Effects of Protein Hydrolysate from Silkworm (Bombyx mori) pupae on the C2C12 Myogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Defatting and Protein Extraction
2.3. Protein Hydrolysis and Ethanol (EtOH) Precipitation
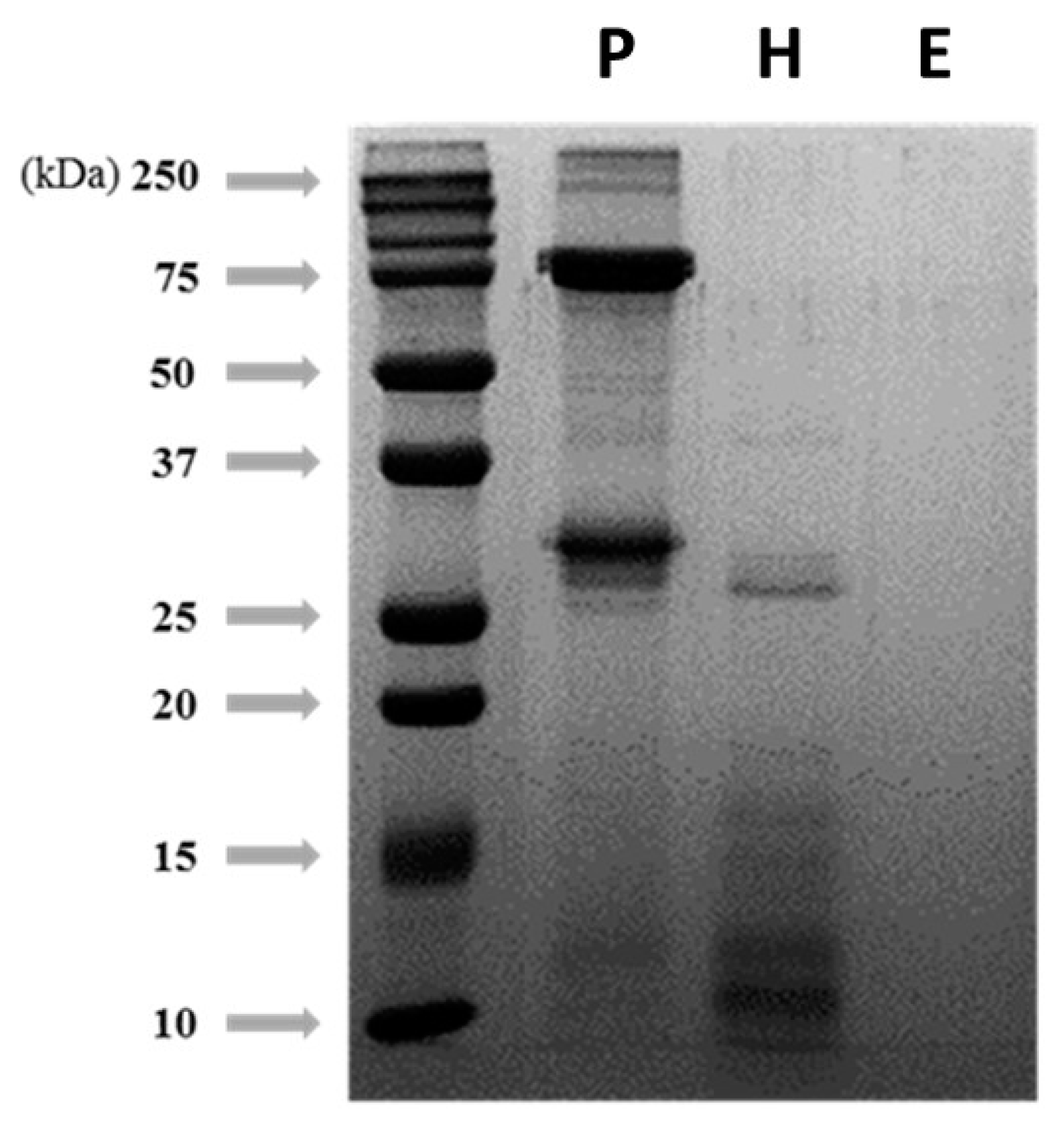
2.4. SDS-PAGE
2.5. Cell Culture
2.6. Immunofluorescence Analysis
2.7. MTT Assay
2.8. Western Blot Analysis
2.9. Fractionation Using Preparative HPLC
2.10. Identification of Active Metabolites by LC-MS/MS
2.11. Quantitative Analysis
2.12. Data Processing and Statistical Analysis
3. Results
3.1. Yields of Each Pretreatment Process of Silkworm pupae (%)
3.2. Degradation Patterns of Silkworm pupae Protein Hydrolysis by Different Pretreatments
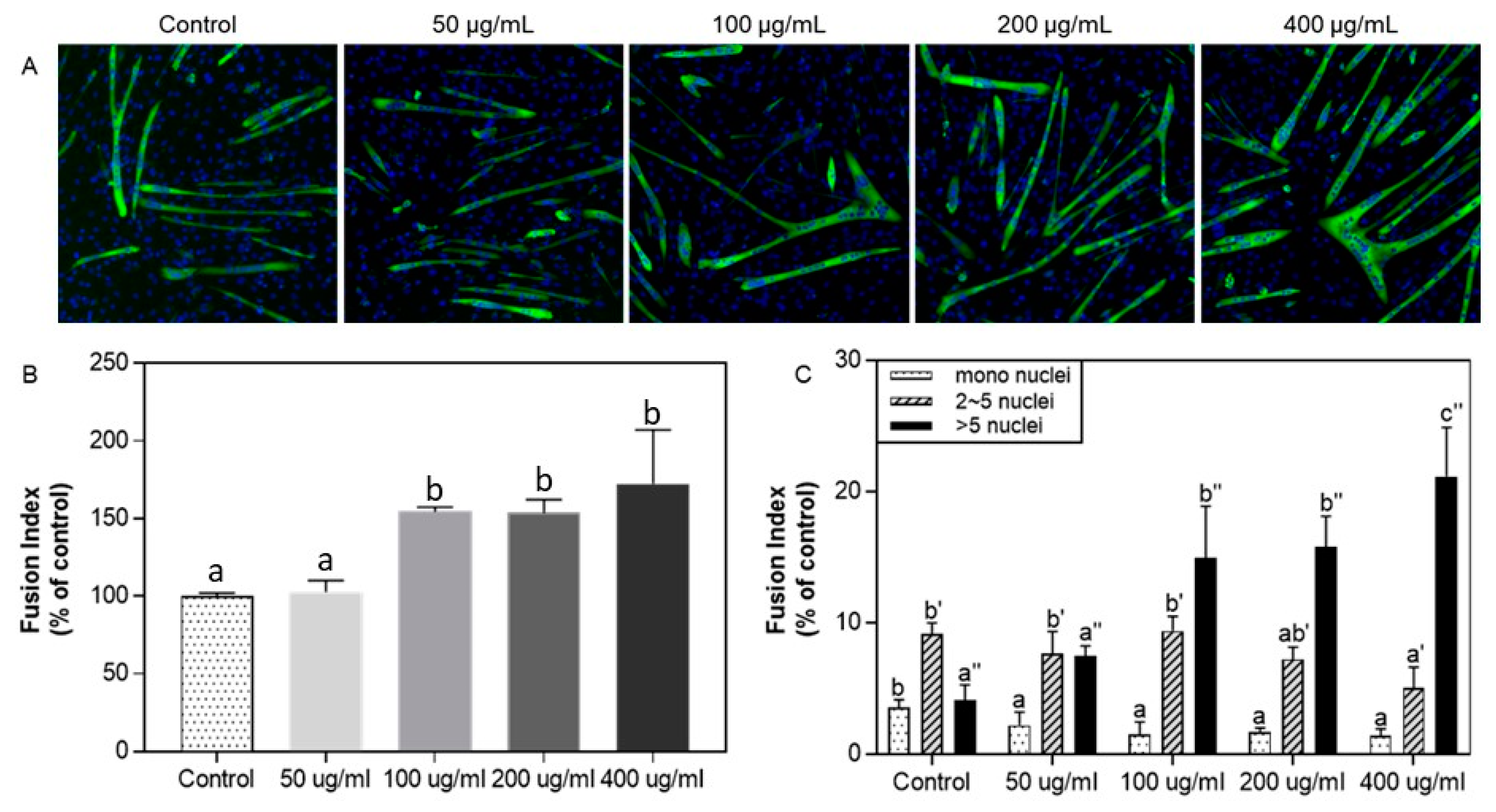
3.3. Effect of Protein Hydrolysate from Silkworm pupae on C2C12 Myogenic Differentiation: Immunofluorescence Assay
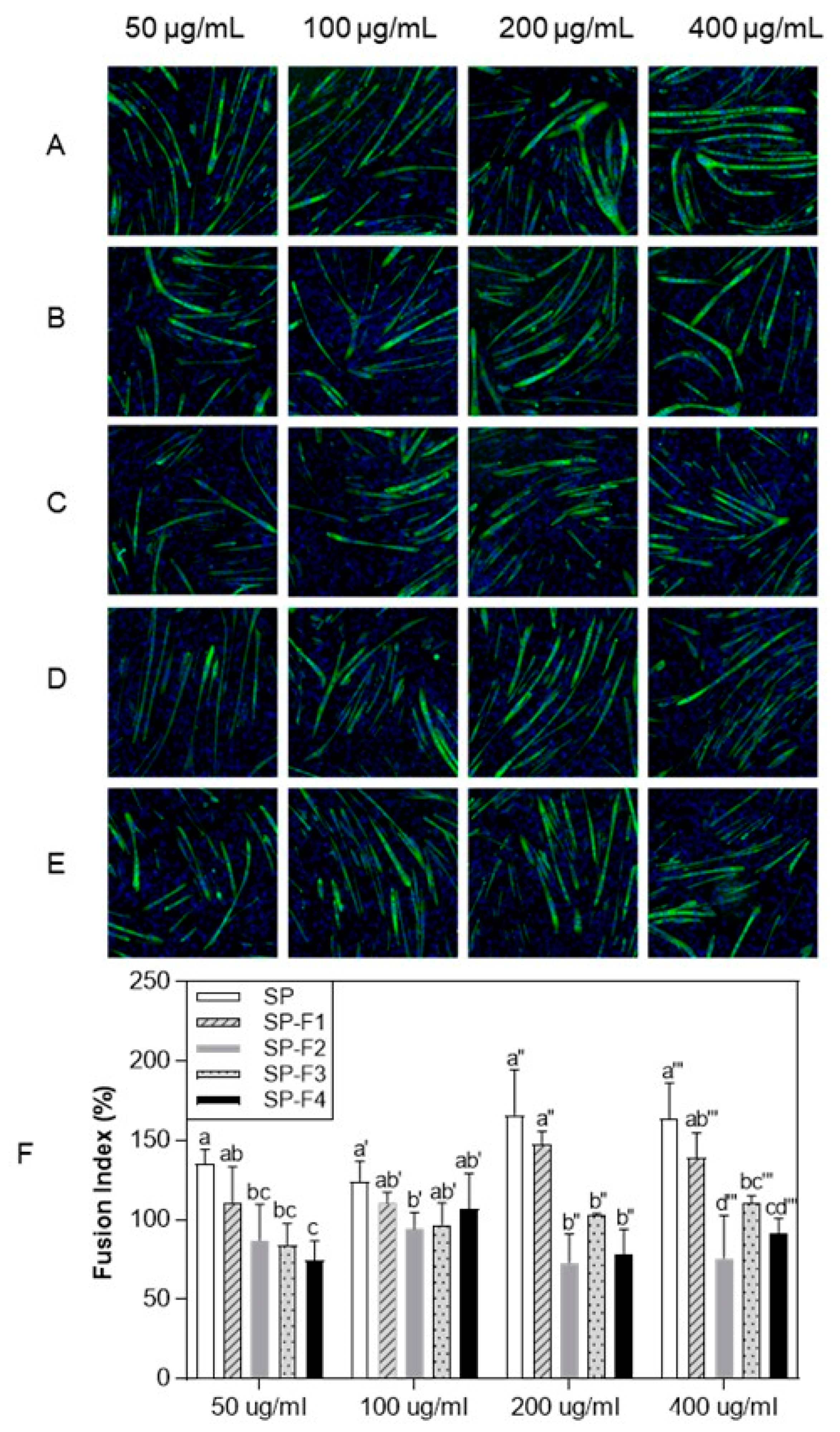
3.4. Effect of Purified Protein Hydrolysate from Silkworm pupae on C2C12 Myogenic Differentiation by the Immunofluorescence Assay
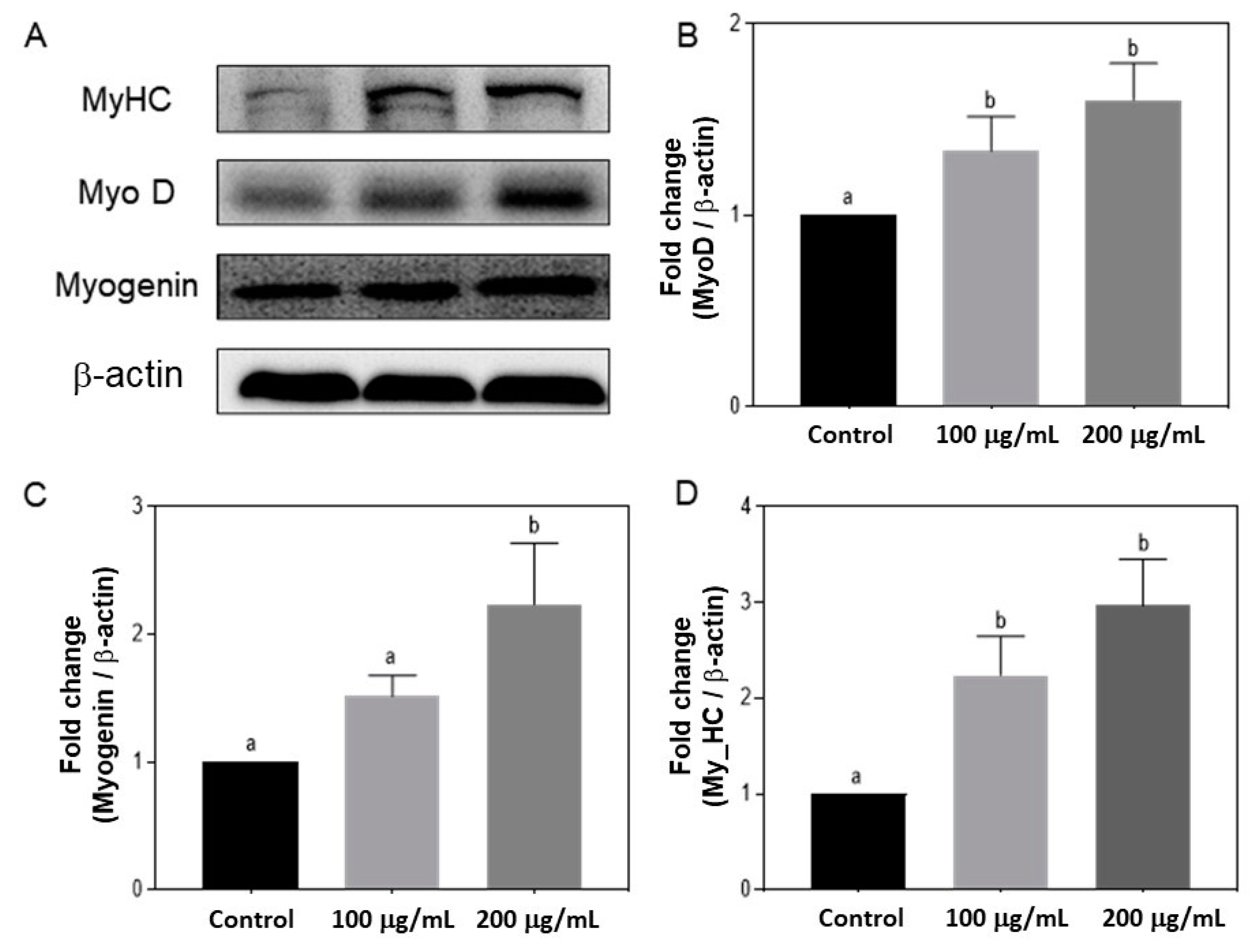
3.5. Effects of Silkworm pupae Protein Hydrolysate on the Expression of MyoD, Myogenin, and MyHC
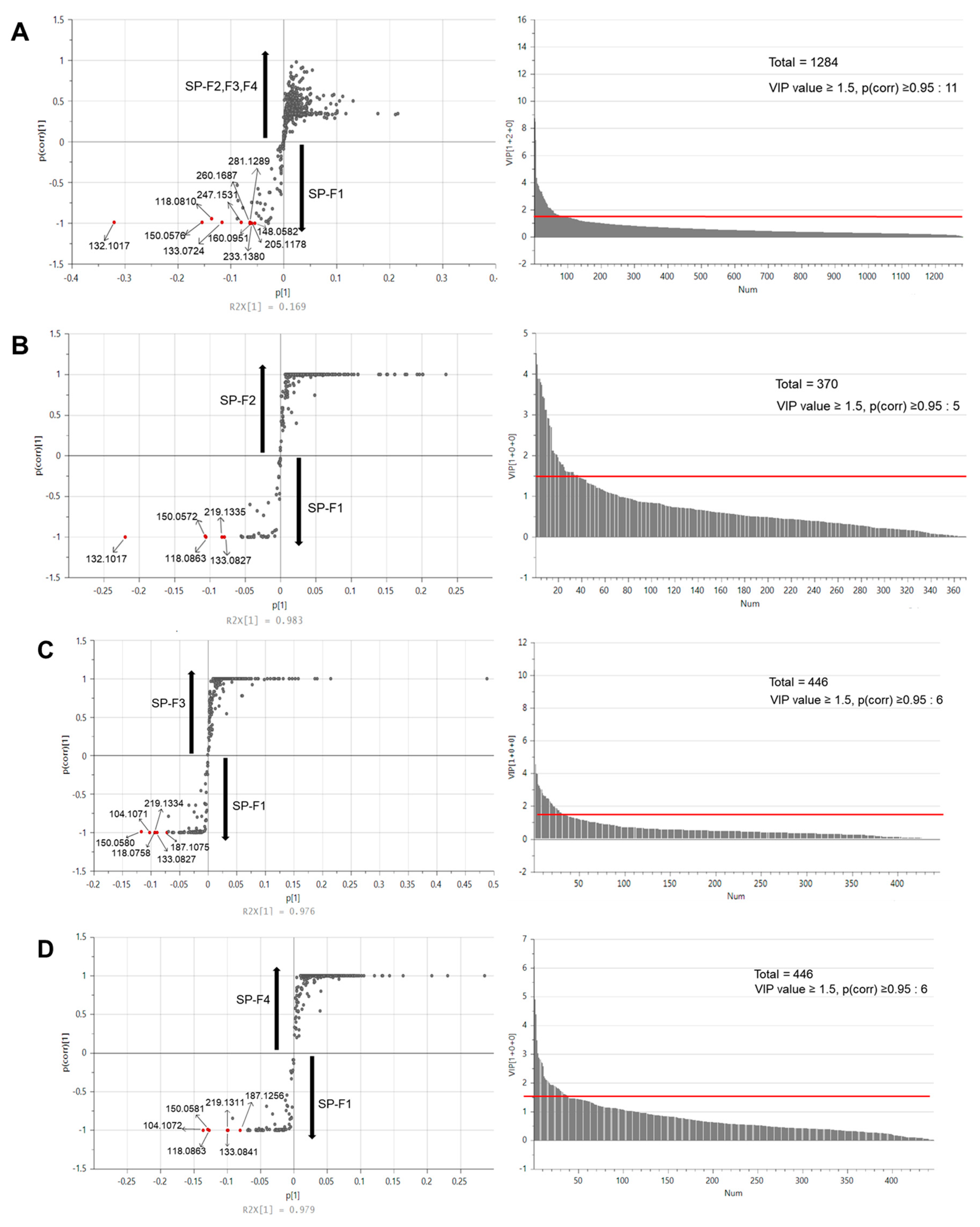
3.6. Metabolomic Analysis by Multivariate Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alami-Durante, H.; Bazin, D.; Cluzeaud, M.; Fontagné-Dicharry, S.; Kaushik, S.; Geurden, I. Effect of dietary methionine level on muscle growth mechanisms in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 483, 273–285. [Google Scholar] [CrossRef]
- Grafton, R.Q.; Daugbjerg, C.; Qureshi, M.E. Towards food security by 2050. Food Secur. 2015, 7, 179–183. [Google Scholar] [CrossRef]
- van Huis, A. Nutrition and health of edible insects. Curr. Opin. Clin. Nutr. Metab. Care. 2020, 23, 228–231. [Google Scholar] [CrossRef]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Hwang, B.B.; Chang, M.H.; Lee, J.H.; Heo, W.; Kim, J.K.; Pan, J.H.; Kim, Y.J.; Kim, J.H. The Edible Insect Gryllus bimaculatus Protects against Gut-Derived Inflammatory Responses and Liver Damage in Mice after Acute Alcohol Exposure. Nutrients 2019, 11, 857. [Google Scholar] [CrossRef] [Green Version]
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Fogliano, V.; Lakemond, C.M.M.; Severini, C. Effects of formulation and process conditions on microstructure, texture and digestibility of extruded insect-riched snacks. Innov. Food Sci. Emerg. Technol. 2018, 45, 344–353. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm Larvae, Adult Crickets, and Silkworm Pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Wang, W.; Wang, J.; Zhu, Z.; Li, X. Molecular mechanisms of novel peptides from silkworm pupae that inhibit alpha-glucosidase. Peptides 2016, 76, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Gahukar, R.T. Chapter 4—Edible Insects Farming: Efficiency and Impact on Family Livelihood, Food Security, and Environment Compared With Livestock and Crops. In Insects as Sustainable Food Ingredients; Dossey, A.T., Morales-Ramos, J.A., Rojas, M.G., Eds.; Academic Press: San Diego, CA, USA, 2016. [Google Scholar]
- Yang, R.; Zhao, X.; Kuang, Z.; Ye, M.; Luo, G.; Xiao, G.; Liao, S.; Li, L.; Xiong, Z. Optimization of antioxidant peptide production in the hydrolysis of silkworm (Bombyx mori L.) pupa protein using response surface methodology. J. Food Agric. Environ. 2013, 11, 952–956. [Google Scholar]
- Zhou, Z.-F.; Ren, Z.-X.; Yu, H.-Y.; Jia, J.-Q.; Gui, Z.-Z. Effects of different modification techniques on molecular structure and bioactivity of Bombyx mori pupa protein. J. Asia-Pacific Entomol. 2017, 20, 35–41. [Google Scholar] [CrossRef]
- Kodaka, M.; Yang, Z.; Nakagawa, K.; Maruyama, J.; Xu, X.; Sarkar, A.; Ichimura, A.; Nasu, Y.; Ozawa, T.; Iwasa, H.; et al. A new cell-based assay to evaluate myogenesis in mouse myoblast C2C12 cells. Exp. Cell Res. 2015, 336, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Langelaan, M.L.; Boonen, K.J.; Rosaria-Chak, K.Y.; van der Schaft, D.W.; Post, M.J.; Baaijens, F.P. Advanced maturation by electrical stimulation: Differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2011, 5, 529–539. [Google Scholar] [CrossRef]
- Bajaj, P.; Reddy, B., Jr.; Millet, L.; Wei, C.; Zorlutuna, P.; Bao, G.; Bashir, R. Patterning the differentiation of C2C12 skeletal myoblasts. Integr. Biol. 2011, 3, 897–909. [Google Scholar] [CrossRef]
- Jones, N.C.; Feforov, Y.V.; Rosenthal, R.S.; Olwin, B.B. ERK1/2 is required for myoblast proliferation but is dispensable for muscle gene expression and cell fusion. J. Cell. Physiol. 2001, 186, 104–115. [Google Scholar] [CrossRef]
- Keren, A.; Tamir, Y.; Bengal, E. The p38 MAPK signaling pathway: A major regulator of skeletal muscle development. Mol. Cell. Endocrinol. 2006, 252, 224–230. [Google Scholar] [CrossRef]
- Chae, J.; Nam, J.-O. Recent Studies on Natural Products that Improve Myogenesis. J. Life Sci. 2020, 30, 202–210. [Google Scholar]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef]
- Choi, B.D.; Wong, N.A.K.; Auh, J.H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Ani. Resour. 2017, 37, 955–961. [Google Scholar]
- Shiota, C.; Abe, T.; Kawai, N.; Ohno, A. Flavones Inhibit LPS-Induced Atrogin-1 MAFbx Expression in Mouse C2C12 Skeletal Myotubes. J. Nutr. Sci. Vitaminol. 2015, 61, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Li, C.; Lian, S.; Xu, B.; Yuan, J.; Lu, J.; Yang, H.; Guo, J.; Ji, H. ActivinA activates Notch1-Shh signaling to regulate proliferation in C2C12 skeletal muscle cells. Mol. Cell. Endocrinol. 2021, 519, 111055. [Google Scholar] [CrossRef]
- Li, H.; Ma, Y.; Xu, W.; Chen, H.; Day, L. MFG-E8 protein promotes C2C12myogenic differentiation by enhancing PI3K/Akt signaling. New J. Chem. 2017, 41, 12061–12070. [Google Scholar] [CrossRef]
- Lin, M.; Zhou, S.; Sakamoto, K. Alpha Mangostin promotes myogenic differentiation of C2C12 mouse myoblast cells. Biochem. Biophys. Res. Commun. 2020, 528, 193–198. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kang, Y.J.; Sung, B.; Jang, J.Y.; Hwang, N.L.; Oh, H.J.; Ahn, Y.R.; Kim, H.J.; Shin, J.H.; Yoo, M.A.; et al. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J. Cell. Physiol. 2018, 233, 736–747. [Google Scholar] [CrossRef]
- Katajamaa, M.; Miettinen, J.; Oresic, M. MZmine: Toolbox for processing and visualization of mass spectrometry based molecular profile data. Int. J. Mol. Med. 2006, 22, 634–636. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, J.; Lancon, A.; Aires, V.; Limagne, E.; Tili, E.; Michaille, J.J.; Latruffe, N. Resveratrol initiates differentiation of mouse skeletal muscle-derived C2C12 myoblasts. Biochem. Pharmacol. 2012, 84, 1251–1259. [Google Scholar] [CrossRef]
- Flamini, V.; Ghadiali, R.S.; Antczak, P.; Rothwell, A.; Turnbull, J.E.; Pisconti, A. The Satellite Cell Niche Regulates the Balance between Myoblast Differentiation and Self-Renewal via p53. Stem Cell Rep. 2018, 10, 970–983. [Google Scholar] [CrossRef] [Green Version]
- Zanou, N.; Gailly, P. Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cell. Mol. Life Sci. 2013, 70, 4117–4130. [Google Scholar] [CrossRef]
- Schmidt, M.; Schüler, S.C.; Hüttner, S.S.; von Eyss, B.; von Maltzahn, J. Adult stem cells at work: Regenerating skeletal muscle. Cell. Mol. Life Sci. 2019, 76, 2559–2570. [Google Scholar] [CrossRef] [Green Version]
- Kim, G.W.; Nam, G.H.; Kim, I.S.; Park, S.Y. Xk-related protein 8 regulates myoblast differentiation and survival. FEBS J. 2017, 284, 3575–3588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitakaze, T.; Sakamoto, T.; Kitano, T.; Inoue, N.; Sugihara, F.; Harada, N.; Yamaji, R. The collagen derived dipeptide hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and myotube hypertrophy. Biochem. Biophys. Res. Commun. 2016, 478, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Anootthato, S.; Therdthai, N.; Ritthiruangdej, P. Characterization of protein hydrolysate from silkworm pupae (Bombyx mori). J. Food Process. Preserv. 2019, 43, e14021. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Zhou, Y.; Zhang, Y.; Xu, L.; Xu, J.; Feng, F.; He, G. Isolation of a novel peptide from silkworm pupae protein components and interaction characteristics to angiotensin I-converting enzyme. Eur. Food Res. Technol. 2010, 232, 29–38. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of Silkworm Pupae Peptide on the Fermentation and Quality of Yogurt. J. Food Process. Preserv. J. Food Process. Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.-K.; Kang, M.-C.; Kim, B.-K.; Choi, Y.-S. Protective effects of edible insect protein extracts from Protaetia brevitarsis against H2O2-induced oxidative stress in mouse C2C12 myoblast cells. Food Biosci. 2023, 52, 102396. [Google Scholar] [CrossRef]
- Gorissen, S.H.M.; Phillips, S.M. Branched-Chain Amino Acids (Leucine, Isoleucine, and Valine) and Skeletal Muscle. In Nutrition and Skeletal Muscle; Academic Press: Cambridge, MA, USA, 2019; pp. 283–298. [Google Scholar] [CrossRef]
- Kim, M.; Sung, B.; Kang, Y.J.; Kim, D.H.; Lee, Y.; Hwang, S.Y.; Yoon, J.H.; Yoo, M.A.; Kim, C.M.; Chung, H.Y.; et al. The combination of ursolic acid and leucine potentiates the differentiation of C2C12 murine myoblasts through the mTOR signaling pathway. Int. J. Mol. Med. 2015, 35, 755–762. [Google Scholar] [CrossRef] [Green Version]
| m/z | Tentative Identification | MS/MS Fragment | Error (ppm) |
|---|---|---|---|
| 118.0863 | Valine | 72.0812, 55.0548, 118.0862 | 0.38 |
| 132.1017 | Isoleucine | 87.0967, 69.0703, 132.1316 | −1.55 |
| 150.0581 | Methionine | 61.0112, 56.0500, 104.0529 | −1.50 |
| 148.0601 | Glutamic acid | 84.0447, 56.0500, 102.0550 | −1.58 |
| 281.1127 | Asp-Phe | 120.0806, 103.0543, 91.0544 | −1.77 |
| 187.1074 | Ala-Pro | 70.0656, 116.0705, 84.0447 | −1.60 |
| 104.1071 | Choline | 104.1070, 60.0813, 58.0656 | 1.05 |
| 219.1334 | unknown | 60.0449, 86.0967, 132.1016 | |
| 205.1180 | unknown | 80.0449, 72.0812, 118.0861 | |
| 247.1283 | unknown | 72.0812, 84.0447, 148.0601 | - |
| 160.1077 | unknown | 55.0548, 60.0813, 101.0598 | - |
| 260.1687 | MS/MS not detected | - | |
| 233.1380 | MS/MS not detected | - | |
| 133.0827 | MS/MS not detected | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.-S.; Park, J.H.; Auh, J.-H. Effects of Protein Hydrolysate from Silkworm (Bombyx mori) pupae on the C2C12 Myogenic Differentiation. Foods 2023, 12, 2840. https://doi.org/10.3390/foods12152840
Kang H-S, Park JH, Auh J-H. Effects of Protein Hydrolysate from Silkworm (Bombyx mori) pupae on the C2C12 Myogenic Differentiation. Foods. 2023; 12(15):2840. https://doi.org/10.3390/foods12152840
Chicago/Turabian StyleKang, Hyeong-Seok, Ji Hye Park, and Joong-Hyuck Auh. 2023. "Effects of Protein Hydrolysate from Silkworm (Bombyx mori) pupae on the C2C12 Myogenic Differentiation" Foods 12, no. 15: 2840. https://doi.org/10.3390/foods12152840
APA StyleKang, H.-S., Park, J. H., & Auh, J.-H. (2023). Effects of Protein Hydrolysate from Silkworm (Bombyx mori) pupae on the C2C12 Myogenic Differentiation. Foods, 12(15), 2840. https://doi.org/10.3390/foods12152840






