Effect of Dynamic High-Pressure Microfluidizer on Physicochemical and Microstructural Properties of Whole-Grain Oat Pulp
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Whole-Grain Oat Pulp
2.3. Dispersion Characteristics of a Stable System
2.4. Particle Characteristics
2.5. Typical Nutrient Content of Whole-Grain Oat Pulp
2.5.1. Determination of β-Glucan Content
2.5.2. Determination of Soluble Protein Content
2.5.3. Determination of Insoluble and Soluble Dietary Fiber Content
2.6. Rheological Properties
2.7. Water Relaxation Properties
2.8. Confocal Laser Scanning Microscope Analysis
2.9. Atomic Force Microscope (AFM) Analysis
2.10. Statistical Analysis
3. Results and Discussion
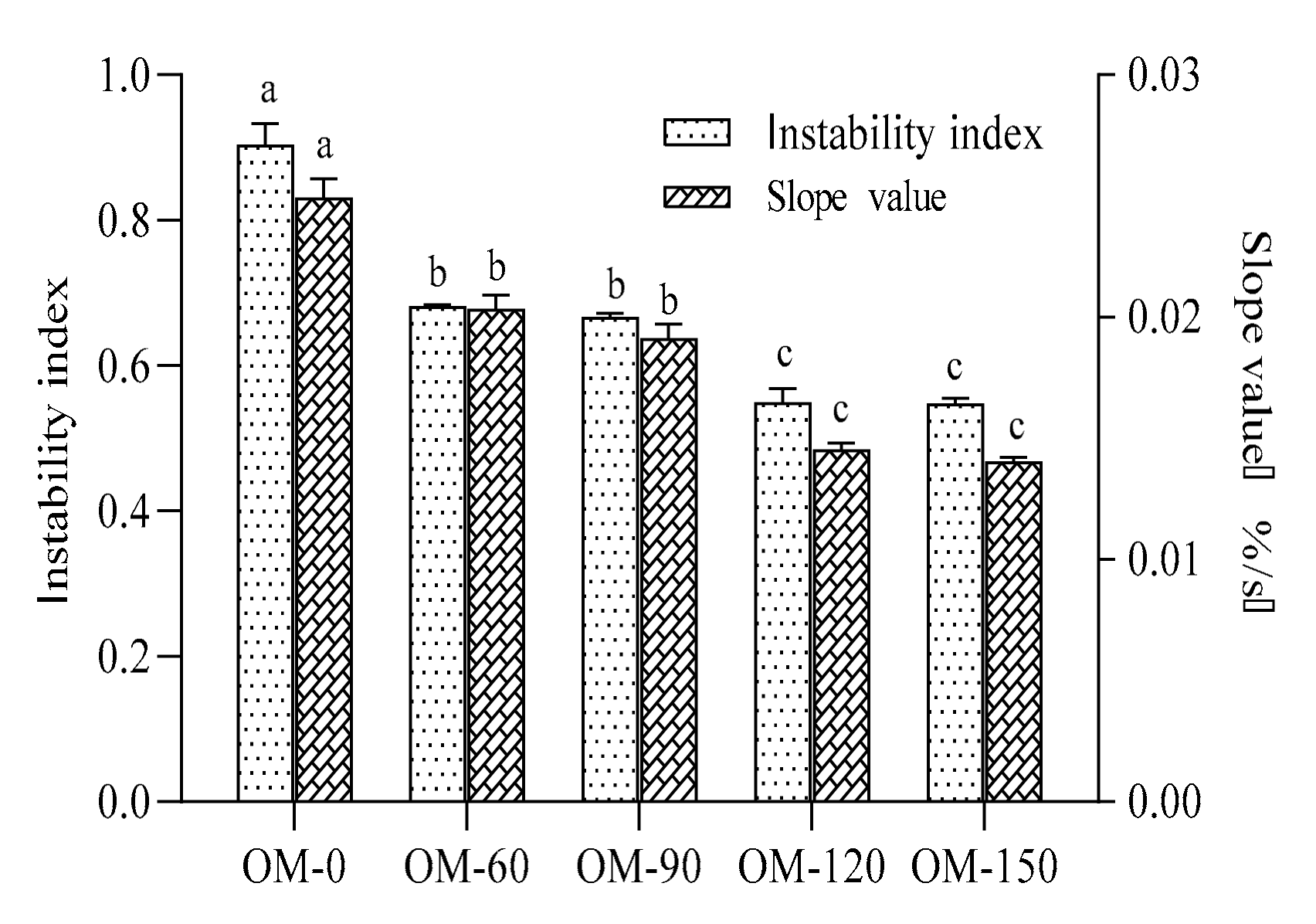
3.1. Stable System Dispersion Characteristics of Whole-Grain Oat Pulp
3.2. Typical Nutrient Content of Whole-Grain Oat Pulp
3.3. Particle Characteristics
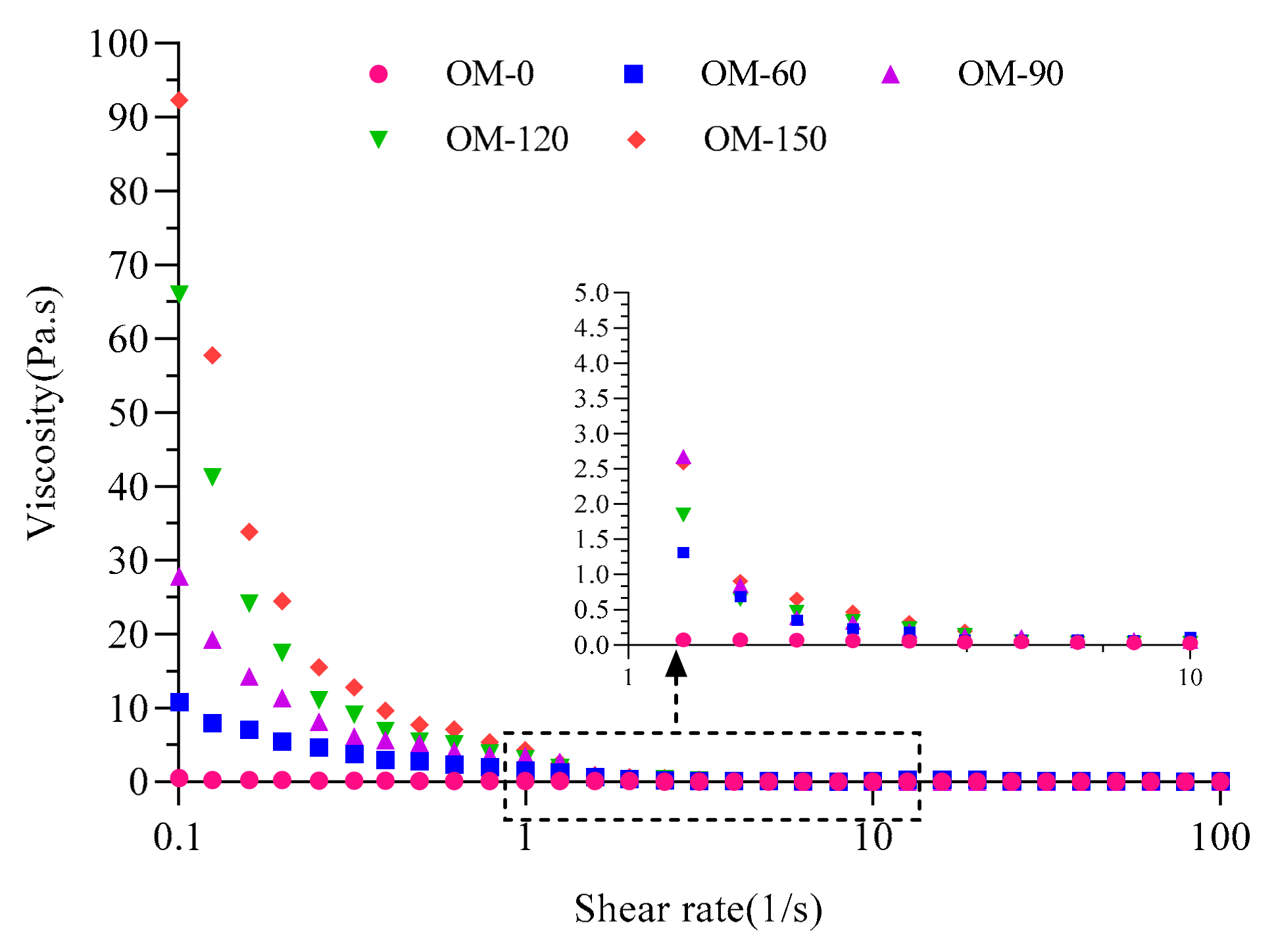
3.4. Rheological Properties
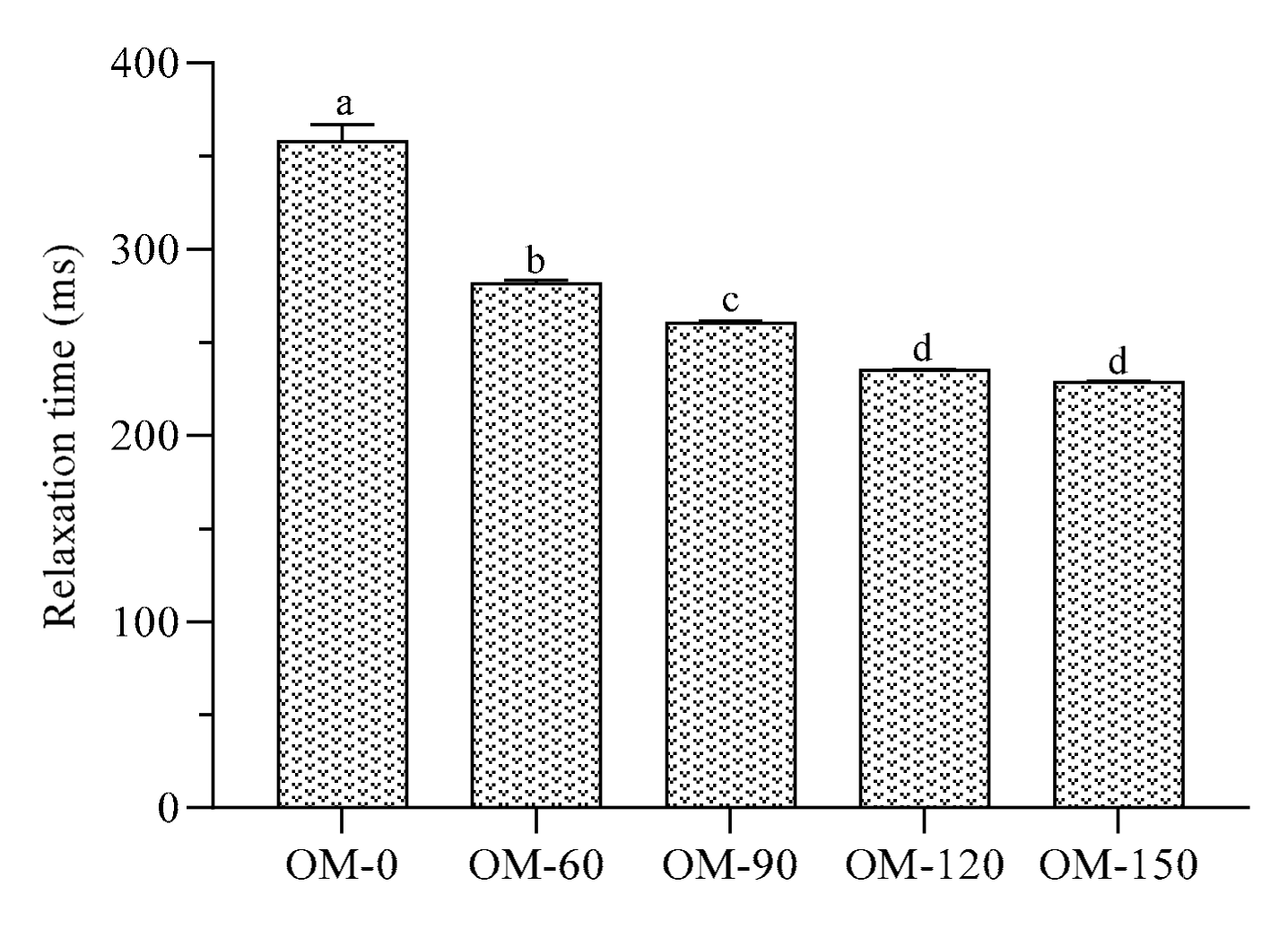
3.5. Water Relaxation Properties
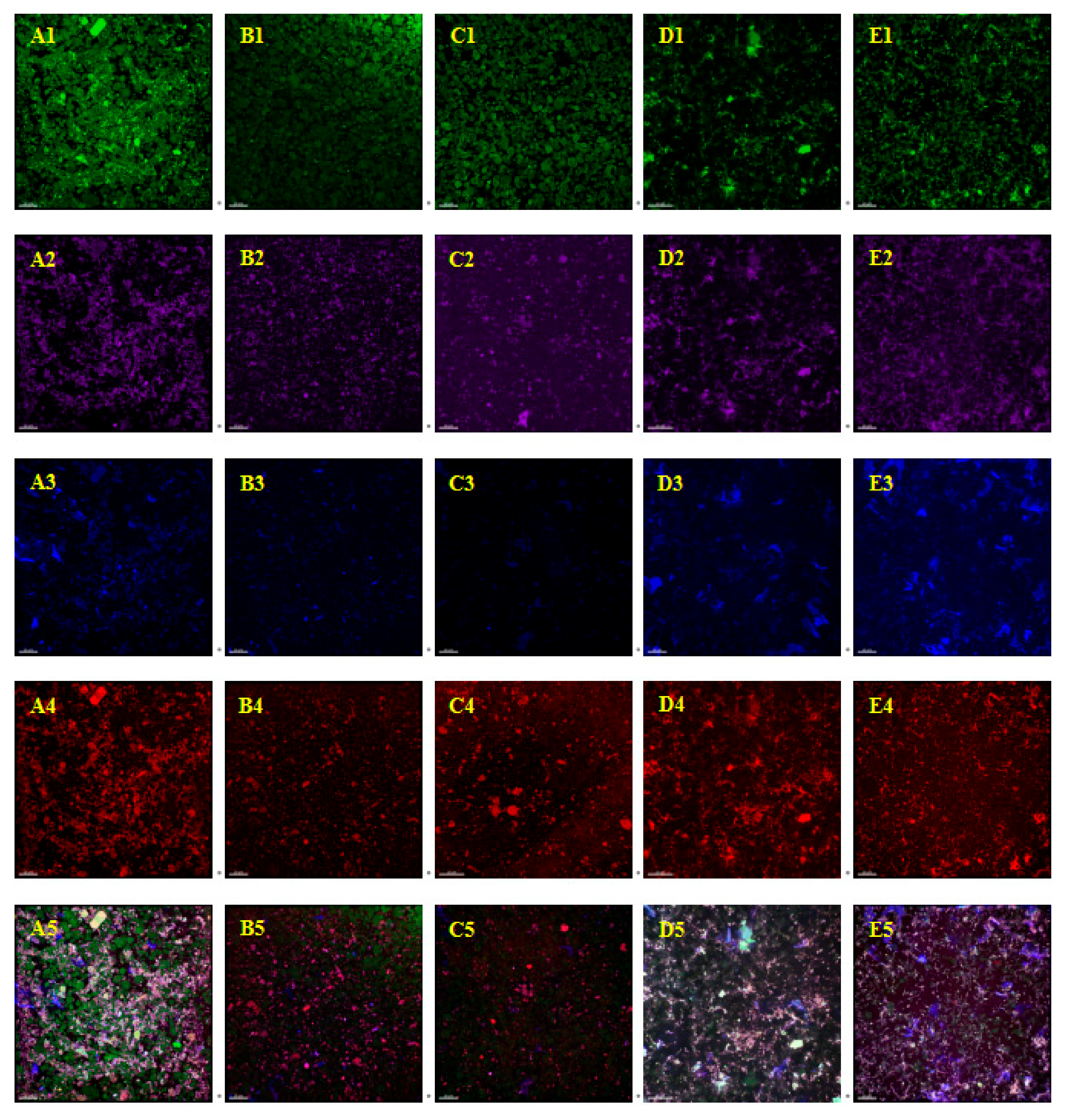
3.6. Confocal Laser Scanning Microscope (CLSM) Analysis
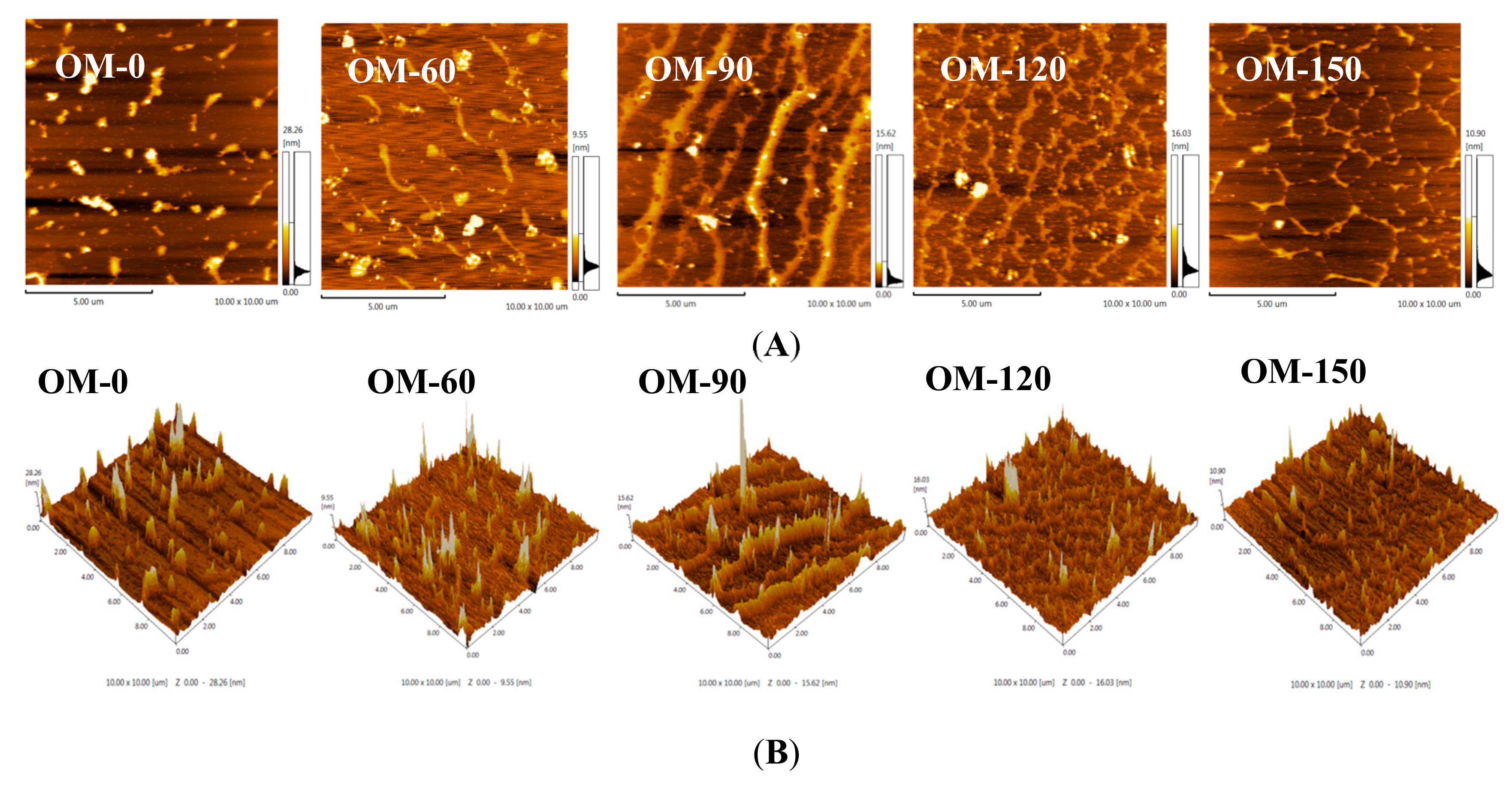
3.7. Atomic Force Microscope (AFM) Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamal, N.; Renhuldt, N.T.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef]
- Kumar, L.; Sehrawat, R.; Kong, Y.Z. Oat proteins: A perspective on functional properties. LWT—Food Sci. Technol. 2021, 152, 112307. [Google Scholar] [CrossRef]
- Hu, C.L.; Tang, Y.; Zhao, Y.T.; Sang, S.M. Quantitative analysis and anti-inflammatory activity evaluation of the a-type avenanthramides in commercial sprouted oat products. J. Agric. Food Chem. 2019, 68, 13068–13075. [Google Scholar] [CrossRef]
- Tamura, K.; Hemsworth, G.R.; Déjean, G.; Rogers, T.E.; Pudlo, N.A.; Urs, K.; Jain, N.; Davies, G.J.; Martens, E.C.; Brumer, H. Molecular mechanism by which prominent human gut bacteroidetes utilize mixed-linkage β-glucans, major health promoting cereal polysaccharides. Cell Rep. 2017, 21, 417–430. [Google Scholar] [CrossRef] [Green Version]
- Grundy, M.M.L.; Quint, J.; Rieder, A.; Ballance, S.; Dreiss, C.A.; Cross, K.L.; Gray, R.; Bajka, B.H.; Butterworth, P.J.; Ellis, P.R.; et al. The impact of oat structure and β-glucan on in vitro lipid digestion. J. Funct. Foods 2017, 38, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Zhang, S.R.; Guan, X.; Li, C.; Li, S.; Liu, Y.Y.; Shi, J.L. Effect of the oat β-glucan on the development of functional quinoa (Chenopodium quinoa wild) milk. Food Chem. 2021, 349, 129201. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Bao, Y.L.; Liu, F.Y.; Wang, H.Y.; Wang, Y.Q. Oat: Current state and challenges in plant-based food applications. Trends Food Sci. Technol. 2023, 134, 56–71. [Google Scholar] [CrossRef]
- Coda, R.; Lanera, A.; TRANI, A.; Gobbetti, M.; Cagno, R.D. Yogurt-like beverages made of a mixture of cereals, soy and grape must: Microbiology, texture, nutritional and sensory properties. Int. J. Food Microbiol. 2012, 155, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Tiffany, P.; Åsmund, R.; Karsten, O. The physical stability of plant-based drinks and the analysis methods thereof. Food Hydrocoll. 2021, 118, 106770. [Google Scholar] [CrossRef]
- Deswal, A.; Deora, N.S.; Mishra, H.N. Optimization of enzymatic production process of oat milk using response surface methodology. Food Bioprocess Technol. 2014, 7, 610–618. [Google Scholar] [CrossRef]
- Li, Y.T.; Chen, M.S.; Deng, L.Z.; Liang, Y.Z.; Liu, Y.K.; Liu, W.; Chen, J.; Liu, C.M. Whole soybean milk produced by a novel industry-scale micofluidizer system without soaking and filtering. J. Food Eng. 2021, 291, 110228. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.H.; Liu, W.; Liu, C.-M.; Li, T.; Tu, Z.-C.; Wan, J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Li, Y.T.; Wang, R.S.; Liang, R.H.; Chen, J.; He, X.H.; Chen, R.Y.; Liu, W.; Liu, C.M. Dynamic high-pressure microfluidization assisting octenyl succinic anhydride modification of rice starch. Carbohydr. Polym. 2018, 193, 336–342. [Google Scholar] [CrossRef]
- Liu, C.M.; Liang, R.H.; Dai, T.T.; Ye, J.P.; Zeng, Z.C.; Luo, S.J.; Chen, J. Effect ofdynamic high pressure microfluidization modified insoluble dietary fiber on gelatinization and rheology of rice starch. Food Hydrocoll. 2016, 57, 55–61. [Google Scholar] [CrossRef]
- Wang, X.M.; Zhu, X.M.; Zhang, N.H.; Tu, Z.C.; Wang, H.; Liu, G.X.; Ye, Y.H. Morphological and structural characteristics of rice amylose by dynamic high-pressure microfluidization modification. J. Food Process Preserv. 2018, 42, e13764. [Google Scholar] [CrossRef]
- Tai, K.D.; Liu, F.G.; He, X.Y.; Ma, P.H.; Mao, L.K.; Gao, Y.X.; Yuan, F. The effect of sterol derivatives on properties of soybean and egg yolk lecithin liposomes: Stability, structure and membrane characteristics. Food Res. Int. 2018, 109, 24–34. [Google Scholar] [CrossRef]
- Lu, X.; Chen, J.; Zheng, M.; Guo, J.; Qi, J.; Chen, Y.; Miao, S.; Zheng, B. Effect of high-intensity ultrasound irradiation on the stability and structural features of coconut-grain milk composite systems utilizing maize kernels and starch with different amylose contents. Ultrason. Sonochem. 2019, 55, 135–148. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Mali, S.; Grossmann, M.V.E.; Yamashita, F.; Garcia, M.A. Effects of plasticizers on the properties of oat starch films. Mat. Sci. Eng. C 2009, 29, 532–538. [Google Scholar] [CrossRef]
- Sharafbafi, N.; Tosh, S.M.; Alexander, M.; Corredig, M. Phase behaviour, rheological properties, and microstructure of oat β-glucan-milk mixtures. Food Hydrocoll. 2014, 41, 274–280. [Google Scholar] [CrossRef]
- Patra, T.; Olsen, K.; Rinnan, Å. A multivariate perspective on the stability of oat-based drinks assessed by spectroscopy. Food Hydrocoll. 2022, 131, 107831. [Google Scholar] [CrossRef]
- Verran, J.; Rowe, D.L.; Cole, D.; Boyd, R.D. The use of the atomic force microscope to visualise and measure wear of food contact surfaces. Int. Biodeterior. Biodegrad. 2000, 46, 99–105. [Google Scholar] [CrossRef]
- Niu, H.; Wang, W.D.; Dou, Z.M.; Chen, X.W.; Chen, X.X.; Chen, H.M.; Fu, X. Multiscale combined techniques for evaluating emulsion stability: A critical review. Adv. Colloid Interface Sci. 2023, 311, 102813. [Google Scholar] [CrossRef]
- Mert, B. Using high pressure microfluidization to improve physical properties and lycopene content of ketchup type products. J. Food Eng. 2012, 109, 579–587. [Google Scholar] [CrossRef]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F-Ananassa) juice. Lwt Food Sci. Technol. 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Doehlert, D.C.; McMullen, M.S.; Hammond, J.J. Genotypic and environmental effects on grain yield and quality of oat grown in North Dakota. Crop Sci. 2001, 41, 1066–1072. [Google Scholar] [CrossRef]
- Kivelae, R.; Pitkaenen, L.; Laine, P.; Aseyev, V.; Sontag-Strohm, T. Influence of homogenisation on the solution properties of oat β-glucan. Food Hydrocoll. 2010, 24, 611–618. [Google Scholar] [CrossRef]
- Guo, X.J.; Chen, M.S.; Li, Y.T.; Dai, T.T.; Shuai, X.X.; Chen, J.; Liu, C.M. Modification of food macromolecules using dynamic high pressure microfluidization: A review. Trends Food Sci. Technol. 2020, 100, 223–234. [Google Scholar] [CrossRef]
- Li, R.Q.; Cao, H.W.; Wang, Y.Q.; Song, H.D.; Huang, K.; Zhang, Y.; Sun, Q.Q.; Sun, Z.L.; Guan, X. Improving physicochemical stability of highland barley-based milk by the addition ofendogenous β-glucan. Food Hydrocoll. 2023, 143, 108875. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.M.; Sun, W.Z.; Zhao, G.L.; Ren, J.Y. Effects of microfluidization treatment and transglutaminase cross-linking on physicochemical, functional, and conformational properties of peanut protein isolate. J. Agric. Food Chem. 2011, 59, 8886–8894. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.X.; Tu, Z.C.; Wang, H.; Zhang, L.; Huang, T.; Ma, D. Monitoring of the functional properties and unfolding change of ovalbumin after DHPM treatment by HDX and FTICR MS functionality and unfolding of oval after DHPM by HDX and FTICR MS. Food Chem. 2017, 227, 413–421. [Google Scholar] [CrossRef]
- He, X.H.; Chen, J.; He, X.M.; Feng, Z.; Li, C.H.; Liu, W.; Dai, T.T.; Liu, C.M. Industry-scale microfluidization as a potential technique to improve solubility and modify structure of pea protein. Innov. Food Sci. Emerg. 2021, 67, 102582. [Google Scholar] [CrossRef]
- Pérez-López, E.; Mateos-Aparicio, I.; Rupérez, P. Okara treated with high hydrostatic pressure assisted by Ultraflo® L: Effect on solubility of dietary fibre. Innov. Food Sci. Emerg. 2016, 33, 32–37. [Google Scholar] [CrossRef]
- Tu, Z.; Chen, L.; Wang, H.; Ruan, C.; Zhang, L.; Kou, Y. Effect of fermentation and dynamic high pressure microfluidization on dietary fibre of soybean residue. J. Food Sci. Technol. 2014, 51, 3285–3292. [Google Scholar] [CrossRef] [Green Version]
- Mcclements, D.J. Food Emulsions: Principles, Practice and Techniques; CRC Press: Boca Raton, FL, USA, 2015; p. 306. [Google Scholar]
- Zhang, Y.; Zhou, F.; Zhao, M.; Lin, L.; Ning, Z.; Sun, B. Soy peptide nanoparticles by ultrasound-induced self-assembly of large peptide aggregates and their role on emulsion stability. Food Hydrocoll. 2017, 74, 62–71. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Guan, Y.J.; Bi, J.F.; Liu, X.; Yi, J.Y.; Chen, Q.Q.; Wu, X.Y.; Zhou, M. Change of the rheological properties of mango juice by high pressure homogenization. Lwt Food Sci. Technol. 2017, 82, 121–130. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Jiang, S.W.; Cao, X.M.; Jiang, S.T.; Pan, L.J. Effect of high pressure homogenization (HPH) on the physical properties of taro (Colocasia esculenta (L). Schott) pulp. J. Food Eng. 2016, 177, 1–8. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, L.; Kong, X.; Zhang, C.; Hua, Y. The properties and the related protein behaviors of oil bodies in soymilk preparation. Eur. Food Res. Technol. 2014, 239, 463–471. [Google Scholar] [CrossRef]
- Peng, X.; Ren, C.; Guo, S. Particle formation and gelation of soymilk: Effect of heat. Trends Food Sci. Technol. 2016, 54, 138–147. [Google Scholar] [CrossRef]
- Chen, B.F.; Cai, Y.J.; Liu, T.X.; Huang, L.H.; Deng, X.L.; Zhao, Q.Z.; Zhao, M.M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocoll. 2019, 93, 167–175. [Google Scholar] [CrossRef]
- Huang, L.; Cai, Y.; Liu, T.; Zhao, X.; Chen, B.; Long, Z.; Zhao, M.; Deng, X.; Zhao, Q. Stability of emulsion stabilized by low-concentration soybean protein isolate: Effects of insoluble soybean fiber. Food Hydrocoll. 2019, 97, 105232. [Google Scholar] [CrossRef]
- Bernat, N.; Cháfer, M.; Rodríguez-García, J.; Chiralt, A.; González-Martínez, C. Effect of high pressure homogenisation and heat treatment on physical properties and stability of almond and hazelnut milks. LWT Food Sci. Technol. 2015, 62, 488–496. [Google Scholar] [CrossRef]
- Pineli, L.L.O.; Botelho, R.B.A.; Zandonadi, R.P.; Solorzano, J.L. Low glycemic index and increased protein content in a novel quinoa milk. LWT Food Sci. Technol. 2015, 63, 1261–1267. [Google Scholar] [CrossRef]
- He, X.H.; Luo, S.J.; Chen, M.S.; Xia, W.; Chen, J.; Liu, C.M. Effect of industry-scale microfluidization on structural and physicochemical properties of potato starch. Innov. Food Sci. Emerg. 2020, 60, 102278. [Google Scholar] [CrossRef]
- Agrahar-Murugkar, D.; Bajpai-Dixit, P.; Kotwaliwale, N. Rheological, nutritional, functional and sensory properties of millets and sprouted legume based beverages. J. Food Sci. Technol. 2020, 5, 1671–1679. [Google Scholar] [CrossRef]
- Silva, K.; Machado, A.; Cardoso, C.; Silva, F.; Freitas, F. Rheological behavior of plant- based beverages. Food Sci. Technol. 2020, 40, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Bucci, A.J.; Van Hekken, D.L.; Tunick, M.H.; Renye, J.A.; Tomasula, P.M. The effects of microfluidization on the physical, microbial, chemical, and coagulation properties of milk. J. Dairy Sci. 2018, 101, 6990–7001. [Google Scholar] [CrossRef] [Green Version]
- Morales-Medina, R.; Dong, D.; Schalow, S.; Drusch, S. Impact of microfluidization on the microstructure and functional properties of pea hull fibre. Food Hydrocoll. 2020, 103, 105660. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, J.; Zhao, P.; Pang, Y.; Ye, H.Q.; Yuan, Y.; Liu, J.B.; Jones, J. Optimized antioxidant peptides fractions preparation and secondary structure analysis by MIR. Int. J. Biol. Macromol. 2013, 59, 151–157. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, L.Y.; Cui, B.; Zhao, H.B.; Liu, P.F. The effects of acetylated distarch phosphate from tapioca starch on rheological properties and microstructure of acidinduced casein gel. Int. J. Biol. Macromol. 2020, 159, 1132–1139. [Google Scholar] [CrossRef]
- Yang, H.S.; Wang, Y.F.; Lai, S.J.; An, H.J.; Li, Y.F.; Chen, F.S. Application of atomic force microscopy as a nanotechnology tool in food science. J. Food Sci. 2007, 72, R65–R75. [Google Scholar] [CrossRef] [PubMed]

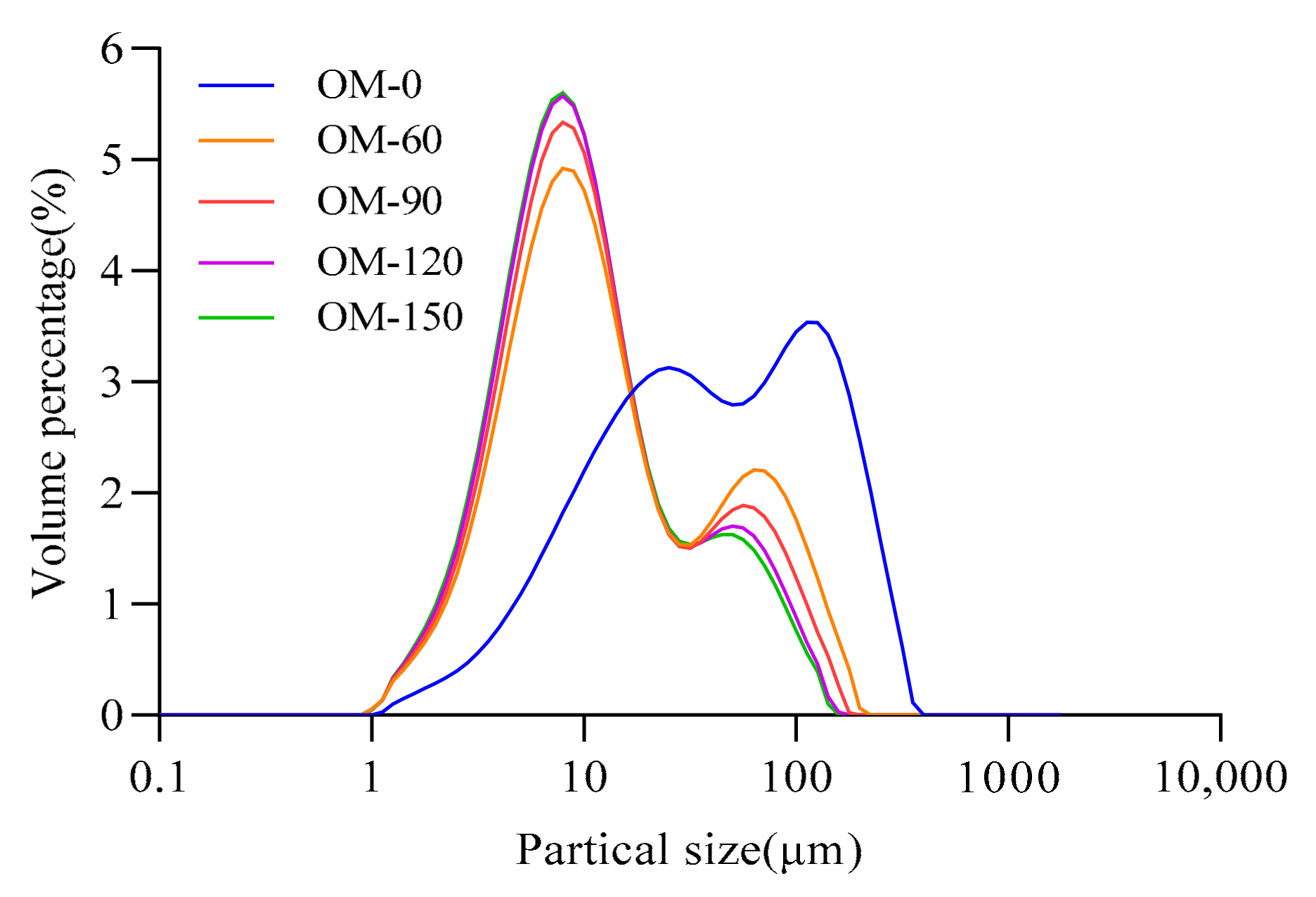

| Particle Size/μm | D [4,3] | D [3,2] | D (0.1) | D (0.5) | D (0.9) | Span |
|---|---|---|---|---|---|---|
| OM-0 | 72.597 ± 0.857 a | 18.618 ± 0.138 a | 7.644 ± 0.046 a | 42.367 ± 0.665 a | 183.160 ± 1.933 a | 4.143 ± 0.033 e |
| OM-60 | 28.176 ± 0.098 b | 8.378 ± 0.008 b | 3.794 ± 0.010 b | 11.496 ± 0.021 b | 82.587 ± 0.478 b | 4.897 ± 0.046 d |
| OM-90 | 22.776 ± 0.283 c | 7.794 ± 0.026 c | 3.645 ± 0.014 c | 10.360 ± 0.037 c | 65.499 ± 1.069 c | 5.190 ± 0.091 c |
| OM-120 | 19.542 ± 0.431 d | 7.42 ± 0.020 d | 3.532 ± 0.010 d | 9.734 ± 0.050 d | 54.052 ± 1.573 d | 5.970 ± 0.127 b |
| OM-150 | 18.608 ± 0.510 e | 7.28 ± 0.021 e | 3.472 ± 0.011 e | 9.552 ± 0.063 d | 50.247 ± 1.670 e | 6.854 ± 0.123 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Kang, Z.; Zhao, S.; Meng, N.; Liu, M.; Tan, B. Effect of Dynamic High-Pressure Microfluidizer on Physicochemical and Microstructural Properties of Whole-Grain Oat Pulp. Foods 2023, 12, 2747. https://doi.org/10.3390/foods12142747
Jiang P, Kang Z, Zhao S, Meng N, Liu M, Tan B. Effect of Dynamic High-Pressure Microfluidizer on Physicochemical and Microstructural Properties of Whole-Grain Oat Pulp. Foods. 2023; 12(14):2747. https://doi.org/10.3390/foods12142747
Chicago/Turabian StyleJiang, Ping, Ziyue Kang, Su Zhao, Ning Meng, Ming Liu, and Bin Tan. 2023. "Effect of Dynamic High-Pressure Microfluidizer on Physicochemical and Microstructural Properties of Whole-Grain Oat Pulp" Foods 12, no. 14: 2747. https://doi.org/10.3390/foods12142747





