Xanthoceras sorbifolia Husk Extract Incorporation for the Improvement in Physical and Antioxidant Properties of Soy Protein Isolate Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of XSHE
2.3. Formation of SPI Films
2.4. Characterization of Films
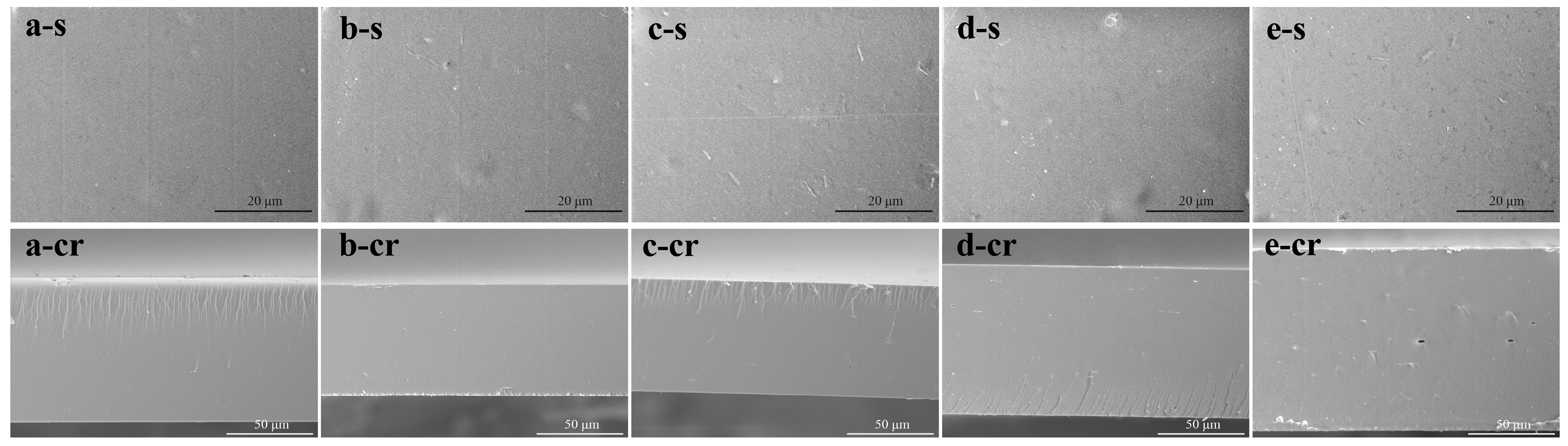
2.4.1. Scanning Electron Microscopy (SEM)
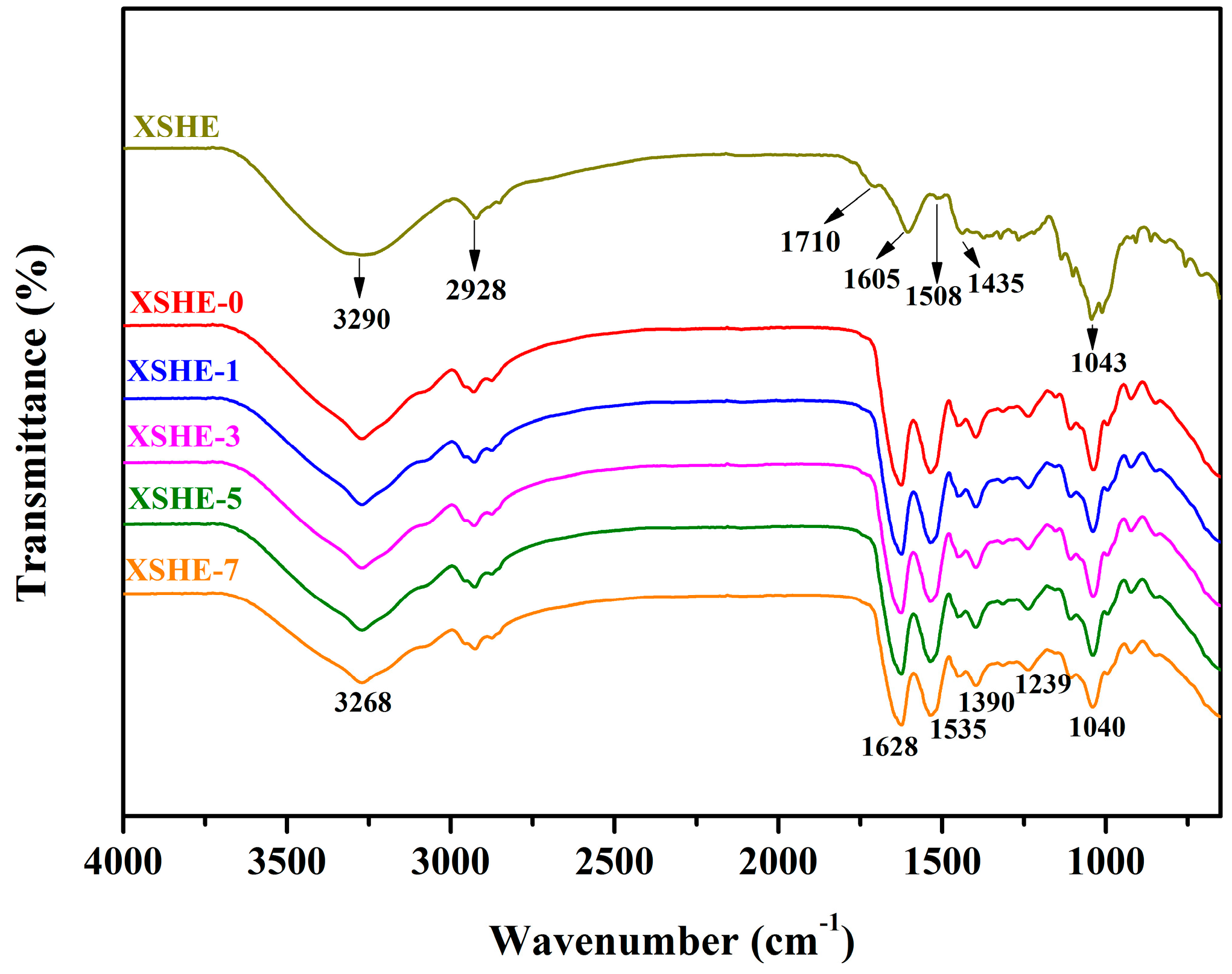
2.4.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.4.3. Colorimetric Analysis
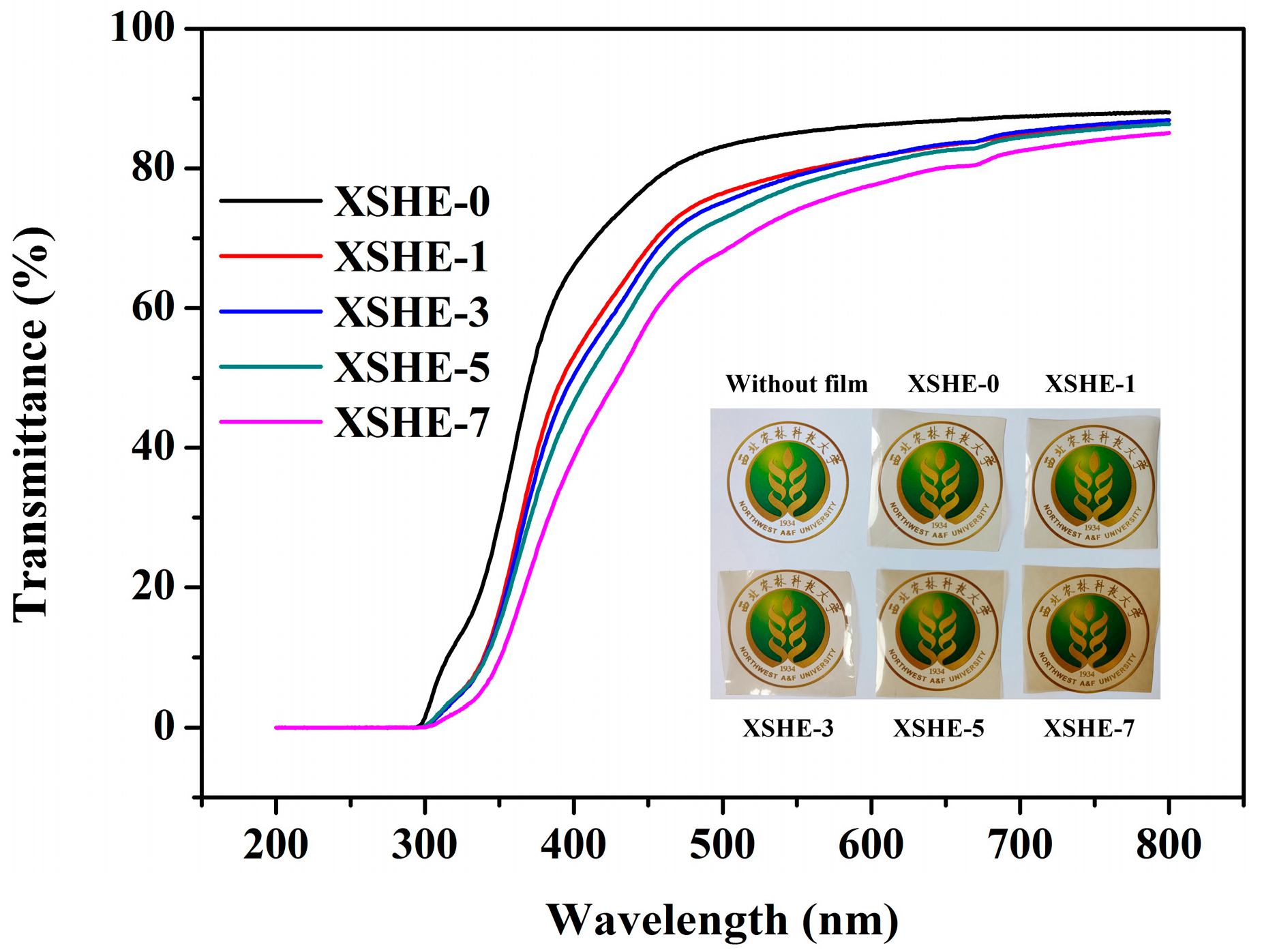
2.4.4. Light Transmittance
2.4.5. Mechanical Performance
2.4.6. Moisture Content (MC) and Water Vapor Permeability (WVP)
2.4.7. Release Test
2.4.8. Antioxidant Activities
2.4.9. Statistical Analysis
3. Results and Discussion
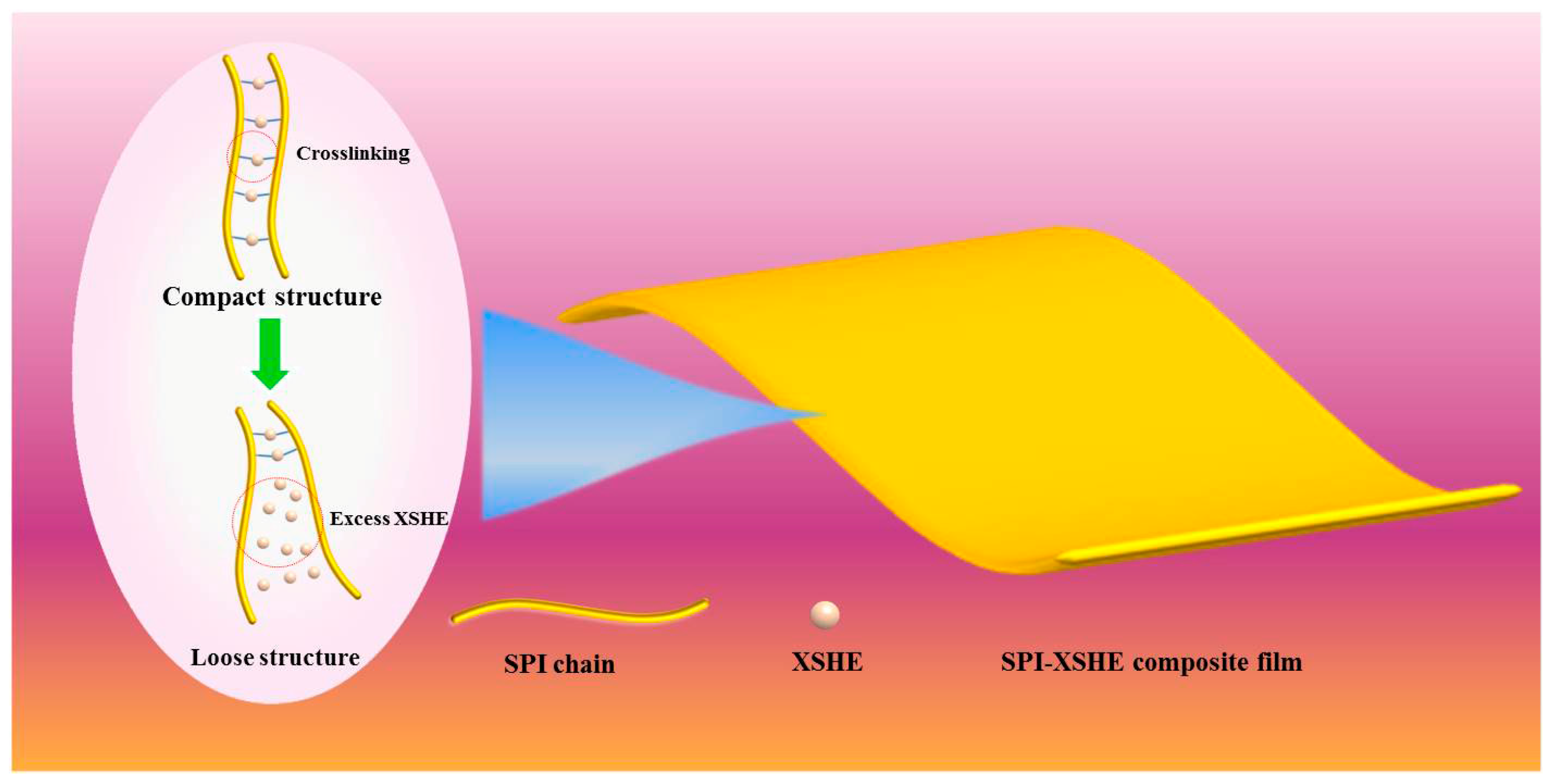
3.1. Morphology of the Films
3.2. FT-IR Spectra
3.3. Film Color
3.4. Light Transmittance of SPI–XSHE Films
3.5. Mechanical Properties
3.6. MC and WVP of SPI–XSHE Films
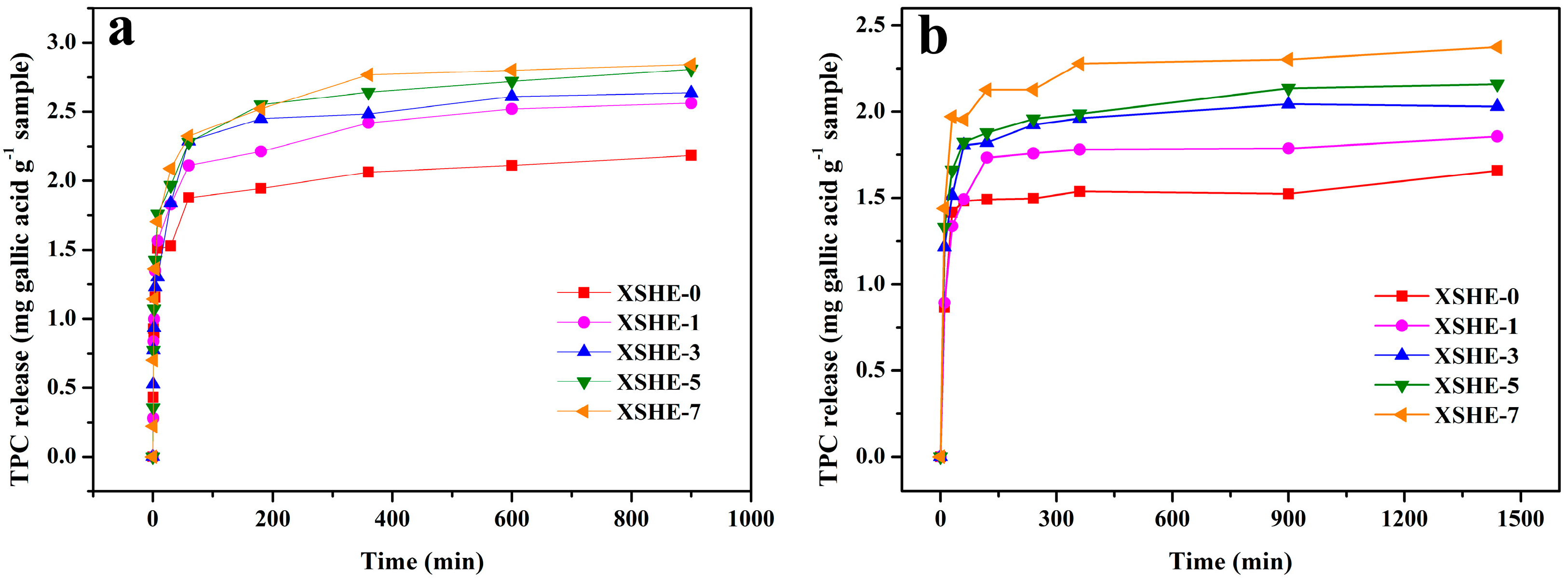
3.7. Food Simulant Release Behavior
3.8. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in take-out food containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Fathi, N.; Almasi, H.; Pirouzifard, M.K. Effect of ultraviolet radiation on morphological and physicochemical properties of sesame protein isolate based edible films. Food Hydrocoll. 2018, 85, 136–143. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Bio-based films prepared with soybean by-products and pine (Pinus densiflora) bark extract. J. Clean. Prod. 2018, 187, 1–8. [Google Scholar] [CrossRef]
- Janjarasskul, T.; Tananuwong, K.; Phupoksakul, T.; Thaiphanit, S. Fast dissolving, hermetically sealable, edible whey protein isolate-based films for instant food and/or dry ingredient pouches. LWT 2020, 134, 110102. [Google Scholar] [CrossRef]
- Meerasri, J.; Sothornvit, R. Characterization of bioactive film from pectin incorporated with gamma-aminobutyric acid. Int. J. Biol. Macromol. 2020, 147, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Abiri, A.B.; Baghaei, H.; Nafchi, A.M. Preparation and application of active bionanocomposite films based on sago starch reinforced with a combination of TiO2 nanoparticles and penganum harmala extract for preserving chicken fillets. Polymers 2023, 15, 2889. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, protein and lipid-based natural edible films in food packaging: A review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, Z.; Cai, Q.; Peng, B. Effects of high hydrostatic pressure on structural and physical properties of nisin-spi film. Int. J. Biol. Macromol. 2018, 111, 976–982. [Google Scholar] [CrossRef]
- Guerrero, P.; Stefani, P.; Ruseckaite, R.; De la Caba, K. Functional properties of films based on soy protein isolate and gelatin processed by compression molding. J. Food Eng. 2011, 105, 65–72. [Google Scholar] [CrossRef]
- Dastidar, T.G.; Netravali, A.N. A soy flour based thermoset resin without the use of any external crosslinker. Green Chem. 2013, 15, 3243–3251. [Google Scholar] [CrossRef]
- Koshy, R.R.; Mary, S.K.; Thomas, S.; Pothan, L.A. Environment friendly green composites based on soy protein isolate–a review. Food Hydrocoll. 2015, 50, 174–192. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Debeaufort, F.; Karbowiak, T. Bioactive edible films for food applications: Mechanisms of antimicrobial and antioxidant activity. Crit. Rev. Food Sci. Nutr. 2019, 59, 3431–3455. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, R. A review on material and antimicrobial properties of soy protein isolate film. J. Polym. Environ. 2019, 27, 1613–1628. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Wu, H.; Lu, J.; Xiao, D.; Yan, Z.; Li, S.; Li, T.; Wan, X.; Zhang, Z.; Liu, Y.; Shen, G. Development and characterization of antimicrobial protein films based on soybean protein isolate incorporating diatomite/thymol complex. Food Hydrocoll. 2021, 110, 106138. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Characterization and in vitro antimicrobial study of soy protein isolate films incorporating carvacrol. Food Hydrocoll. 2022, 122, 107091. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, Y.; Kang, S.; Wang, K.; Xu, H. Development and evaluation of soy protein isolate-based antibacterial nanocomposite films containing cellulose nanocrystals and zinc oxide nanoparticles. Food Hydrocoll. 2020, 106, 105898. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Karbowiak, T.; Debeaufort, F. Bioactive edible films for food applications: Influence of the bioactive compounds on film structure and properties. Crit. Rev. Food Sci. Nutr. 2019, 59, 1137–1153. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ullah, A. Recent advances in protein derived bionanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 406–434. [Google Scholar] [CrossRef]
- Chang-Bravo, L.; López-Córdoba, A.; Martino, M. Biopolymeric matrices made of carrageenan and corn starch for the antioxidant extracts delivery of cuban red propolis and yerba mate. React. Funct. Polym. 2014, 85, 11–19. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, S.; Xu, S.; Li, J.; Wang, L. Characterization of antioxidant properties of soy bean protein-based films with cortex phellodendri extract in extending the shelf life of lipid. Food Packag. Shelf Life 2019, 22, 100413. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and antioxidant properties of flexible soy protein isolate films by incorporating chestnut (Castanea mollissima) bur extracts. LWT 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L. Developing a bio-based packaging film from soya by-products incorporated with valonea tannin. J. Clean. Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite-clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Hanani, Z.N. Storage stability of soy protein isolate films incorporated with mango kernel extract at different temperature. Food Hydrocoll. 2019, 87, 541–549. [Google Scholar] [CrossRef]
- Adilah, Z.M.; Jamilah, B.; Hanani, Z.N. Functional and antioxidant properties of protein-based films incorporated with mango kernel extract for active packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Qu, X.-J.; Fu, Y.-J.; Luo, M.; Zhao, C.-J.; Zu, Y.-G.; Li, C.-Y.; Wang, W.; Li, J.; Wei, Z.-F. Acidic ph based microwave-assisted aqueous extraction of seed oil from yellow horn (Xanthoceras sorbifolia bunge.). Ind. Crops Prod. 2013, 43, 420–426. [Google Scholar] [CrossRef]
- Ruan, C.-J.; Yan, R.; Wang, B.-X.; Mopper, S.; Guan, W.-K.; Zhang, J. The importance of yellow horn (Xanthoceras sorbifolia) for restoration of arid habitats and production of bioactive seed oils. Ecol. Eng. 2017, 99, 504–512. [Google Scholar] [CrossRef]
- Ma, Y.; Bi, Q.; Li, G.; Liu, X.; Fu, G.; Zhao, Y.; Wang, L. Provenance variations in kernel oil content, fatty acid profile and biodiesel properties of Xanthoceras sorbifolium bunge in northern china. Ind. Crops Prod. 2020, 151, 112487. [Google Scholar] [CrossRef]
- Gu, L.-B.; Zhang, G.-J.; Du, L.; Du, J.; Qi, K.; Zhu, X.-L.; Zhang, X.-Y.; Jiang, Z.-H. Comparative study on the extraction of Xanthoceras sorbifolia bunge (yellow horn) seed oil using subcritical n-butane, supercritical CO2, and the soxhlet method. LWT 2019, 111, 548–554. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Ha, W.; Lin, Y.; Jiang, K.; Yang, J.-L.; Shi, Y.-P. Polyphenols isolated from Xanthoceras sorbifolia husks and their anti-tumor and radical-scavenging activities. Molecules 2016, 21, 1694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Su, D.; Yu, B.; Chen, C.; Cheng, L.; Li, X.; Xi, R.; Gao, H.; Wang, X. Novel anti-tumour barringenol-like triterpenoids from the husks of Xanthoceras sorbifolia bunge and their three dimensional quantitative structure activity relationships analysis. Fitoterapia 2017, 116, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, Q. Inhibitory effect of flavonoids consituents from Xanthoceras sorbifolia nutshell on tyrosinase. J. Chin. Cereals Oils Assoc 2013, 28, 96–100. [Google Scholar]
- Liu, X. Improvement of ethanol extract from husk of Xanthoceras sorbifolia on rats with learning and memory dysfunction. Chin Tradit Herb Drugs 2007, 12, 106–110. [Google Scholar]
- Wang, D.; Yu, B.; Chen, C.; Duan, J.; Di, D.; Xiong, X.; Yang, Y.; Gao, H. New natural barrigenol-like triterpenoid isolated from the husks of Xanthoceras sorbifolia bunge. Nat. Prod. Res. 2018, 32, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Liu, L.; Wang, Y.; Li, Z.; Liu, R.; Li, Q.; Wang, Y.; Yang, B.; Chen, X.; Bi, K. Characterization and quantification of the triterpenoids in different parts of Xanthoceras sorbifolia by hplc–esi-ms. J. Pharm. Biomed. Anal. 2011, 55, 259–264. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, F.; Ma, S.; Qi, G.; Shang, H.; Zheng, Z.; Yuan, H.; Yang, L. Chemical constituents of the fruit husk of Xanthoceras sorbifolia. Chem. Nat. Compd. 2020, 56, 325–327. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L. Preparation of a visual ph-sensing film based on tara gum incorporating cellulose and extracts from grape skins. Sens. Actuators B Chem. 2016, 235, 401–407. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Pham, B.-T.T.; Le, H.N.; Bach, L.G.; Thuc, C.H. Comparative characterization and release study of edible films of chitosan and natural extracts. Food Packag. Shelf Life 2022, 32, 100830. [Google Scholar] [CrossRef]
- Kevij, H.T.; Salami, M.; Mohammadian, M.; Khodadadi, M. Fabrication and investigation of physicochemical, food simulant release, and antioxidant properties of whey protein isolate-based films activated by loading with curcumin through the ph-driven method. Food Hydrocoll. 2020, 108, 106026. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Ferreira, W.H.; Goycoolea, F.M.; Murray, B.S.; Andrade, C.T. Improved antioxidant and mechanical properties of food packaging films based on chitosan/deep eutectic solvent, containing açaí-filled microcapsules. Molecules 2023, 28, 1507. [Google Scholar] [CrossRef]
- Wang, D.; Su, D.; Li, X.-Z.; Liu, D.; Xi, R.G.; Gao, H.Y.; Wang, X.B. Barrigenol triterpenes from the husks of Xanthoceras sorbifolia bunge and their antitumor activities. RSC Adv. 2016, 6, 27434–27446. [Google Scholar] [CrossRef]
- Hergert, H.L. Infrared spectra of lignin and related compounds. Ii. Conifer lignin and model compounds1, 2. J. Org. Chem. 1960, 25, 405–413. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Chen, H.; He, J.; Li, J. A high-performance soy protein isolate-based nanocomposite film modified with microcrystalline cellulose and cu and zn nanoclusters. Polymers 2017, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Qin, Q.; Li, W.; Zhang, X.; Gao, B.; Han, L.; Liu, X. Feasibility of bionanocomposite films fabricated using capsicum leaf protein and cellulose nanofibers. Food Chem. 2022, 387, 132769. [Google Scholar] [CrossRef]
- Yu, Z.; Sun, L.; Wang, W.; Zeng, W.; Mustapha, A.; Lin, M. Soy protein-based films incorporated with cellulose nanocrystals and pine needle extract for active packaging. Ind. Crops Prod. 2018, 112, 412–419. [Google Scholar] [CrossRef]
- Tong, C.; Wu, Z.; Sun, J.; Lin, L.; Wang, L.; Guo, Y.; Huang, Z.; Wu, C.; Pang, J. Effect of carboxylation cellulose nanocrystal and grape peel extracts on the physical, mechanical and antioxidant properties of konjac glucomannan films. Int. J. Biol. Macromol. 2020, 156, 874–884. [Google Scholar] [CrossRef]
- Yong, H.; Wang, X.; Bai, R.; Miao, Z.; Zhang, X.; Liu, J. Development of antioxidant and intelligent ph-sensing packaging films by incorporating purple-fleshed sweet potato extract into chitosan matrix. Food Hydrocoll. 2019, 90, 216–224. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch–chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, K.; Ma, Y.; Ma, D.; Li, X.; Zhao, X. Incorporations of blueberry extracts into soybean-protein-isolate film preserve qualities of packaged lard. Int. J. Food Sci. Technol. 2010, 45, 1801–1806. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Lime peel pectin integrated with coconut water and lime peel extract as a new bioactive film sachet to retard soybean oil oxidation. Food Hydrocoll. 2019, 97, 105173. [Google Scholar] [CrossRef]
- Pruneda, E.; Peralta-Hernández, J.; Esquivel, K.; Lee, S.; Godínez, L.; Mendoza, S. Water vapor permeability, mechanical properties and antioxidant effect of mexican oregano–soy based edible films. J. Food Sci. 2008, 73, C488–C493. [Google Scholar] [CrossRef]
- Li, T.; Xia, N.; Xu, L.; Zhang, H.; Zhang, H.; Chi, Y.; Zhang, Y.; Li, L.; Li, H. Preparation, characterization and application of spi-based blend film with antioxidant activity. Food Packag. Shelf Life 2021, 27, 100614. [Google Scholar] [CrossRef]
- Buonocore, G.G.; Del Nobile, M.A.; Panizza, A.; Corbo, M.R.; Nicolais, L. A general approach to describe the antimicrobial agent release from highly swellable films intended for food packaging applications. J. Control. Release 2003, 90, 97–107. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; González-Martínez, C.; Chiralt, A.; Desobry, S. Study of the release of limonene present in chitosan films enriched with bergamot oil in food simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of abts/dpph assays to measure antioxidant capacity in popular antioxidant-rich us foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]

| Film Sample | Color Parameters | |||
|---|---|---|---|---|
| L | a | b | ΔE | |
| XSHE-0 | 94.21 ± 0.04 a | −1.91 ± 0.02 e | 9.84 ± 0.04 e | 10.07 ± 0.02 e |
| XSHE-1 | 90.15 ± 0.57 b | −1.81 ± 0.02 d | 14.99 ± 0.36 d | 16.30 ± 0.54 d |
| XSHE-3 | 89.57 ± 0.08 c | −1.59 ± 0.03 c | 17.00 ± 0.19 c | 18.31 ± 0.17 c |
| XSHE-5 | 88.37 ± 0.12 d | −1.42 ± 0.06 b | 21.41 ± 0.09 b | 22.81 ± 0.12 b |
| XSHE-7 | 86.77 ± 0.22 e | −0.37 ± 0.09 a | 22.65 ± 0.36 a | 24.56 ± 0.42 a |
| Film Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| XSHE-0 | 4.99 ± 0.03 d | 169.33 ± 7.02 a |
| XSHE-1 | 5.59 ± 0.19 c | 144.67 ± 10.26 b |
| XSHE-3 | 6.27 ± 0.09 b | 148.00 ± 7.21 b |
| XSHE-5 | 7.37 ± 0.25 a | 126.67 ± 3.06 c |
| XSHE-7 | 6.03 ± 0.05 b | 154.67 ± 5.03 b |
| Film Sample | MC (%) | WVP (g m−1 s−1 Pa−1 × 10−10) |
|---|---|---|
| XSHE-0 | 17.21 ± 0.36 a | 1.45 ± 0.04 a |
| XSHE-1 | 14.76 ± 0.59 b | 1.38 ± 0.02 b |
| XSHE-3 | 14.35 ± 1.65 b,c | 1.14 ± 0.01 d |
| XSHE-5 | 13.24 ± 0.32 c | 1.13 ± 0.02 d |
| XSHE-7 | 13.97 ± 0.45 b,c | 1.20 ± 0.03 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Yan, W.; Hou, Y.; Wang, D.; Yu, M. Xanthoceras sorbifolia Husk Extract Incorporation for the Improvement in Physical and Antioxidant Properties of Soy Protein Isolate Films. Foods 2023, 12, 2842. https://doi.org/10.3390/foods12152842
Han Y, Yan W, Hou Y, Wang D, Yu M. Xanthoceras sorbifolia Husk Extract Incorporation for the Improvement in Physical and Antioxidant Properties of Soy Protein Isolate Films. Foods. 2023; 12(15):2842. https://doi.org/10.3390/foods12152842
Chicago/Turabian StyleHan, Yingying, Wentao Yan, Yuping Hou, Dongmei Wang, and Miao Yu. 2023. "Xanthoceras sorbifolia Husk Extract Incorporation for the Improvement in Physical and Antioxidant Properties of Soy Protein Isolate Films" Foods 12, no. 15: 2842. https://doi.org/10.3390/foods12152842
APA StyleHan, Y., Yan, W., Hou, Y., Wang, D., & Yu, M. (2023). Xanthoceras sorbifolia Husk Extract Incorporation for the Improvement in Physical and Antioxidant Properties of Soy Protein Isolate Films. Foods, 12(15), 2842. https://doi.org/10.3390/foods12152842





