1. Introduction
The blueberry is a fruit that stands out in the world fruit crop due to its excellent nutritional value. Worldwide, it is considered the fruit of longevity since its anthocyanin content is high, which prevents several degenerative diseases (due to the high antioxidant capacity of the anthocyanins) and, thus, contributes to a healthier life [
1,
2,
3,
4]. Despite this, anthocyanins are phenolic compounds, which can have negative impacts on the sensory quality of the fruit, impairing its consumer acceptance. In this context, the manufacture of jams is an alternative to soften the astringent taste [
5,
6] and, in addition, the production of fruit jellies is considered a leading industrially important product, especially in European countries, such as England, which assumes a prominent role both in terms of consumption and quality [
7,
8].
The literature defines fruit jam as the product obtained by cooking fruit (whole, in pieces or fruit juice) with sugar and water and concentrated until it achieves a gelatinous consistency. It cannot be artificially colored or flavored, but the addition of acidulants and pectin is tolerated, if necessary, to compensate for any deficiency in the natural acidity and/or pectin content [
8,
9].
Pectin, used mainly in the food industry as a gelling agent in jellies and jams, is classified according to the degree of esterification (DE) as low (LM, <50% DE) and high (HM, >50% DE) methoxy. LM pectin is often used in low sugar jams and is obtained by controlled de-esterification of HM pectin under acidic or alkaline conditions. However, pectin processing (e.g., extraction, heat, or pH condition) and functional group distribution vary between factories. Pectin is an important component in texture variation, which represents a critical factor for food acceptability [
10]
.Even industrialized foods remain susceptible to biological activities evidenced by variations (which can be microbiological, physical, chemical, and even enzymatic), which can result in loss of nutrients, as well as physical, chemical (pH, acidity), and rheological (texture and viscosity) changes in properties [
11,
12]. One of the main quality attributes that influences the acceptability of jam is texture, which influences its appearance, flavor, and sensory impressions (gustatory and tactile) [
13,
14]. That is, the consistency of a jam must maintain its semisolid state when removed from its flask, with a smooth texture and without resistance to cutting [
15,
16].
Texture is defined as the manifestation of the rheological properties of the material, considered an important attribute of food, considering that it affects the process, storage, handling, and acceptance of the product by the consumer [
17]. Knowledge of the rheological properties of semisolid foods such as jellies is important for process design, quality control and new product development [
18].
Previous research reports the main factors responsible for the rheological behavior of fruit-derived products as the type of fruit, temperature, and solid content such as sugars, pectins and fibers [
19].
Broomes and Badrie [
20] reported an increase in gel firmness and a decrease in jelly acceptability the higher the added LM pectin content when investigating the effects of adding low methoxyl (LM) pectin on the physical and sensory properties of light jellies. The authors mentioned that all sensory attributes (color, odor, appearance, flavor, texture and general acceptability) were significantly affected by the presence of LM pectin.
In comparison, Basu and Shivhare [
21], when investigating the effects of adding sorbitol on the rheological and sensory properties of mango jam, found that increasing the content of this sweetener resulted in a decrease in hardness and an increase in the sensory evaluation of spreadability.
Despite the availability of some studies involving the stability of phenolic compounds in blueberry jams [
22,
23,
24], there are few studies on the effect of storage time on the rheological properties of this type of product.
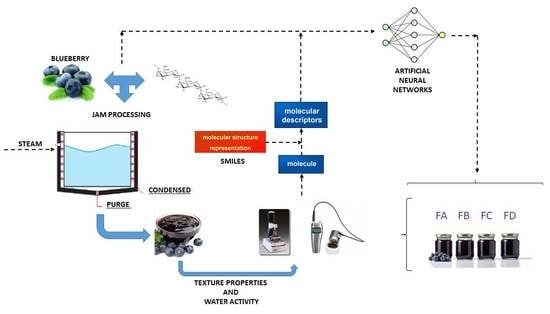
In general, the properties can be predicted through a mathematical model. There are several methods for identifying a great model that can predict such properties. One successful method in the academic environment is artificial neural networks (ANN). In this context, technical information provided to the ANNs can be obtained from specific properties of the involved substances and from the so-called molecular descriptors.
An artificial neural network is a system or information-processing paradigm, composed of highly interconnected processing elements or “neurons”, called artificial (nodes), that work to solve specific problems. It is inspired from the biological nervous system, similar to how the brain processes information. In engineering, they greatly resemble human neurons and are also interconnected, just as their human counterparts (
Figure 1). These ensembles are arranged into interlinked layers along specified architectures. The most common for the engineering applications is multilayer perception (MLP), which is a feed forward neural network. It consists of input and output layers with hidden layers in between. Each layer has several artificial neurons. The outputs from the input layer are fed to hidden nodes. Input nodes receive some type of information (stimulus) from the outside or other neighboring neurons and process it by sending a hidden link and then the output to neighboring neurons through their related links [
25,
26].
The field of molecular descriptors is based on the mass of different theories such as algebra, graph theory, information theory, computational chemistry, theories of organic reactivity and physical chemistry. The molecular descriptor is a logic and mathematical procedure transforming chemical information encoded within a symbolic representation of a molecule into a useful number (such as code SMILES) or the result of some standardized experiment. They are fundamental tools used in several areas such as chemistry, pharmaceutical sciences, environmental protection policy, health research, quality control, etc. [
28,
29].
Molecular descriptors consider the invariance with respect to labeling and numbering of the molecule atoms and to the molecule rototranslation, an unambiguous algorithmically computable definition, and values in a suitable numerical range for the set of molecules. All of them consider the connectivity of atoms in molecules, molecular size, shape, atom distributions, number of atoms, bond count, atom type, ring count, and molecular weight, etc. Thus, they are called topological indexes, geometrical, constitutional, and thermodynamic descriptors, etc., defining the chemical structures [
30].
Dragon 7.0 [
31] is one of the most important computer programs used to calculate molecular descriptors of the properties of compounds. Dragon calculates the molecular descriptors and fingerprints for more than 5000 molecular descriptors. They are divided into 30 logical blocks, each one divided into subblocks to allow easy retrieval of molecular descriptors. In this work, it is used to obtain the molecular descriptors of five components of blueberry jam (water, citric acid, glucose, sucrose, and anthocyanin). Dragon requires technical information from molecular structure files for calculating the molecular descriptors. These structure files are previously generated by other specific chemical drawing programs. The SMILES format file (.smi) is the most used to obtain the molecular descriptors.
The combination of artificial neural networks (ANN) and molecular descriptors (MD) is a technique recently used for the prediction of physical, chemical, and thermodynamic properties of pure fluids and solutions or mixtures. The authors have already used this technique in several research studies [
32,
33]. SMILES (Simplified Molecular Input Line Entry System) is a specific, easy, and flexible chemical notation to represent a chemical structure in a way that can be interpreted by the Dragon [
34,
35]. The SMILES notation requires that the user learns a set of rules. SMILES is used to translate a chemical’s three-dimensional structure (atoms, bonds, aromatic and nonaromatic rings, stereoisomers, isotopes, etc.) into a type of symbol string that is easily understood by the computer software. Other computer programs are available to translate a chemical structure into SMILES codes [
36,
37].
The present study includes four blueberry jam formulations based on the Climax and Powder Blue varieties. They were prepared as conventional and light jams. High methoxyl pectin (HM) and low methoxyl (LM) pectin were used for gelling and the products were analyzed according to their physical and chemical properties, water activity and texture properties (hardness and adhesiveness), immediately after processing and after 30, 90 and 120 days to analyze the storage effect on jam characteristics. Considering the need for studies that correlate storage time with changes in physical and chemical properties, this study evaluates the influence of storage time on these properties of traditional and light blueberry jam formulations (Climax and Powder Blue). Experimental data were submitted to the mathematical simulation. This approach used artificial neural networks, with which the study was performed based on several configurations to find the optimal configuration. Independent variables assumed in this work were: time (days), % water, % citric acid, % glucose, % sucrose, % anthocyanin, % HM pectin, % LM pectin, % xanthan gum, pH and acidity (%). The jelly molecule is complex, for this reason it was necessary to study and use the molecular descriptors (Dragon 7.0) as independent variables. In the mathematical simulation approach, hardness (N), water activity and adhesiveness (mJ) were assumed as the dependent variables.
4. Conclusions
This study demonstrated that longer cooking times and higher amounts of sucrose in traditional formulations, despite resulting in jellies with less anthocyanin content (compared to light products), can contribute to the inhibition of possible interactions of pigment (with oxygen, light or ascorbic acid). The inhibition of these interactions resulted in lower losses of this phenolic compound over the storage period.
The traditional jelly made from Climax fruits and HM pectin extracted from passion fruit peel (FC) was the most influenced by storage time with respect to physical and chemical properties. In this case, in addition to the reduction in the anthocyanin content, the storage time significantly influenced the content of reduced sugars and soluble solids, as well as water activity.
Regarding the rheological properties, with the exception of the hardness of the FC product (made from Climax fruits variety and HM pectin extracted from the passion fruit peel), practically all the texture parameters were influenced by storage time. In this context, the conventional product prepared with fruits of the Powder Blue variety and synthetic HM pectin (FA) was the most affected, in terms of hardness and adhesiveness of the final product.
The variables of time (days), % water, % citric acid, % glucose, % sucrose, % anthocyanin, % pectin HM, % Pectin LM, % xanthan gum, pH and acidity (%) were used as independent variables in an artificial neural network model to predict the physical, chemical and rheological properties of four types of blueberry jam. Mathematical simulations, using different architectures of an ANN model, were also implemented using molecular descriptors as independent variables to predict the physical, chemical and rheological properties of blueberry jam. Experimental data composed of 680 points were used (independent and dependent variables), 585 points were used in the learning step, 55 points were used in testing and 40 points were set aside for the prediction step. ANN architectures were developed with three and four layers, and the four-layer architecture was found to be the most accurate, represented as 10-30-10-3 (10 is the number of nodes or independent variables in the input layer, 30 and 10 are the numbers of nodes in the hidden layers, and 3 is the number of nodes or dependent variables in the output layer). Numerical results were obtained in function of general mean absolute deviations, which were less than 0.06%, 0.07% and 0.13% for each dependent variable: hardness (N), water activity and adhesiveness (mJ).