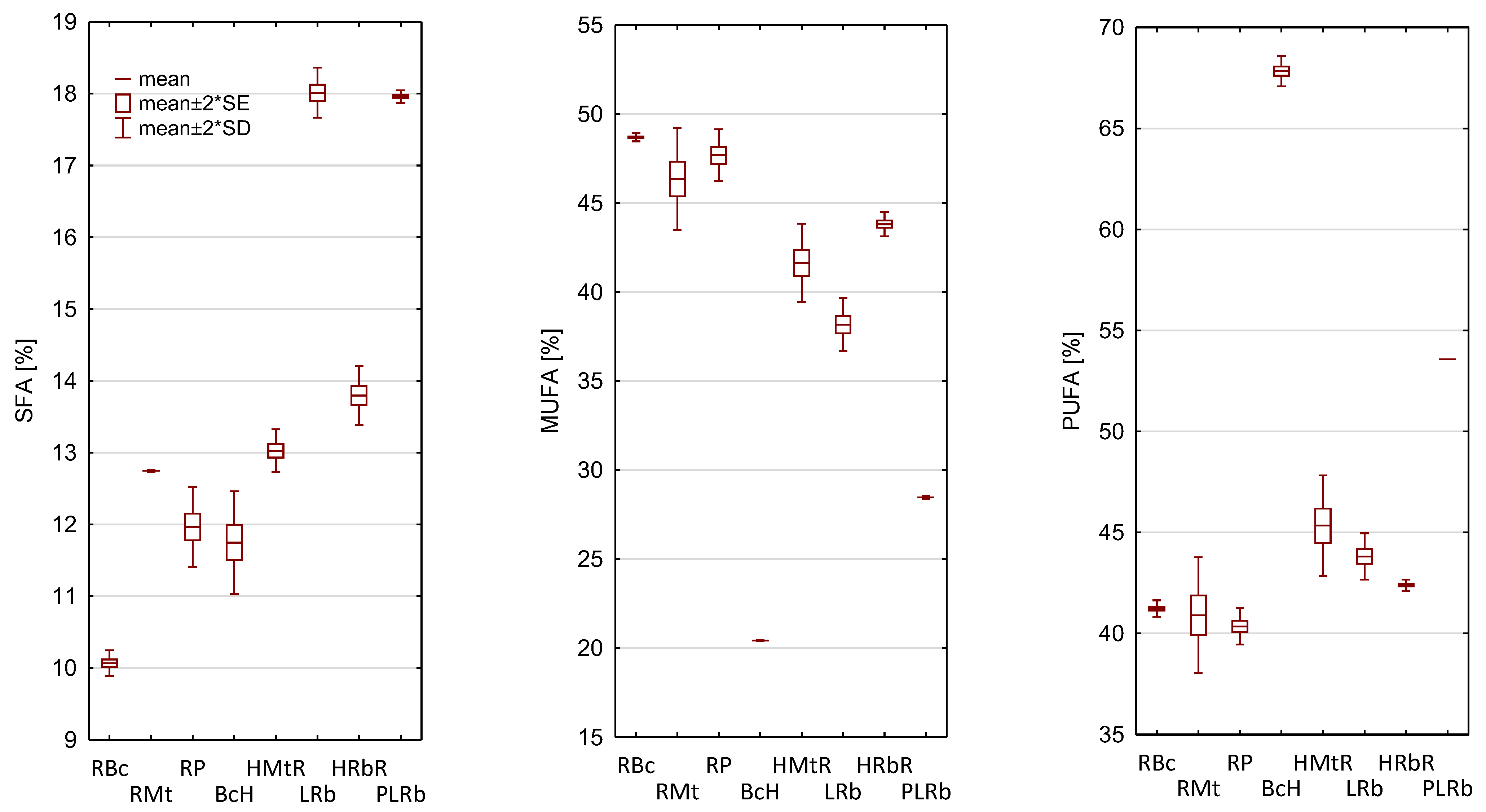
3.1. Composition of Fatty Acids in the Analyzed Blends
3.2. Nutritional Quality Indicators of Oils
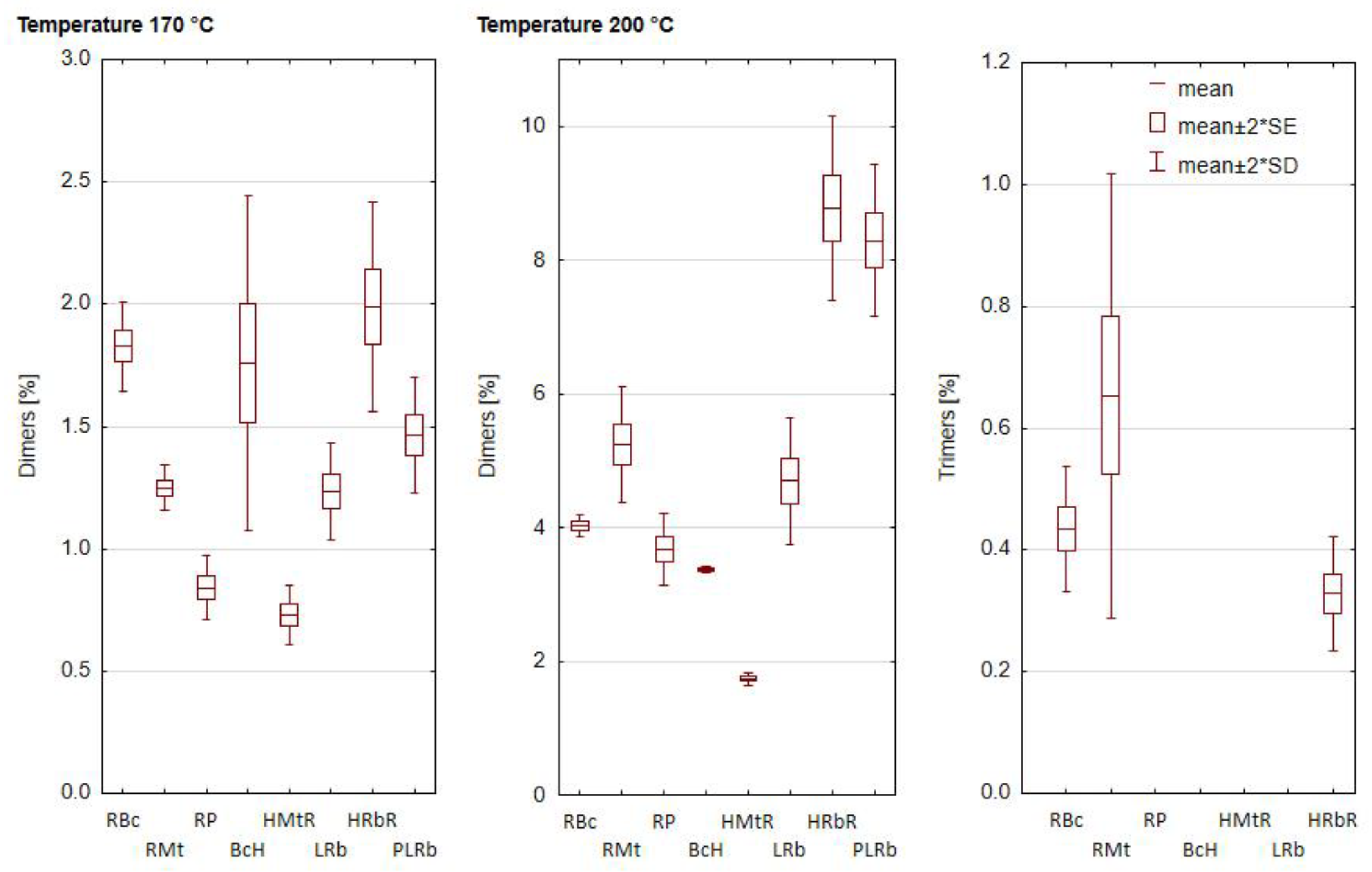
3.3. Influence of the Heating Process on the Formation of Total Polar Compounds (TPC) and Polymerized Triacylglycerols (PTG)
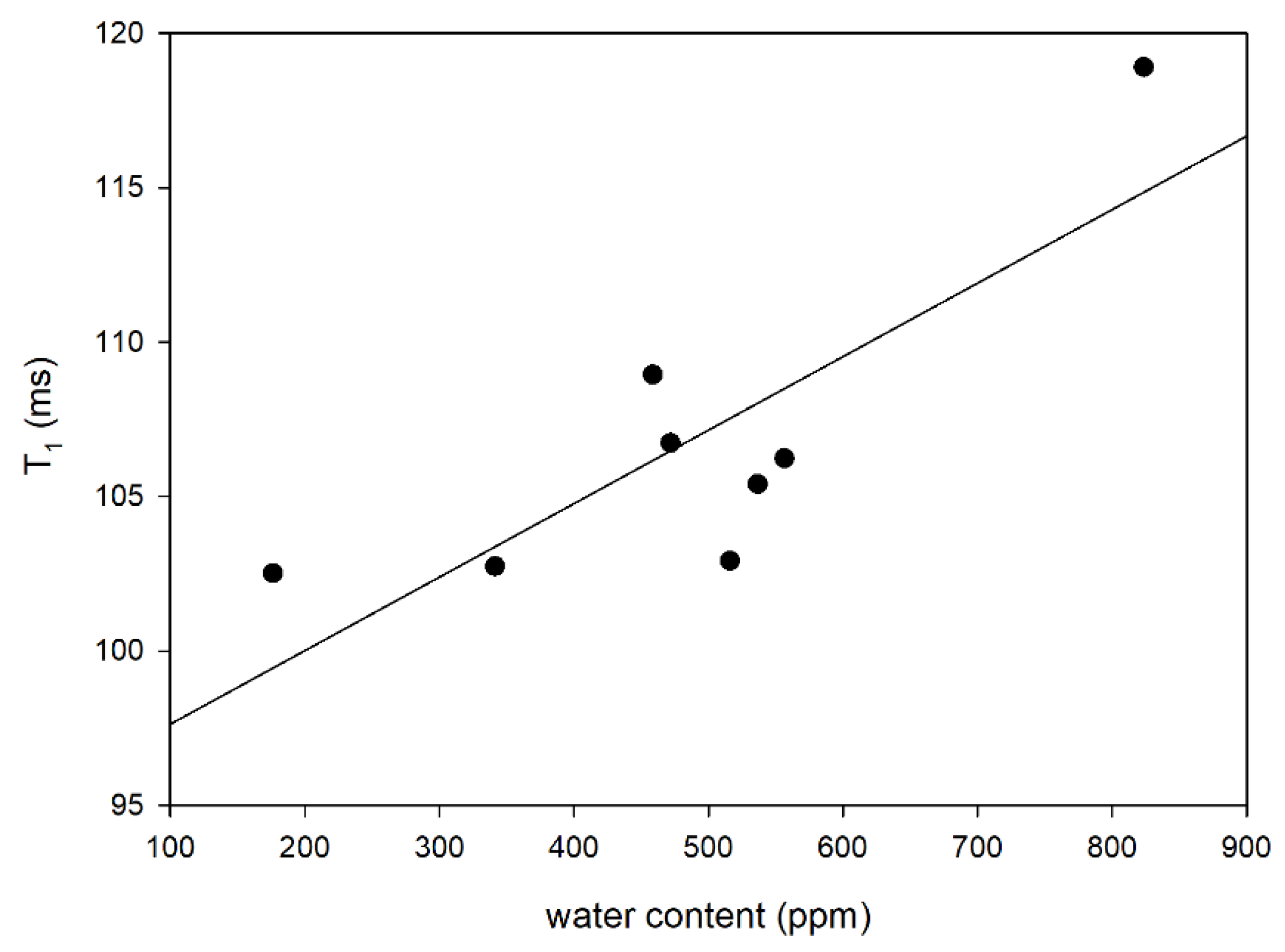
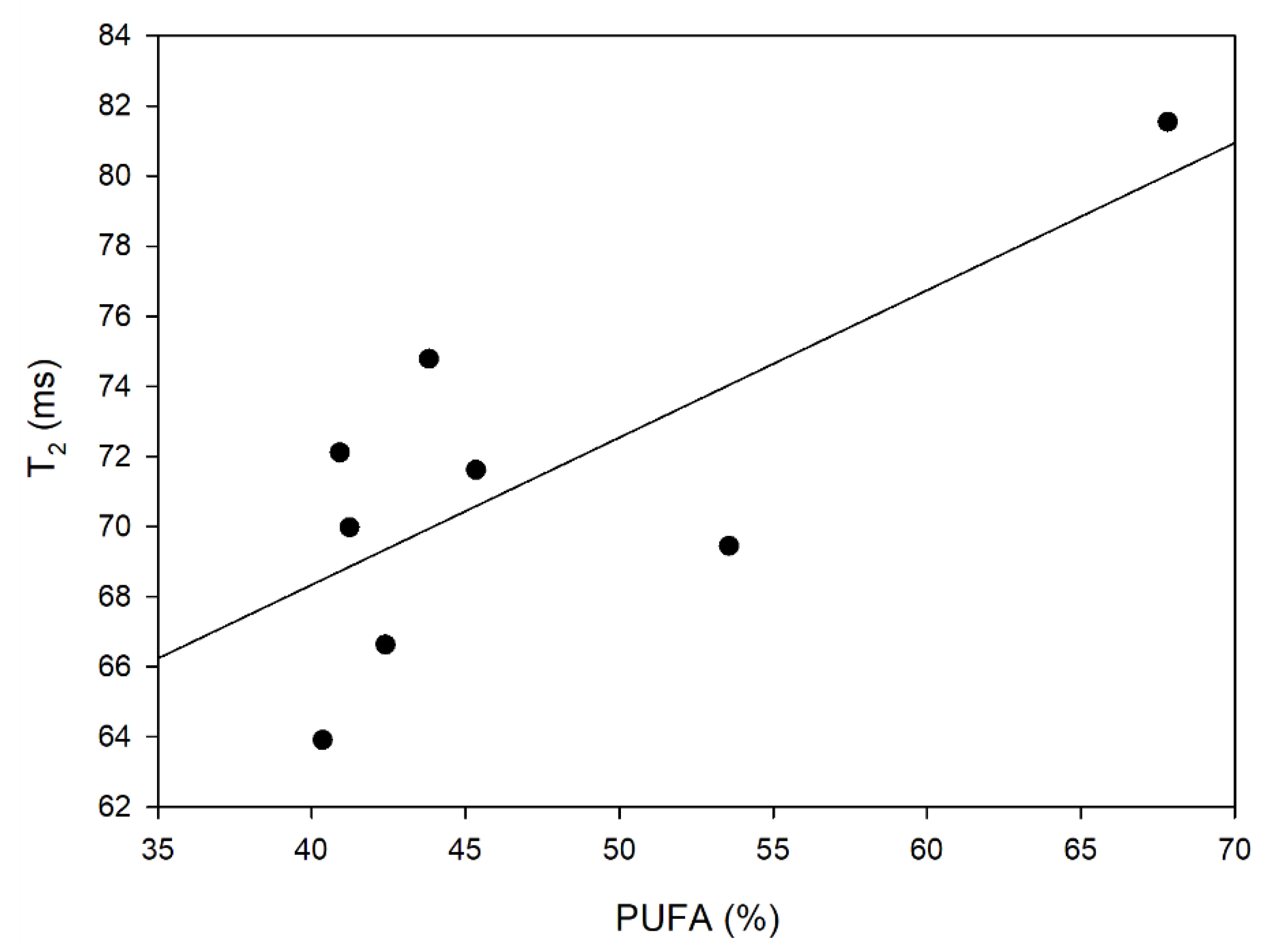
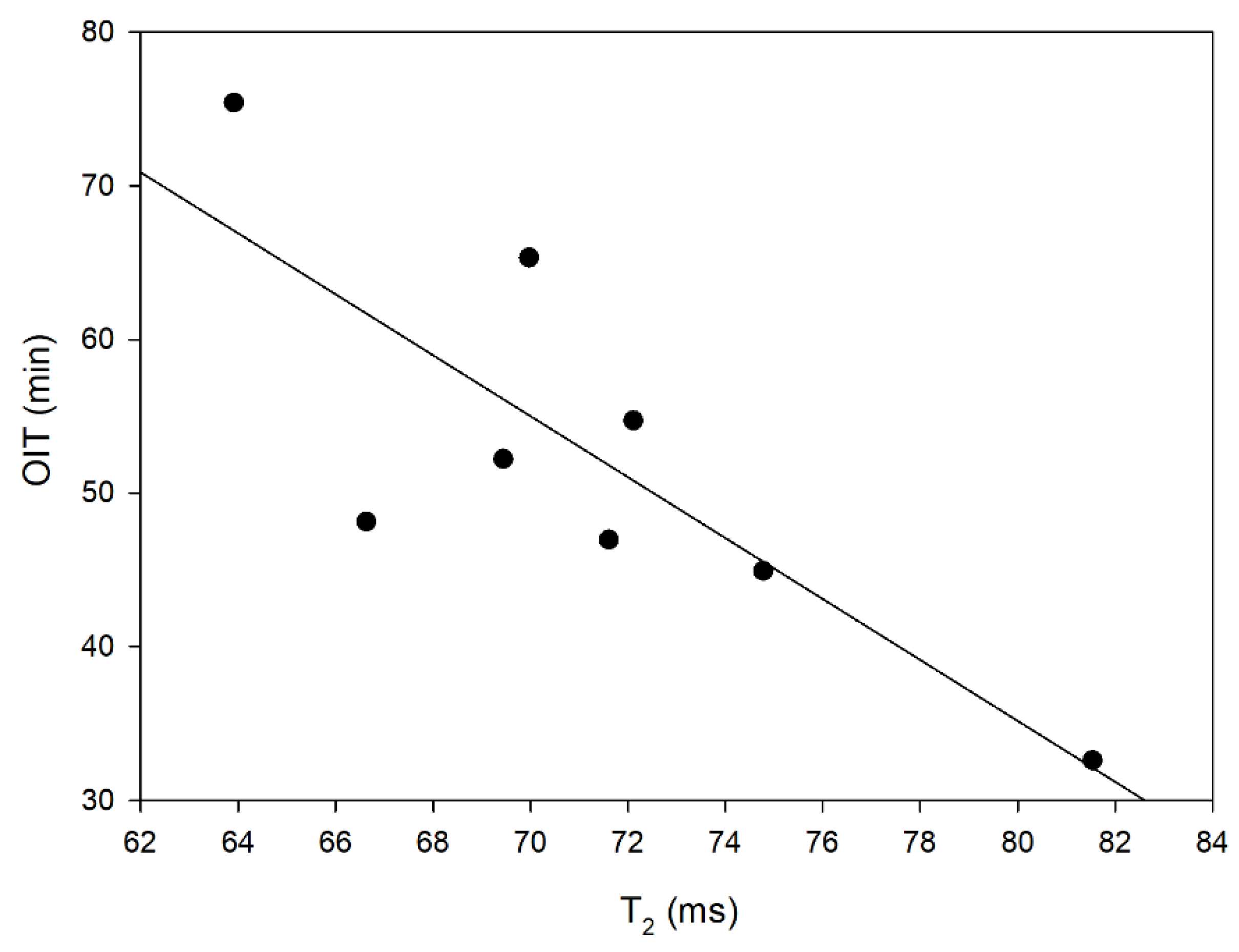
3.4. LF NMR Relaxometry and DSC Oxidation Induction Time
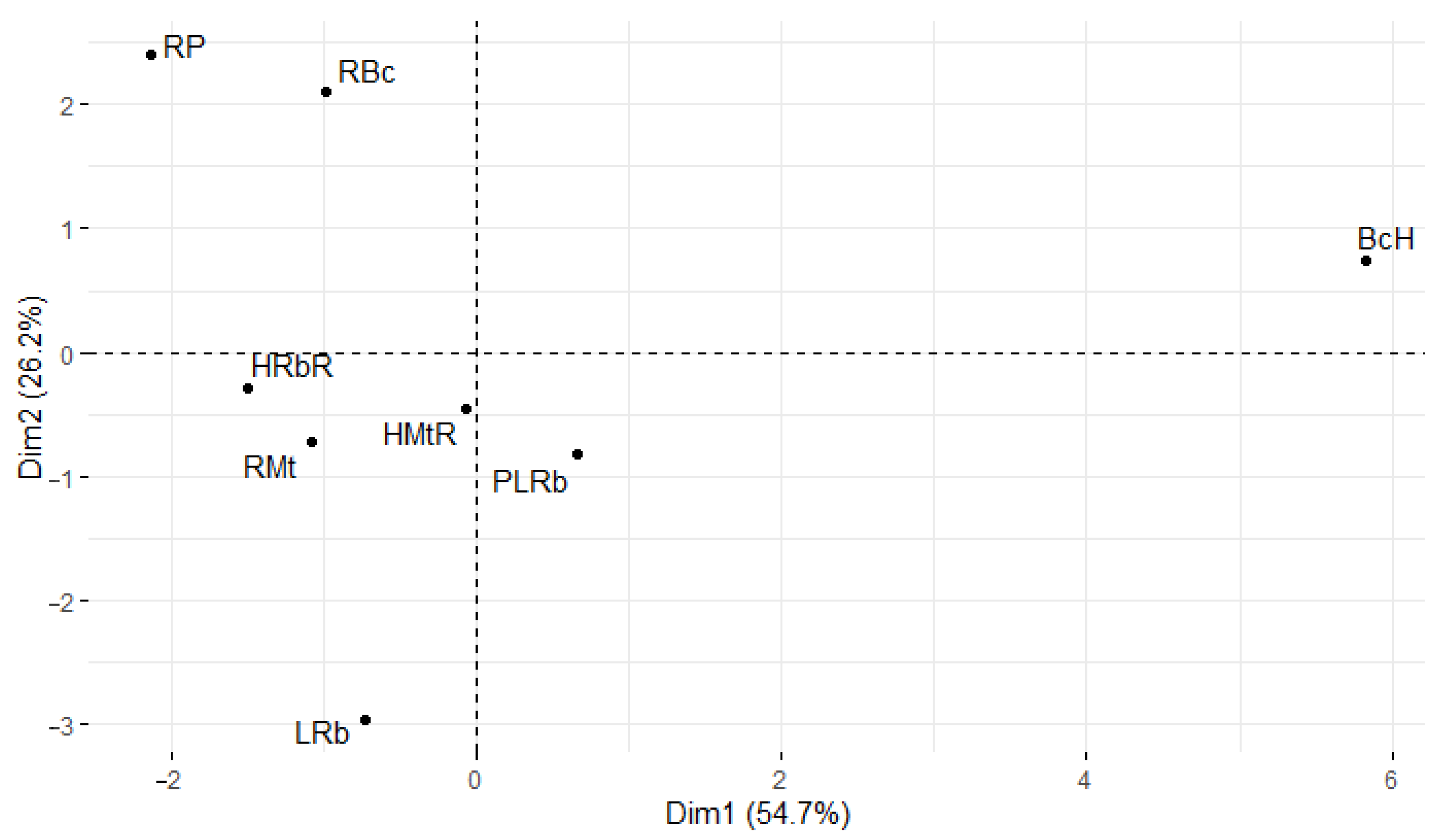
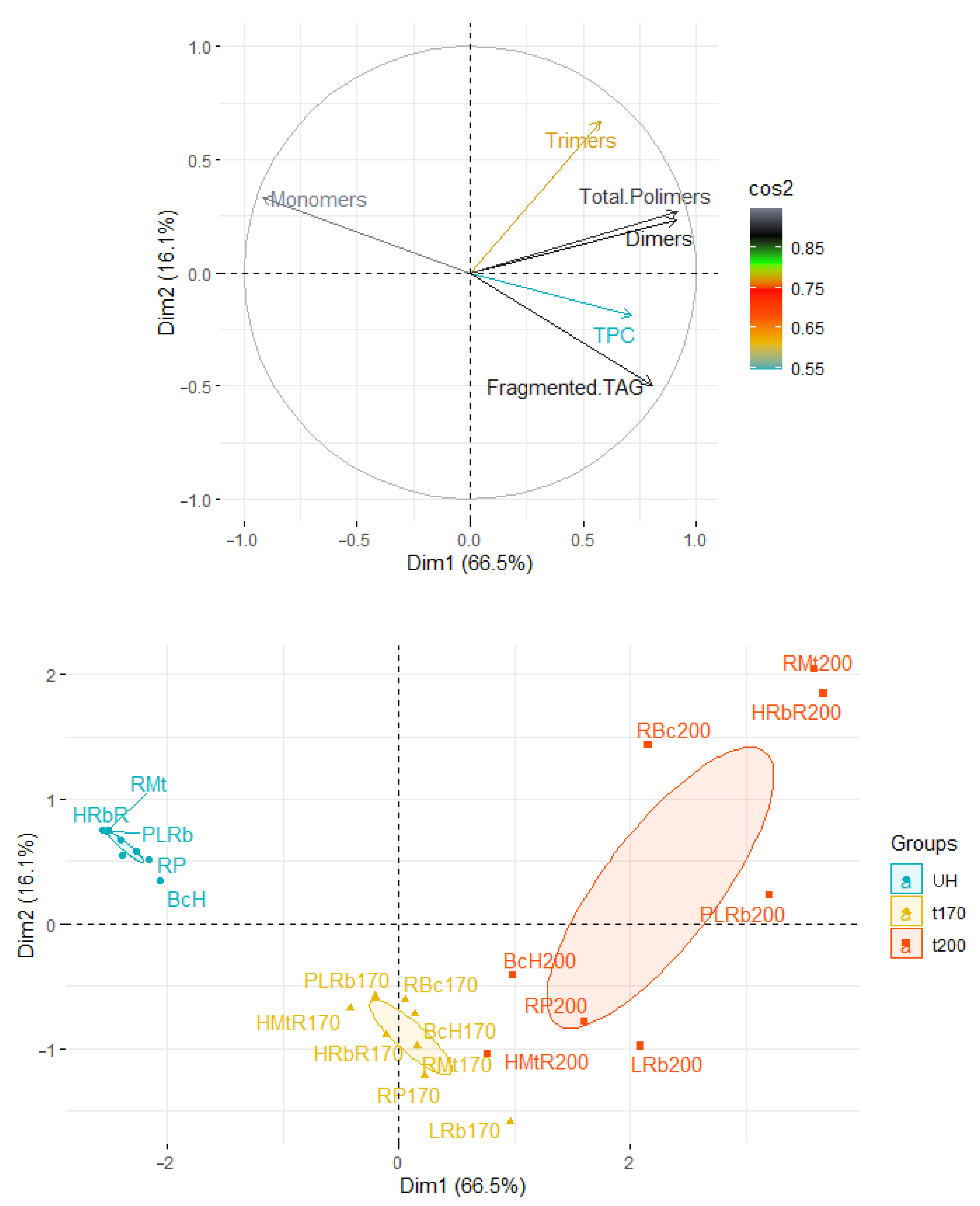
3.5. Statistical Considerations on the Influence of the Analyzed Parameters on the Stability of Oils
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Micić, D.M.; Ostojić, S.B.; Simonović, M.B.; Krstić, G.; Pezo, L.L.; Simonović, B.R. Kinetics of blackberry and raspberry seed oils oxidation by DSC. Thermochim. Acta 2015, 601, 39–44. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filoda, P.F.; Chaves, F.C.; Hoffmann, J.F.; Rombaldi, C.V. Olive oil: A review on the identity and quality of olive oils produced in Brazil. Rev. Bras. Frutic. 2021, 43, e847. [Google Scholar] [CrossRef]
- Rani, H.; Sharma, S.; Bala, M. Technologies for extraction of oil from oilseeds and other plant sources in retrospect and prospects: A review. J. Food Process. Eng. 2021, 44, e13851. [Google Scholar] [CrossRef]
- Matthäus, B. Oil Technology. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2007; pp. 483–527. [Google Scholar]
- Kmiecik, D.; Fedko, M.; Siger, A.; Kowalczewski, P.Ł. Nutritional Quality and Oxidative Stability during Thermal Processing of Cold-Pressed Oil Blends with 5:1 Ratio of ω6/ω3 Fatty Acids. Foods 2022, 11, 1081. [Google Scholar] [CrossRef] [PubMed]
- Chouaibi, M.; Rezig, L.; Gaout, N.; Ben Daoued, K.; Msaada, K.; Hamdi, S. Cold pressed Pistacia lentiscus seed oils. In Cold Pressed Oils; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–384. [Google Scholar]
- Zhang, Y.-Y.; Zhang, F.; Thakur, K.; Ci, A.-T.; Wang, H.; Zhang, J.-G.; Wei, Z.-J. Effect of natural polyphenol on the oxidative stability of pecan oil. Food Chem. Toxicol. 2018, 119, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Masui, E.; Tamogami, S.; Chen, J.; Zhang, H. Analysis of the Dynamic Decomposition of Unsaturated Fatty Acids and Tocopherols in Commercial Oils during Deep Frying. Anal. Lett. 2019, 52, 1991–2005. [Google Scholar] [CrossRef]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostagland. Leukot. Essent. Fat. Acids 2018, 132, 34–40. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Evolutionary Aspects of Diet: The Omega-6/Omega-3 Ratio and the Brain. Mol. Neurobiol. 2011, 44, 203–215. [Google Scholar] [CrossRef]
- Cichocki, W.; Czerniak, A.; Smarzyński, K.; Jeżowski, P.; Kmiecik, D.; Baranowska, H.M.; Walkowiak, K.; Ostrowska-Ligęza, E.; Różańska, M.B.; Lesiecki, M.; et al. Physicochemical and Morphological Study of the Saccharomyces cerevisiae Cell-Based Microcapsules with Novel Cold-Pressed Oil Blends. Appl. Sci. 2022, 12, 6577. [Google Scholar] [CrossRef]
- Innis, S.M. Omega-3 Fatty Acid Biochemistry: Perspectives from Human Nutrition. Mil. Med. 2014, 179, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, I.; Kornsteiner, M. Fats and Fatty Acid Requirements for Adults. Ann. Nutr. Metab. 2009, 55, 56–75. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.; Afonso, C.; Bandarra, N.M. Seafood lipids and cardiovascular health. Nutrire 2016, 41, 7. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Akhilender Naidu, K.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R.; American Dietetic Association. Position of the American Dietetic Association: Vegetarian Diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, D.; Fedko, M.; Małecka, J.; Siger, A.; Kowalczewski, P.Ł. Effect of Heating Temperature of High-Quality Arbequina, Picual, Manzanilla and Cornicabra Olive Oils on Changes in Nutritional Indices of Lipid, Tocopherol Content and Triacylglycerol Polymerization Process. Molecules 2023, 28, 4247. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Imran, A.; Nosheen, F.; Fatima, M.; Arshad, M.U.; Afzaal, M.; Ijaz, N.; Noreen, R.; Mehta, S.; Biswas, S.; et al. Functional roles and novel tools for improving-oxidative stability of polyunsaturated fatty acids: A comprehensive review. Food Sci. Nutr. 2023, 11, 2471–2482. [Google Scholar] [CrossRef]
- Jackson, V.; Penumetcha, M. Dietary oxidised lipids, health consequences and novel food technologies that thwart food lipid oxidation: An update. Int. J. Food Sci. Technol. 2019, 54, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Karrar, E.; Mahdi, A.A.; Sheth, S.; Mohamed Ahmed, I.A.; Manzoor, M.F.; Wei, W.; Wang, X. Effect of maltodextrin combination with gum arabic and whey protein isolate on the microencapsulation of gurum seed oil using a spray-drying method. Int. J. Biol. Macromol. 2021, 171, 208–216. [Google Scholar] [CrossRef]
- Różańska, M.B.; Kowalczewski, P.Ł.; Tomaszewska-Gras, J.; Dwiecki, K.; Mildner-Szkudlarz, S. Seed-Roasting Process Affects Oxidative Stability of Cold-Pressed Oils. Antioxidants 2019, 8, 313. [Google Scholar] [CrossRef] [Green Version]
- Erickson, M.D.; Yevtushenko, D.P.; Lu, Z.-X. Oxidation and Thermal Degradation of Oil during Frying: A Review of Natural Antioxidant Use. Food Rev. Int. 2022, 1–32. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Staton, J.A.; Farkas, B.E. Effect of frying oil degradation on surface tension and wettability. LWT 2019, 99, 519–524. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef] [PubMed]
- Valdés García, A.; Beltrán Sanahuja, A.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G.; Garrigós, M.C. Effect of Frying and Roasting Processes on the Oxidative Stability of Sunflower Seeds (Helianthus annuus) under Normal and Accelerated Storage Conditions. Foods 2021, 10, 944. [Google Scholar] [CrossRef]
- Sumnu, S.G.; Sahin, S. (Eds.) Advances in Deep-Fat Frying of Foods; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-42913-881-2. [Google Scholar]
- AOCS Official Method Ce 1h-05. Determination of cis-, trans-, Saturated, Monounsaturated and Polyunsaturated Fatty Acids in Vegetable or Non-Ruminant Animal Oils and Fats by Capillary GLC. In Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Champaign, IL, USA, 2009; pp. 1–29. [Google Scholar]
- AOCS Official Method 982.27. Polar Components in Frying Fats, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- AOCS Official Method 993.25. Polymerized Triglycerides in Oils and Fats, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- AOCS Official Method Cd 1c-85. Calculated Iodine Value, 6th ed.; American Oil Chemists’ Society: Champaign, IL, USA, 2009. [Google Scholar]
- ISO-8534:2008; Animal and Vegetable Fats and Oils. Determination of Water Content—Karl Fischer Method. ISO: Geneva, Switzerland, 2008.
- Fukushima, E.; Roeder, S.B.W. Experimental Pulse NMR; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-0-42949-386-7. [Google Scholar]
- Małyszek, Z.; Lewandowicz, J.; Le Thanh-Blicharz, J.; Walkowiak, K.; Kowalczewski, P.Ł.; Baranowska, H.M. Water Behavior of Emulsions Stabilized by Modified Potato Starch. Polymers 2021, 13, 2200. [Google Scholar] [CrossRef]
- Lee, H.; Woo, J.; Chen, Z.; Leung, S.; Peng, X. Serum fatty acid, lipid profile and dietary intake of Hong Kong Chinese omnivores and vegetarians. Eur. J. Clin. Nutr. 2000, 54, 768–773. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, M.; Jovanović, M.; Nikolić, K. Advantages and disadvantages of vegetarian nutrition. Zdr. Zast. 2019, 48, 51–56. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations; World Health Oragnization. WHO and FAO Joint Consultation: Fats and Oils in Human Nutrition. Nutr. Rev. 2009, 53, 202–205. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640S–646S. [Google Scholar] [CrossRef] [Green Version]
- Knothe, G. Structure indices in FA chemistry. How relevant is the iodine value? J. Am. Oil Chem. Soc. 2002, 79, 847–854. [Google Scholar] [CrossRef]
- Lam, H.-Y.; Roy, P.K.; Chattopadhyay, S. Thermal degradation in edible oils by surface enhanced Raman spectroscopy calibrated with iodine values. Vib. Spectrosc. 2020, 106, 103018. [Google Scholar] [CrossRef]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Gillingham, L.G.; Harris-Janz, S.; Jones, P.J.H. Dietary Monounsaturated Fatty Acids Are Protective against Metabolic Syndrome and Cardiovascular Disease Risk Factors. Lipids 2011, 46, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary Polyunsaturated Fatty Acids (PUFAs): Uses and Potential Health Benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef]
- Filip, S.; Hribar, J.; Vidrih, R. Influence of natural antioxidants on the formation of trans -fatty-acid isomers during heat treatment of sunflower oil. Eur. J. Lipid Sci. Technol. 2011, 113, 224–230. [Google Scholar] [CrossRef]
- Zhang, Q.; Saleh, A.S.M.; Chen, J.; Shen, Q. Chemical alterations taken place during deep-fat frying based on certain reaction products: A review. Chem. Phys. Lipids 2012, 165, 662–681. [Google Scholar] [CrossRef]
- Rudzińska, M.; Hassanein, M.M.M.; Abdel-Razek, A.G.; Kmiecik, D.; Siger, A.; Ratusz, K. Influence of composition on degradation during repeated deep-fat frying of binary and ternary blends of palm, sunflower and soybean oils with health-optimised saturated-to-unsaturated fatty acid ratios. Int. J. Food Sci. Technol. 2018, 53, 1021–1029. [Google Scholar] [CrossRef]
- Grajzer, M.; Szmalcel, K.; Kuźmiński, Ł.; Witkowski, M.; Kulma, A.; Prescha, A. Characteristics and Antioxidant Potential of Cold-Pressed Oils—Possible Strategies to Improve Oil Stability. Foods 2020, 9, 1630. [Google Scholar] [CrossRef]
- Machado, M.; Rodriguez-Alcalá, L.M.; Gomes, A.M.; Pintado, M. Vegetable oils oxidation: Mechanisms, consequences and protective strategies. Food Rev. Int. 2022, 1–18. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Salta, F.N.; Chiou, A.; Andrikopoulos, N.K. Formation and distribution of oxidized fatty acids during deep- and pan-frying of potatoes. Eur. J. Lipid Sci. Technol. 2007, 109, 1111–1123. [Google Scholar] [CrossRef]
- Lampi, A.-M.; Kamal-Eldin, A. Effect of α- and γ-tocopherols on thermal polymerization of purified high-oleic sunflower triacylglycerols. J. Am. Oil Chem. Soc. 1998, 75, 1699–1703. [Google Scholar] [CrossRef]
- Budilarto, E.S.; Kamal-Eldin, A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. Eur. J. Lipid Sci. Technol. 2015, 117, 1095–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosio, E.; Gianferri, R.R. Low-resolution NMR—An analytical tool in foods characterization and traceability. In Basic NMR in Foods Characterization; Brosio, E., Ed.; Research Signpost: Kerala, India, 2009; pp. 9–37. ISBN 978-81-308-0303-6. [Google Scholar]
- Osheter, T.; Campisi-Pinto, S.; Resende, M.T.; Linder, C.; Wiesman, Z. 1H LF-NMR Self-Diffusion Measurements for Rapid Monitoring of an Edible Oil’s Food Quality with Respect to Its Oxidation Status. Molecules 2022, 27, 6064. [Google Scholar] [CrossRef] [PubMed]
- Resende, M.T.; Linder, C.; Wiesman, Z. 1H LF-NMR Energy Relaxation Time Characterization of the Chemical and Morphological Structure of PUFA-Rich Linseed Oil During Oxidation with and without Antioxidants. Eur. J. Lipid Sci. Technol. 2019, 121, 1800339. [Google Scholar] [CrossRef]
- Makowska, A.; Dwiecki, K.; Kubiak, P.; Baranowska, H.M.; Lewandowicz, G. Polymer-Solvent Interactions in Modified Starches Pastes–Electrokinetic, Dynamic Light Scattering, Rheological and Low Field Nuclear Magnetic Resonance Approach. Polymers 2022, 14, 2977. [Google Scholar] [CrossRef] [PubMed]
- Pignitter, M.; Somoza, V. Critical evaluation of methods for the measurement of oxidative rancidity in vegetable oils. J. Food Drug Anal. 2020, 20, 772–777. [Google Scholar] [CrossRef]
- Tan, C.; Che Man, Y. Recent developments in differential scanning calorimetry for assessing oxidative deterioration of vegetable oils. Trends Food Sci. Technol. 2002, 13, 312–318. [Google Scholar] [CrossRef]
- Vecchio Ciprioti, S.; Paciulli, M.; Chiavaro, E. Application of different thermal analysis techniques to characterize oxidized olive oils. Eur. J. Lipid Sci. Technol. 2017, 119, 1600074. [Google Scholar] [CrossRef]
- Flores, M.; Avendaño, V.; Bravo, J.; Valdés, C.; Forero-Doria, O.; Quitral, V.; Vilcanqui, Y.; Ortiz-Viedma, J. Edible Oil Parameters during Deterioration Processes. Int. J. Food Sci. 2021, 2021, 7105170. [Google Scholar] [CrossRef] [PubMed]
- Stauffer, C.E. Emulsifiers; American Association of Cereal Chemists, Inc.: Saint Paul, MN, USA, 1999; ISBN 1-89-11270-20. [Google Scholar]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Code | Type of Oil | Percentage (%) | ||||
|---|---|---|---|---|---|---|
| RBc | Rapeseed oil | Black cumin oil | - | 60 | 40 | - |
| RMt | Rapeseed oil | Milk thistle seed oil | - | 50 | 50 | - |
| RP | Rapeseed oil | Pumpkin seed oil | - | 58 | 42 | - |
| BcH | Black cumin oil | Hemp oil | - | 40 | 60 | - |
| HMtR | Hemp oil | Milk thistle seed oil | Rapeseed oil | 10 | 50 | 40 |
| LRb | Linseed oil | Rice bran oil | - | 11 | 89 | - |
| HRbR | Hemp oil | Rice bran oil | Rapeseed oil | 15 | 55 | 30 |
| PLRb | Pumpkin seed oil | Linseed oil | Rice bran oil | 60 | 15 | 25 |
| Oil Blends 1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | Ratio ω6/ω3 | CIV |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RBc | 7.76 ± 0.13 | 0.18 ± 0.03 | 2.21 ± 0.04 | 48.52 ± 0.08 | 34.17 ± 0.26 | 7.07 ± 0.06 | 0.10 ± 0.01 | nd | nd | 4.84:1 | 119.56 ± 0.20 |
| RMt | 6.52 ± 0.05 | 0.12 ± 0.01 | 3.88 ± 0.05 | 46.23 ± 1.45 | 34.36 ± 1.09 | 6.55 ± 0.34 | 1.55 ± 0.07 | nd | 0.80 ± 0.01 | 5.25:1 | 116.51 ± 1.55 |
| RP | 8.23 ± 0.24 | 0.15 ± 0.01 | 3.57 ± 0.04 | 47.54 ± 0.73 | 33.65 ± 0.46 | 6.70 ± 0.01 | 0.14 ± 0.01 | nd | 0.03 ± 0.01 | 5.02:1 | 116.83 ± 0.14 |
| BcH | 8.56 ± 0.34 | 0.12 ± 0.03 | 2.69 ± 0.04 | 18.46 ± 0.08 | 56.40 ± 0.54 | 11.43 ± 0.16 | 0.37 ± 0.01 | 1.84 ± 0.07 | 0.13 ± 0.02 | 4.93:1 | 145.03 ± 0.49 |
| HMtR | 6.65 ± 0.24 | 0.11 ± 0.00 | 3.96 ± 0.07 | 41.22 ± 1.10 | 38.06 ± 0.87 | 7.27 ± 0.38 | 1.60 ± 0.13 | 0.31 ± 0.00 | 0.82 ± 0.03 | 5.24:1 | 120.75 ± 1.55 |
| LRb | 15.64 ± 0.06 | 0.13 ± 0.03 | 1.79 ± 0.12 | 37.49 ± 0.81 | 36.67 ± 0.95 | 7.14 ± 0.38 | 0.57 ± 0.00 | 0.55 ± 0.03 | 0.01 ± 0.00 | 5.14:1 | 115.00 ± 0.02 |
| HRbR | 11.61 ± 0.09 | 0.14 ± 0.02 | 1.75 ± 0.08 | 42.88 ± 0.33 | 35.47 ± 0.02 | 6.92 ± 0.16 | 0.41 ± 0.03 | 0.80 ± 0.00 | 0.03 ± 0.00 | 5.13:1 | 117.17 ± 0.08 |
| PLRb | 12.91 ± 0.07 | 0.11 ± 0.00 | 4.63 ± 0.10 | 28.21 ± 0.05 | 44.93 ± 0.05 | 8.64 ± 0.05 | 0.37 ± 0.01 | 0.16 ± 0.01 | 0.05 ± 0.00 | 5.20:1 | 124.90 ± 0.09 |
| Oil Blends 1 | PUFA/SFA | IA | IT | HH |
|---|---|---|---|---|
| RBc | 4.09 | 0.09 | 0.15 | 11.57 |
| RMt | 3.21 | 0.07 | 0.16 | 13.36 |
| RP | 3.37 | 0.09 | 0.18 | 10.68 |
| BcH | 5.77 | 0.10 | 0.14 | 10.08 |
| HMtR | 3.48 | 0.08 | 0.16 | 13.01 |
| LRb | 2.43 | 0.19 | 0.27 | 5.20 |
| HRbR | 3.07 | 0.13 | 0.20 | 7.34 |
| PLRb | 2.98 | 0.16 | 0.26 | 6.33 |
| Oil Blends 1 | Unheated | Heating Temperature (°C) | |
|---|---|---|---|
| 170 | 200 | ||
| RBc | 5.41 ± 0.10 aE | 7.41 ± 0.14 bCD | 10.74 ± 0.17 cB |
| RMt | 3.78 ± 0.14 aC | 8.99 ± 0.10 bE | 11.74 ± 0.14 cC |
| RP | 6.45 ± 0.30 aF | 10.54 ± 0.13 bG | 14.53 ± 0.23 cF |
| BcH | 4.98 ± 0.15 aDE | 7.84 ± 0.17 bD | 12.74 ± 0.06 cD |
| HMtR | 4.62 ± 0.16 aD | 9.57 ± 0.17 bF | 14.98 ± 0.27 cF |
| LRb | 1.84 ± 0.14 aA | 4.01 ± 0.11 bA | 7.96 ± 0.04 cA |
| HRbR | 2.54 ± 0.04 aB | 5.29 ± 0.13 bB | 7.88 ± 0.23 cA |
| PLRb | 3.41 ± 0.04 aC | 7.05 ± 0.14 bC | 13.45 ± 0.00 cE |
| Oil Blends 1 | Water Content (ppm) | T1 Relaxation Time (ms) | T2 Relaxation Time (ms) | τc (s) | OIT (min) |
|---|---|---|---|---|---|
| RBc | 536.5 ± 4.9 b | 106.73 ± 0.33 c | 69.97 ± 1.22 d | 4.9298 × 10−9 | 65.33 |
| RMt | 471.7 ± 6.6 c | 102.91 ± 0.43 d | 72.11 ± 1.56 cd | 4.4037 × 10−9 | 54.72 |
| RP | 823.5 ± 10.5 a | 105.40 ± 0.38 c | 63.91 ± 1.40 f | 5.5434 × 10−9 | 75.40 |
| BcH | 516.1 ± 18.1 b | 118.90 ± 0.33 a | 81.54 ± 1.42 a | 4.5746 × 10−9 | 32.60 |
| HMtR | 341.4 ± 14.6 d | 106.23 ± 0.34 c | 71.61 ± 1.41 d | 4.7103 × 10−9 | 46.95 |
| LRb | 176.4 ± 4.7 e | 102.51 ± 0.28 d | 74.78 ± 1.45 bc | 4.0809 × 10−9 | 44.93 |
| HRbR | 556.6 ± 20.9 b | 102.74 ± 0.29 d | 66.63 ± 1.23 e | 5.0148 × 10−9 | 48.13 |
| PLRb | 458.6 ± 13.6 c | 108.95 ± 0.32 b | 69.44 ± 1.34 d | 5.1513 × 10−9 | 52.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cichocki, W.; Kmiecik, D.; Baranowska, H.M.; Staroszczyk, H.; Sommer, A.; Kowalczewski, P.Ł. Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies. Foods 2023, 12, 2660. https://doi.org/10.3390/foods12142660
Cichocki W, Kmiecik D, Baranowska HM, Staroszczyk H, Sommer A, Kowalczewski PŁ. Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies. Foods. 2023; 12(14):2660. https://doi.org/10.3390/foods12142660
Chicago/Turabian StyleCichocki, Wojciech, Dominik Kmiecik, Hanna Maria Baranowska, Hanna Staroszczyk, Agata Sommer, and Przemysław Łukasz Kowalczewski. 2023. "Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies" Foods 12, no. 14: 2660. https://doi.org/10.3390/foods12142660
APA StyleCichocki, W., Kmiecik, D., Baranowska, H. M., Staroszczyk, H., Sommer, A., & Kowalczewski, P. Ł. (2023). Chemical Characteristics and Thermal Oxidative Stability of Novel Cold-Pressed Oil Blends: GC, LF NMR, and DSC Studies. Foods, 12(14), 2660. https://doi.org/10.3390/foods12142660











