Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses
Abstract
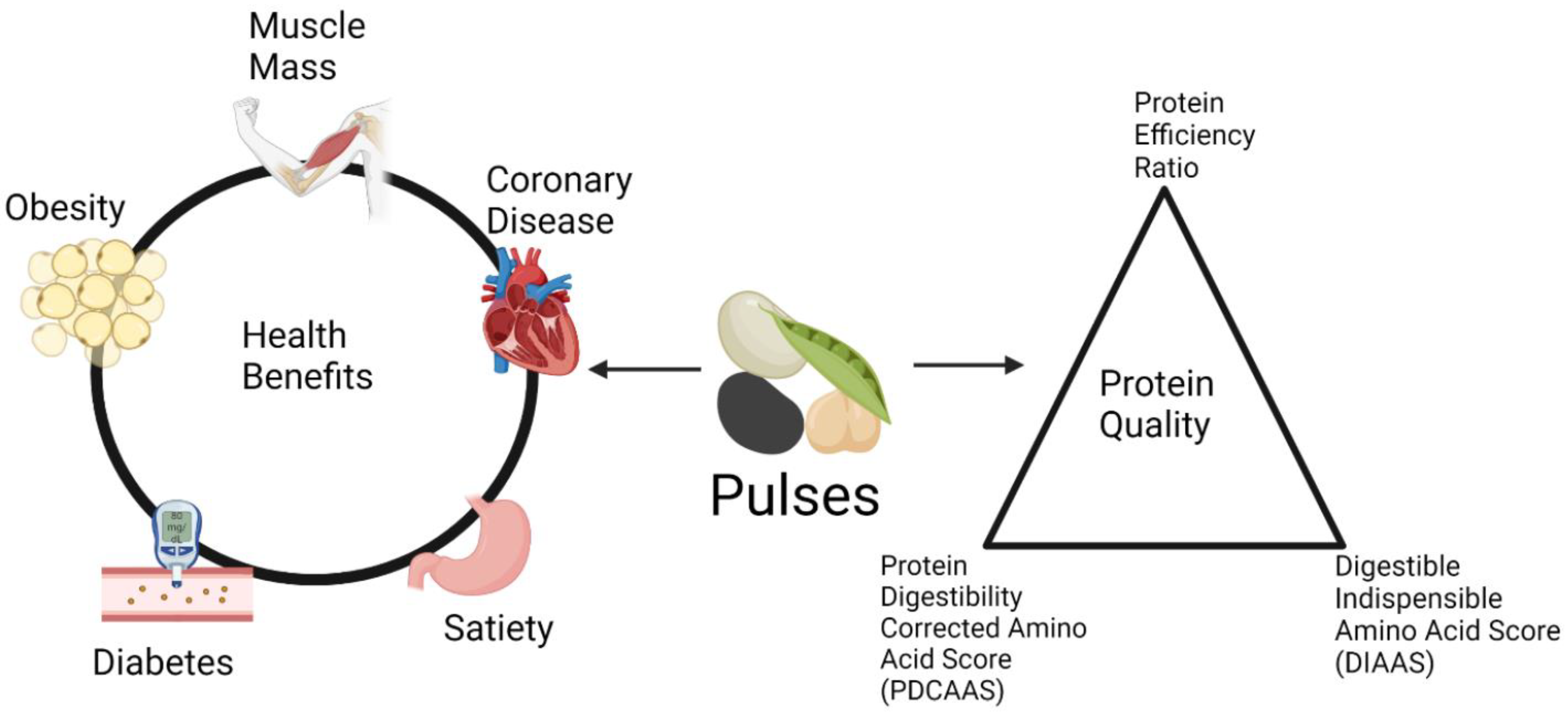
1. Introduction
Plant Protein Sources
2. Determination of Protein Quality and Regulation of Protein Content Claims
2.1. Protein Efficiency Ratio
2.2. Protein Digestibility Corrected Amino Acid Score
2.3. Digestible Indispensable Amino Acid Score
2.4. Advantages and Disadvantages of PER, PDCAAS, and DIAAS
| Protein Quality Measurement | Benefits | Detriments |
|---|---|---|
| PER | ||
| PDCAAS | ||
| DIAAS |
|
2.5. Alternative Options for the Regulation of Protein Content Claims
2.6. Amino Acid Composition and Protein Quality of Protein-Containing Fractions
3. Effects of Pulses on Human Health
3.1. Cardiovascular Disease (CVD)
3.2. Satiety
3.3. Lipid Metabolism
3.4. Diabetes Mellitus
3.5. Muscle Protein Synthesis
3.6. Gut Health
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- United Nations. Revision of World Population Prospects. 2017. Available online: https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_KeyFindings.pdf (accessed on 16 November 2022).
- Nosworthy, M.G.; House, J.D. Factors influencing the quality of dietary proteins: Implications for pulses. Cereal Chem. 2017, 94, 49–57. [Google Scholar] [CrossRef]
- Garcia-Mora, P.; Peñas, E.; Frías, J.; Gomez, R.; Martinez-Villaluenga, C. High-pressure improves enzymatic proteolysis and the release of peptides with angiotensin I converting enzyme inhibitory and antioxidant activities from lentil proteins. Food Chem. 2015, 171, 224–232. [Google Scholar] [CrossRef]
- Malik, V.S.; Li, Y.; Tobias, D.K.; Pan, A.; Hu, F.B. Dietary protein intake and risk of type 2 diabetes in US men and women. Am. J. Epidemiol. 2016, 183, 715–728. [Google Scholar] [CrossRef]
- Food and Drug Administration. Food labeling: Health claims; soy protein and coronary heart disease. Fed. Regist. 1999, 64, 57700–57733. [Google Scholar]
- Millward, D.J. The nutritional value of plant-based diets in relation to human amino acid and protein requirements. Proc. Nutr. Soc. 1999, 58, 249–260. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation, Bethesda, Md., USA 4–8 December 1989; Food & Agriculture Org.: Rome, Italy, 1991. [Google Scholar]
- Berkow, S.E.; Barnard, N. Vegetarian diets and weight status. Nutr. Rev. 2006, 64, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Farmer, B.; Larson, B.T.; Fulgoni III, V.L.; Rainville, A.J.; Liepa, G.U. A vegetarian dietary pattern as a nutrient-dense approach to weight management: An analysis of the national health and nutrition examination survey 1999–2004. J. Am. Diet. Assoc. 2011, 111, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Sabaté, J.; Wien, M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010, 91, 1525S–1529S. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Phillips, R.L. Does a vegetarian diet reduce the occurrence of diabetes? Am. J. Public Health 1985, 75, 507–512. [Google Scholar] [CrossRef]
- Ornish, D.; Brown, S.E.; Billings, J.; Scherwitz, L.; Armstrong, W.T.; Ports, T.A.; McLanahan, S.M.; Kirkeeide, R.L.; Gould, K.; Brand, R. Can lifestyle changes reverse coronary heart disease?: The Lifestyle Heart Trial. Lancet 1990, 336, 129–133. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sasaki, S.; Okubo, S.; Hayashi, M.; Tsugane, S. Blood pressure change in a free-living population-based dietary modification study in Japan. J. Hypertens. 2006, 24, 451–458. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAO Expert Consultation, 31 March–2 April 2011, Auckland, New Zealand.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Gilani, G.S. Background on international activities on protein quality assessment of foods. Br. J. Nutr. 2012, 108, S168–S182. [Google Scholar] [CrossRef]
- Health Canada. Report No.: FO-1. Health Canada. 1981. Available online: http://www.hc-sc.gc.ca/fn-an/alt_formats/hpfb-dgpsa/pdf/res-rech/fo-1-eng.pdf (accessed on 23 January 2023).
- CFIA. Elements within the Nutrition Facts Table. CFIA. 2014. Available online: http://www.inspection.gc.ca/food/labelling/food-labelling-for-industry/nutrition-labelling/elements-within-the-nutrition-facts-table/eng/1389206763218/1389206811747?chap=7 (accessed on 10 November 2022).
- Marinangeli, C.P.; House, J.D. Potential impact of the digestible indispensable amino acid score as a measure of protein quality on dietary regulations and health. Nutr. Rev. 2017, 75, 658–667. [Google Scholar] [CrossRef]
- Nosworthy, M.G.; Neufeld, J.; Frohlich, P.; Young, G.; Malcolmson, L.; House, J.D. Determination of the protein quality of cooked Canadian pulses. Food Sci. Nutr. 2017, 5, 896–903. [Google Scholar] [CrossRef] [PubMed]
- FDA. Food Label Revis Nutr Supplement Facts Labels. Available online: https://www.federalregister.gov/documents/2016/05/27/2016-11867/food-labeling-revision-of-the-nutrition-and-supplement-facts-labels2016 (accessed on 7 October 2022).
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Joint, F.; World Health Organization. Protein and Amino Acid Requirements in Human Nutrition: Report of a Joint FAO/WHO/UNU Expert Consultation; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Sarwar, G. Digestibility of protein and bioavailability of amino acids in foods. World Rev. Nutr. Diet. 1987, 54, 26–70. [Google Scholar]
- Low, A.G. Nutrient absorption in pigs. J. Sci. Food Agric. 1980, 31, 1087–1130. [Google Scholar] [CrossRef] [PubMed]
- Moughan, P.; Souffrant, W.; Hodgkinson, S. Physiological approaches to determining gut endogenous amino acid flows in the mammal. Arch. Anim. Nutr. 1998, 51, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Moughan, P. The digestible amino acid composition of several milk proteins: Application of a new bioassay. J. Dairy Sci. 1998, 81, 909–917. [Google Scholar] [CrossRef]
- Commission, E. Regulation (EC) No. 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; European Parliament and the Council of the European Union: Rome, Italy, 2006. [Google Scholar]
- Food Standards Australia New Zealand. Nutrition and Health Claims v157pdf; Federal Register of Legislative Instruments: Canberra, Australia, 2015. Available online: https://www.foodstandards.gov.au/code/Documents/1.2.7%20Nutrition%20and%20health%20claims%20v157.pdf (accessed on 3 December 2022).
- Anderson, G.H.; Ashley, D.V.; Jones, J.D. Utilization of L-methionine sulfoxide, L-methionine sulfone and cysteic acid by the weanling rat. J. Nutr. 1976, 106, 1108–1114. [Google Scholar] [CrossRef]
- Marshall, H.; Chang, K.; Miller, K.; Satterlee, L. Sulfur amino acid stability. Effects of processing on legume proteins. J. Food Sci. 1982, 47, 1170–1174. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Moughan, P.J. Determination of sulfur amino acids in foods as related to bioavailability. J. AOAC Int. 2008, 91, 907–913. [Google Scholar] [CrossRef]
- HEARTS Technical Package for Cardiovascular Disease Management in Primary Health Care; World Health Organization: Geneva, Switzerland, 2018; Available online: https://www.who.int/cardiovascular_diseases/hearts/en/ (accessed on 3 January 2023).
- HEARTS-D. Diagnosis and Management of Type 2 Diabetes; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. World Health Organization Obesity and Overweight Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 3 January 2023).
- World Health Organization. Time to Deliver: Report of the WHO Independent High-Level Commission on Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Didinger, C.; Thompson, H.J. The role of pulses in improving human health: A review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Didinger, C.; Foster, M.T.; Bunning, M.; Thompson, H.J. Nutrition and human health benefits of dry beans and other pulses. Dry Beans Pulses Prod. Process. Nutr. 2022, 481–504. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Marinangeli, C.P.; Jones, P.J.; Carlberg, J.G. Canadian potential healthcare and societal cost savings from consumption of pulses: A cost-of-illness analysis. Nutrients 2017, 9, 793. [Google Scholar] [CrossRef]
- Didinger, C.; Thompson, H. Motivating pulse-centric eating patterns to benefit human and environmental well-being. Nutrients 2020, 12, 3500. [Google Scholar] [CrossRef]
- Messina, V. Nutritional and health benefits of dried beans. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 437S–442S. [Google Scholar] [CrossRef]
- Byass, P. Correlation between noncommunicable disease mortality in people aged 30–69 years and those aged 70–89 years. Bull. World Health Organ. 2019, 97, 589. [Google Scholar] [CrossRef] [PubMed]
- Members, T.F.; Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. J. Prev. Cardiol. 2016, 23, NP1–NP96. [Google Scholar]
- Members, A.T.F.; Hamm, C.W.; Bassand, J.-P.; Agewall, S.; Bax, J.; Boersma, E.; Bueno, H.; Caso, P.; Dudek, D.; Gielen, S. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2011, 32, 2999–3054. [Google Scholar]
- Francula-Zaninovic, S.; Nola, I.A. Management of measurable variable cardiovascular disease’risk factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-Y.; Meng, X.; Li, Y.; Zhao, C.-N.; Liu, Q.; Li, H.-B. Effects of vegetables on cardiovascular diseases and related mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Caulfield, L.E.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Rebholz, C.M. Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and all-cause mortality in a general population of middle-aged adults. J. Am. Heart Assoc. 2019, 8, e012865. [Google Scholar] [CrossRef]
- Padhi, E.M.; Ramdath, D.D. A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. J. Funct. Foods 2017, 38, 635–643. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Ruiz-Narvarez, E.; Siles, X.; Campos, H. Decreased consumption of dried mature beans is positively associated with urbanization and nonfatal acute myocardial infarction. J. Nutr. 2005, 135, 1770–1775. [Google Scholar] [CrossRef]
- Bazzano, L.A.; He, J.; Ogden, L.G.; Loria, C.; Vupputuri, S.; Myers, L.; Whelton, P.K. Legume consumption and risk of coronary heart disease in US men and women: NHANES I Epidemiologic Follow-up Study. Arch. Intern. Med. 2001, 161, 2573–2578. [Google Scholar] [CrossRef]
- Clark, J.L.; Taylor, C.G.; Zahradka, P. Black beans and red kidney beans induce positive postprandial vascular responses in healthy adults: A pilot randomized cross-over study. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 216–226. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Fulgoni III, V.L. Bean consumption is associated with greater nutrient intake, reduced systolic blood pressure, lower body weight, and a smaller waist circumference in adults: Results from the National Health and Nutrition Examination Survey 1999–2002. J. Am. Coll. Nutr. 2008, 27, 569–576. [Google Scholar] [CrossRef]
- Oosthuizen, W.; Scholtz, C.; Vorster, H.; Jerling, J.; Vermaak, W. Extruded dry beans and serum lipoprotein and plasma haemostatic factors in hyperlipidaemic men. Eur. J. Clin. Nutr. 2000, 54, 373–379. [Google Scholar] [CrossRef][Green Version]
- Lukus, P.K.; Doma, K.M.; Duncan, A.M. The role of pulses in cardiovascular disease risk for adults with diabetes. Am. J. Lifestyle Med. 2020, 14, 571–584. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef]
- Anderson, G.H.; Moore, S.E. Dietary Proteins in the Regulation of Food Intake and Body Weight in Humans. J. Nutr. 2004, 134, 974S–979S. [Google Scholar] [CrossRef] [PubMed]
- Leidy, H.J.; Lepping, R.J.; Savage, C.R.; Harris, C.T. Neural Responses to Visual Food Stimuli After a Normal vs. Higher Protein Breakfast in Breakfast-Skipping Teens: A Pilot fMRI Study. Obesity 2011, 19, 2019–2025. [Google Scholar] [CrossRef]
- Leidy, H.J.; Ortinau, L.C.; Douglas, S.M.; Hoertel, H.A. Beneficial effects of a higher-protein breakfast on the appetitive, hormonal, and neural signals controlling energy intake regulation in overweight/obese, “breakfast-skipping”, late-adolescent girls. Am. J. Clin. Nutr. 2013, 97, 677–688. [Google Scholar] [CrossRef]
- Douglas, S.M.; Lasley, T.R.; Leidy, H.J. Consuming Beef vs. Soy Protein Has Little Effect on Appetite, Satiety, and Food Intake in Healthy Adults. J. Nutr. 2015, 145, 1010–1016. [Google Scholar] [CrossRef]
- Pai, S.; Ghugre, P.S.; Udipi, S.A. Satiety from rice-based, wheat-based and rice–pulse combination preparations. Appetite 2005, 44, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Zykus, A.; Luhovyy, B.L.; Nunez, M.F.; Wong, C.L.; Anderson, G.H. The acute effects of a pulse-containing meal on glycaemic responses and measures of satiety and satiation within and at a later meal. Br. J. Nutr. 2012, 108, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Mollard, R.C.; Luhovyy, B.L.; Smith, C.; Anderson, G.H. Acute effects of pea protein and hull fibre alone and combined on blood glucose, appetite, and food intake in healthy young men—A randomized crossover trial. Appl. Physiol. Nutr. Metab. 2014, 39, 1360–1365. [Google Scholar] [CrossRef]
- Abou-Samra, R.; Keersmaekers, L.; Brienza, D.; Mukherjee, R.; Macé, K. Effect of different protein sources on satiation and short-term satiety when consumed as a starter. Nutr. J. 2011, 10, 139. [Google Scholar] [CrossRef]
- Koba, S.; Sasaki, J. Treatment of Hyperlipidemia from Japanese Evidence. J. Atheroscler. Thromb. 2006, 13, 267–280. [Google Scholar] [CrossRef]
- Li, S.S.; Blanco Mejia, S.; Lytvyn, L.; Stewart, S.E.; Viguiliouk, E.; Ha, V.; De Souza, R.J.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017, 6, e006659. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. Soybean Foods and Their Benefits: Potential Mechanisms of Action. Nutr. Rev. 2005, 63, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.L.F.; Leung, S.S.F.; Sham, A.L.K.; Lam, T.H.; Janus, E.D. Intake of Soy Products Is Associated with Better Plasma Lipid Profiles in the Hong Kong Chinese Population. J. Nutr. 2000, 130, 2590–2593. [Google Scholar] [CrossRef]
- Erdman, J.W. Soy Protein and Cardiovascular Disease. Circulation 2000, 102, 2555–2559. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kurisu, Y.; Shimizu, H. Decreased Serum Total Cholesterol Concentration Is Associated with High Intake of Soy Products in Japanese Men and Women. J. Nutr. 1998, 128, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Nachvak, S.M.; Moradi, S.; Anjom-Shoae, J.; Rahmani, J.; Nasiri, M.; Maleki, V.; Sadeghi, O. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: A systematic review and dose–response meta-analysis of prospective cohort studies. J. Acad. Nutr. Diet. 2019, 119, 1483–1500.e1417. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, X.; Li, C.; Jiao, S.; Dong, W. Association between consumption of soy and risk of cardiovascular disease: A meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2017, 24, 735–747. [Google Scholar] [CrossRef]
- Potter, S.M. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J. Nutr. 1995, 125 (Suppl. 3), 606S–611S. [Google Scholar]
- Xiao, C.W.; Mei, J.; Wood, C.M. Effect of soy proteins and isoflavones on lipid metabolism and involved gene expression. Front. Biosci. 2008, 13, 2660–2673. [Google Scholar] [CrossRef]
- Takahashi, Y.; Konishi, T. Tofu (Soybean Curd) Lowers Serum Lipid Levels and Modulates Hepatic Gene Expression Involved in Lipogenesis Primarily through Its Protein, Not Isoflavone, Component in Rats. J. Agric. Food Chem. 2011, 59, 8976–8984. [Google Scholar] [CrossRef]
- Huf, M.W.; Hamilton, R.M.G.; Carroll, K.K. Plasma cholesterol levels in rabbits fed low fat, cholesterol-free, semipurified diets: Effects of dietary proteins, protein hydrolysates and amino acid mixtures. Atherosclerosis 1977, 28, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Duranti, M.; Lovati, M.R.; Dani, V.; Barbiroli, A.; Scarafoni, A.; Castiglioni, S.; Ponzone, C.; Morazzoni, P. The α’ Subunit from Soybean 7S Globulin Lowers Plasma Lipids and Upregulates Liver β-VLDL Receptors in Rats Fed a Hypercholesterolemic Diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of White Lupin Seed, a Naturally Isoflavone-Poor Legume, Reduce Cholesterolemia in Rats and Increase LDL Receptor Activity in HepG2 Cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary Lupin Protein Lowers Triglyceride Concentrations in Liver and Plasma in Rats by Reducing Hepatic Gene Expression of Sterol Regulatory Element-Binding Protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef]
- Weisse, K.; Brandsch, C.; Zernsdorf, B.; Nembongwe, G.S.N.; Hofmann, K.; Eder, K.; Stangl, G.I. Lupin protein compared to casein lowers the LDL cholesterol: HDL cholesterol-ratio of hypercholesterolemic adults. Eur. J. Nutr. 2010, 49, 65–71. [Google Scholar] [CrossRef]
- Macarulla, M.T.; Medina, C.; Diego, M.A.D.; Chávarri, M.; Zulet, M.Á.; Martínez, J.A.; Nöel-Suberville, C.; Higueret, P.; Portillo, M.P. Effects of the whole seed and a protein isolate of faba bean (Vicia faba) on the cholesterol metabolism of hypercholesterolaemic rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Lasekan, J.B.; Gueth, L.; Khan, S. Influence of dietary golden pea proteins versus casein on plasma and hepatic lipids in rats. Nutr. Res. 1995, 15, 71–84. [Google Scholar] [CrossRef]
- Alonso, R.; Grant, G.; Marzo, F. Thermal treatment improves nutritional quality of pea seeds (Pisum sativum L.) without reducing their hypocholesterolemic properties. Nutr. Res. 2001, 21, 1067–1077. [Google Scholar] [CrossRef]
- Macarulla, Z.; Martínez, H. Lipid and Glucose Utilization in Hypercholesterolemic Rats Fed a Diet Containing Heated Chickpea (Cicer Aretinum L.): A Potential Functional Food. Int. J. Vitam. Nutr. Res. 1999, 69, 403–411. [Google Scholar] [CrossRef]
- Boualga, A.; Prost, J.; Taleb-Senouci, D.; Krouf, D.; Kharoubi, O.; Lamri-Senhadji, M.; Belleville, J.; Bouchenak, M. Purified chickpea or lentil proteins impair VLDL metabolism and lipoprotein lipase activity in epididymal fat, but not in muscle, compared to casein, in growing rats. Eur. J. Nutr. 2009, 48, 162–169. [Google Scholar] [CrossRef]
- Abeysekara, S.; Chilibeck, P.D.; Vatanparast, H.; Zello, G.A. A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. Br. J. Nutr. 2012, 108, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Hermsdorff, H.H.M.; Zulet, M.Á.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Singh, N. Pulse Chemistry and Technology; Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Shevkani, K.; Kaur, A.; Kumar, S.; Singh, N. Cowpea protein isolates: Functional properties and application in gluten-free rice muffins. LWT-Food Sci. Technol. 2015, 63, 927–933. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Chen, Y.; Kaur, A.; Yu, L. Pulse proteins: Secondary structure, functionality and applications. J. Food Sci. Technol. 2019, 56, 2787–2798. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Luna-Vital, D.A.; Mojica, L.; de Mejía, E.G.; Mendoza, S.; Loarca-Piña, G. Biological potential of protein hydrolysates and peptides from common bean (Phaseolus vulgaris L.): A review. Food Res. Int. 2015, 76, 39–50. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-chemical properties, antioxidant activities and angiotensin-I converting enzyme inhibitory of protein hydrolysates from Mung bean (Vigna radiate). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Lima, S.L.S.; Alves, N.E.G.; Assis, A.; Moreira, M.E.C.; Toledo, R.C.L.; Rosa, C.O.B.; Teixeira, O.R.; Bassinello, P.Z.; De Mejía, E.G.; et al. Common bean protein hydrolysate modulates lipid metabolism and prevents endothelial dysfunction in BALB/c mice fed an atherogenic diet. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 141–150. [Google Scholar] [CrossRef]
- Leterme, P. Recommendations by health organizations for pulse consumption. Br. J. Nutr. 2002, 88, 239–242. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Kendall, C.W.; Esfahani, A.; Wong, J.M.; Carleton, A.J.; Jiang, H.Y.; Bazinet, R.P.; Vidgen, E.; Jenkins, D.J. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia 2009, 52, 1479–1495. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Wernbom, M.; Augustsson, J.; Thome, R. The Influence of Frequency, Intensity, Volume and Mode of Strength Training on Whole Muscle Cross-Sectional Area in Humans. Sports Med. 2007, 37, 225–264. [Google Scholar] [CrossRef]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Jefferson, L.S. Signaling Pathways Involved in Translational Control of Protein Synthesis in Skeletal Muscle by Leucine. J. Nutr. 2001, 131, 856S–860S. [Google Scholar] [CrossRef]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine Stimulates Translation Initiation in Skeletal Muscle of Postabsorptive Rats via a Rapamycin-Sensitive Pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Leucine and Protein Synthesis: mTOR and Beyond. Nutr. Rev. 2008, 65, 122–129. [Google Scholar] [CrossRef]
- Devries, M.C.; McGlory, C.; Bolster, D.R.; Kamil, A.; Rahn, M.; Harkness, L.; Baker, S.K.; Phillips, S.M. Protein leucine content is a determinant of shorter- and longer-term muscle protein synthetic responses at rest and following resistance exercise in healthy older women: A randomized, controlled trial. Am. J. Clin. Nutr. 2018, 107, 217–226. [Google Scholar] [CrossRef]
- Phillips, S.M. The impact of protein quality on the promotion of resistance exercise-induced changes in muscle mass. Nutr. Metab. 2016, 13, s12986–s13016. [Google Scholar] [CrossRef]
- Van Vliet, S.; Burd, N.A.; Van Loon, L.J. The Skeletal Muscle Anabolic Response to Plant- versus Animal-Based Protein Consumption. J. Nutr. 2015, 145, 1981–1991. [Google Scholar] [CrossRef]
- Norton, L.E.; Layman, D.K.; Bunpo, P.; Anthony, T.G.; Brana, D.V.; Garlick, P.J. The Leucine Content of a Complete Meal Directs Peak Activation but Not Duration of Skeletal Muscle Protein Synthesis and Mammalian Target of Rapamycin Signaling in Rats. J. Nutr. 2009, 139, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Churchward-Venne, T.A.; Burd, N.A.; Breen, L.; Tarnopolsky, M.A.; Phillips, S.M. Myofibrillar protein synthesis following ingestion of soy protein isolate at rest and after resistance exercise in elderly men. Nutr. Metab. 2012, 9, 57. [Google Scholar] [CrossRef]
- Phillips, S.M. Nutrient-rich meat proteins in offsetting age-related muscle loss. Meat Sci. 2012, 92, 174–178. [Google Scholar] [CrossRef]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D.V.; Azcárate-Peril, M.A.; Chapkin, R.S.; Turner, N.D. Shaping functional gut microbiota using dietary bioactives to reduce colon cancer risk. Semin. Cancer Biol. 2017, 46, 191–204. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet–Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Lepp, D.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Pauls, K.P.; Tsao, R.; Wood, G.A.; Robinson, L.E.; et al. Diets enriched with cranberry beans alter the microbiota and mitigate colitis severity and associated inflammation. J. Nutr. Biochem. 2016, 28, 129–139. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Graf, D.; McGillis, L.H.; Hussain, A.; Carey, C.; Robinson, L.E.; Liu, R.; Tsao, R.; et al. Chickpea-supplemented diet alters the gut microbiome and enhances gut barrier integrity in C57Bl/6 male mice. J. Funct. Foods 2017, 38, 663–674. [Google Scholar] [CrossRef]
- Neil, E.S.; McGinley, J.N.; Fitzgerald, V.K.; Lauck, C.A.; Tabke, J.A.; Streeter-McDonald, M.R.; Yao, L.; Broeckling, C.D.; Weir, T.L.; Foster, M.T.; et al. White Kidney Bean (Phaseolus vulgaris L.) Consumption Reduces Fat Accumulation in a Polygenic Mouse Model of Obesity. Nutrients 2019, 11, 2780. [Google Scholar] [CrossRef]
- Ojo, B.A.; Lu, P.; Alake, S.E.; Keirns, B.; Anderson, K.; Gallucci, G.; Hart, M.D.; El-Rassi, G.D.; Ritchey, J.W.; Chowanadisai, W.; et al. Pinto beans modulate the gut microbiome, augment MHC II protein, and antimicrobial peptide gene expression in mice fed a normal or western-style diet. J. Nutr. Biochem. 2021, 88, 108543. [Google Scholar] [CrossRef]
- Monk, J.M.; Wu, W.; Lepp, D.; Wellings, H.R.; Hutchinson, A.L.; Liddle, D.M.; Graf, D.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy bean supplemented high-fat diet improves intestinal health, epithelial barrier integrity and critical aspects of the obese inflammatory phenotype. J. Nutr. Biochem. 2019, 70, 91–104. [Google Scholar] [CrossRef]
- Monk, J.M.; Lepp, D.; Wu, W.; Pauls, K.P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation primes the colonic mucosal microenvironment to improve gut health. J. Nutr. Biochem. 2017, 49, 89–100. [Google Scholar] [CrossRef]
- Monk, J.M.; Wu, W.; Hutchinson, A.L.; Pauls, P.; Robinson, L.E.; Power, K.A. Navy and black bean supplementation attenuates colitis-associated inflammation and colonic epithelial damage. J. Nutr. Biochem. 2018, 56, 215–223. [Google Scholar] [CrossRef]
- Zhang, C.; Monk, J.M.; Lu, J.T.; Zarepoor, L.; Wu, W.; Liu, R.; Pauls, K.P.; Wood, G.A.; Robinson, L.; Tsao, R.; et al. Cooked navy and black bean diets improve biomarkers of colon health and reduce inflammation during colitis. Br. J. Nutr. 2014, 111, 1549–1563. [Google Scholar] [CrossRef]
- McGinley, J.N.; Fitzgerald, V.K.; Neil, E.S.; Omerigic, H.M.; Heuberger, A.L.; Weir, T.L.; McGee, R.; Vandemark, G.; Thompson, H.J. Pulse crop effects on gut microbial populations, intestinal function, and adiposity in a mouse model of diet-induced obesity. Nutrients 2020, 12, 593. [Google Scholar] [CrossRef]
- Siva, N.; Thavarajah, P.; Kumar, S.; Thavarajah, D. Variability in prebiotic carbohydrates in different market classes of chickpea, common bean, and lentil collected from the American local market. Front. Nutr. 2019, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chang, S.K.; Zhang, Y.; Hsu, C.-Y.; Nannapaneni, R. Gut microbiota and short chain fatty acid composition as affected by legume type and processing methods as assessed by simulated in vitro digestion assays. Food Chem. 2020, 312, 126040. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Olias, R. Beneficial effects of legumes in gut health. Curr. Opin. Food Sci. 2017, 14, 32–36. [Google Scholar] [CrossRef]
| Adjusted PER | PDCAAS | DIAAS | |
|---|---|---|---|
| Milk a | 2.50 | 1 | 114 |
| Eggs a | 3.10 | 1 | 113 |
| Chicken a | 2.70 | 1 | 108 |
| Oatmeal a | 1.80 | 0.82 | 84 |
| White bread a | 1.00 | 0.28 | 29 |
| White Rice a | 1.50 | 0.56 | 57 |
| Tofu a | 2.30 | 0.56 | 52 |
| Red Kidney Beans b | 1.55 | 0.55 | 51 |
| Navy Beans b | 1.51 | 0.67 | 65 |
| Whole Green Lentils b | 1.30 | 0.63 | 58 |
| Split Red Lentils b | 0.98 | 0.54 | 50 |
| Split Yellow Peas b | 1.42 | 0.64 | 73 |
| Split Green Peas b | 0.86 | 0.50 | 46 |
| Black Beans b | 1.61 | 0.53 | 49 |
| Chickpeas b | 2.32 | 0.52 | 85 |
| Pinto Beans b | 1.64 | 0.59 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosworthy, M.G.; Medina, G.; Lu, Z.-H.; House, J.D. Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses. Foods 2023, 12, 2816. https://doi.org/10.3390/foods12152816
Nosworthy MG, Medina G, Lu Z-H, House JD. Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses. Foods. 2023; 12(15):2816. https://doi.org/10.3390/foods12152816
Chicago/Turabian StyleNosworthy, Matthew G., Gerardo Medina, Zhan-Hui Lu, and James D. House. 2023. "Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses" Foods 12, no. 15: 2816. https://doi.org/10.3390/foods12152816
APA StyleNosworthy, M. G., Medina, G., Lu, Z.-H., & House, J. D. (2023). Plant Proteins: Methods of Quality Assessment and the Human Health Benefits of Pulses. Foods, 12(15), 2816. https://doi.org/10.3390/foods12152816






