Co-Encapsulation of Curcumin and Diosmetin in Nanoparticles Formed by Plant-Food-Protein Interaction Using a pH-Driven Method
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Different Characterizations of zein-SPI NPs
2.3.1. Particle Characterization
2.3.2. TEM
2.3.3. FTIR
2.3.4. Fluorescence of Free Bioactive Substance and Encapsulate Bioactive Substance
2.3.5. XRD
2.4. pH Stability
2.5. Encapsulate and Loading
2.6. Storage Stability
2.7. Bioactive Substance Release
2.7.1. In Vitro Gastrointestinal Digestion
2.7.2. MTT
2.8. Re-Dispersibility of zein-SPI NPs
2.9. Statistical Analysis
3. Results and Discussion
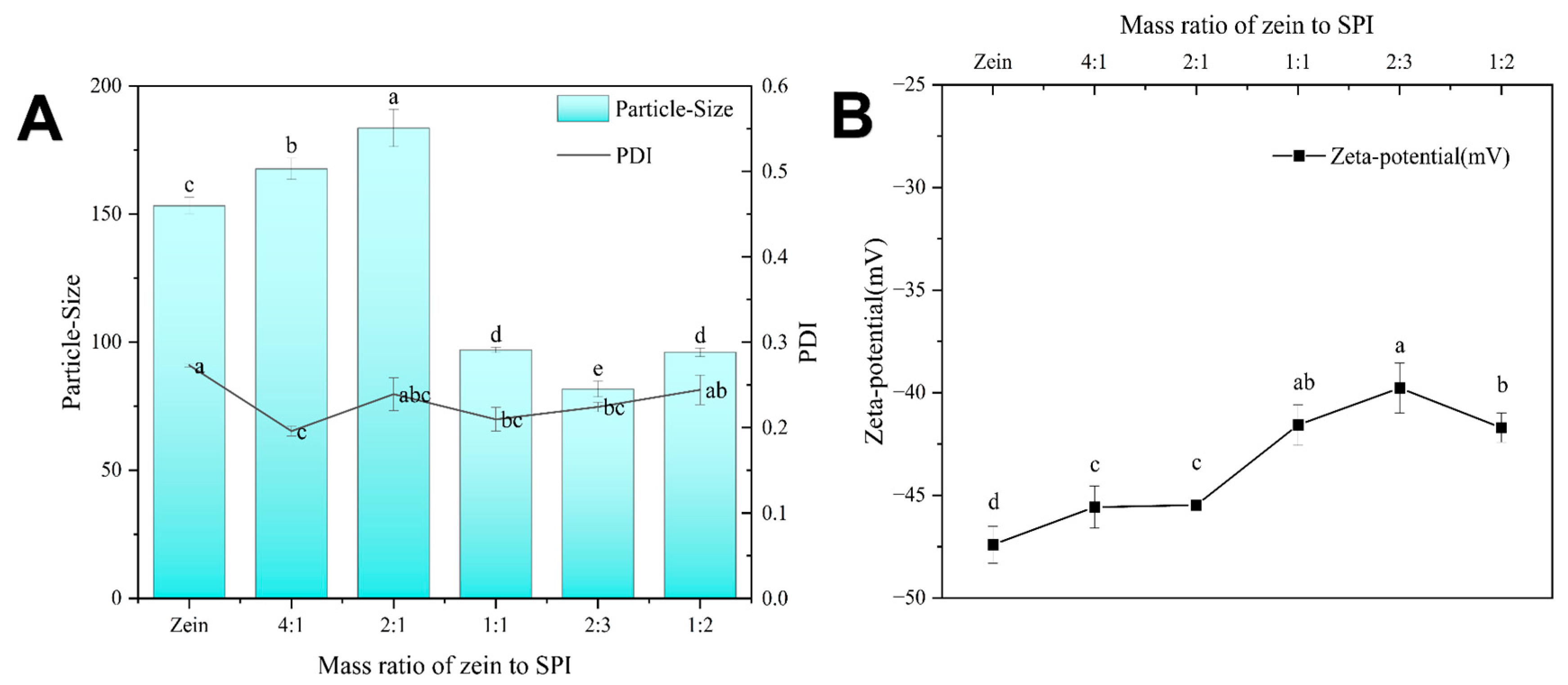
3.1. Co-Assembled zein-SPI Complex NPs
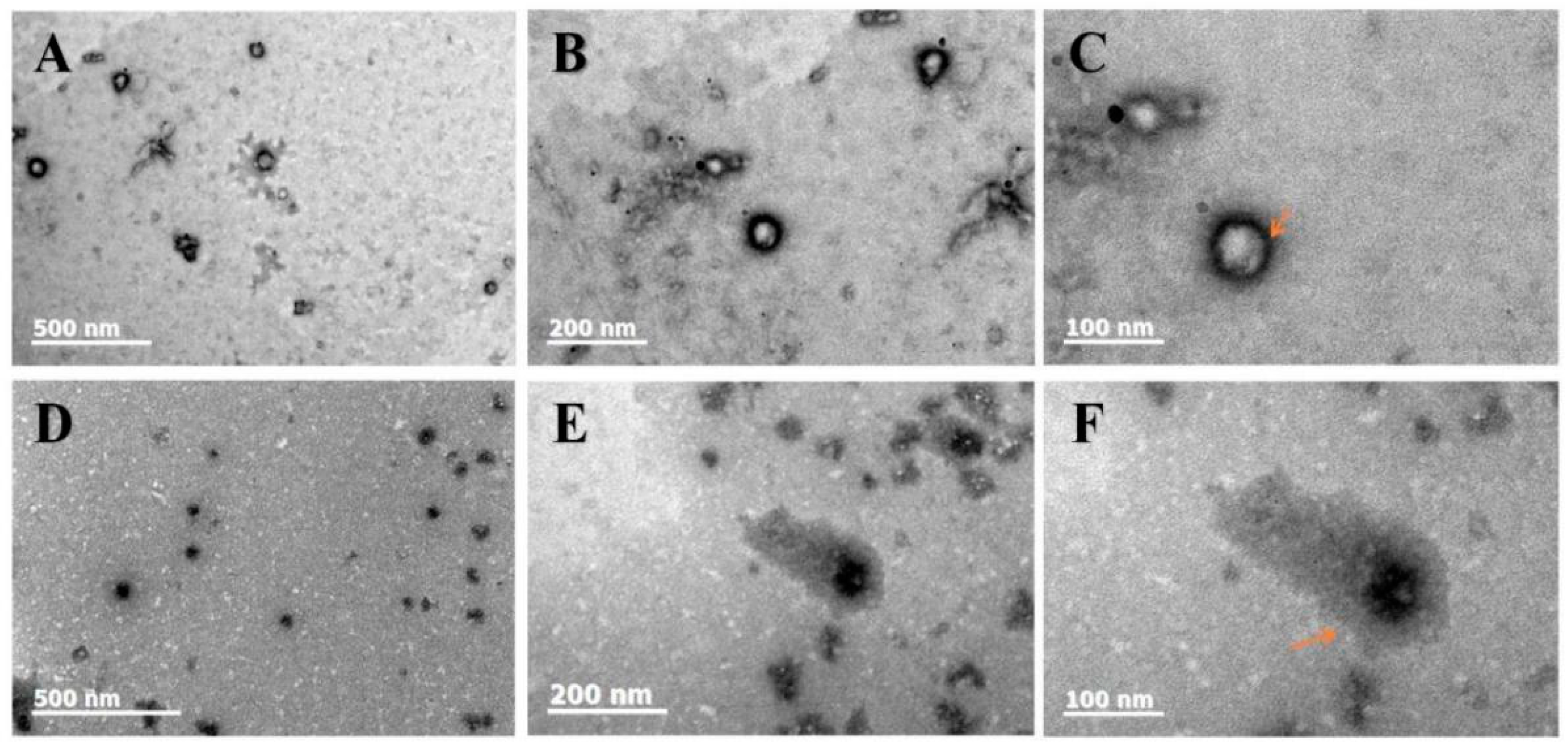
3.2. TEM
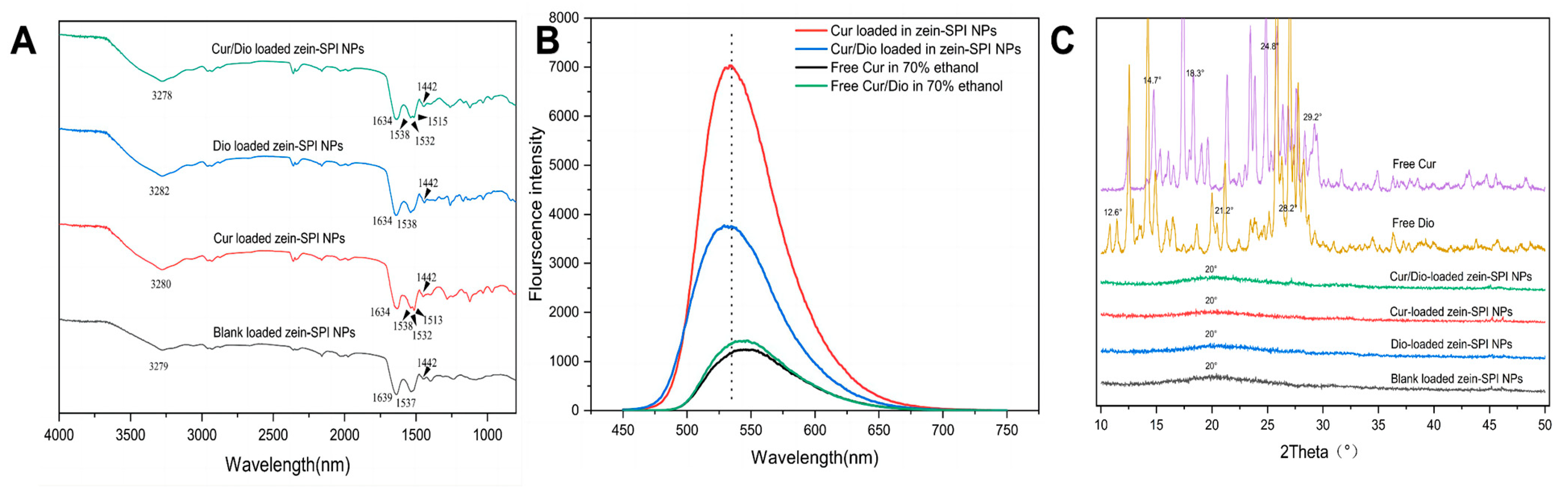
3.3. FTIR
3.4. Fluorescence of Free Bioactive Substance and Encapsulated Bioactive Substance
3.5. XRD
3.6. pH Stability
3.7. Bioactive Substance EE and LE
3.8. Storage Stability Also Related to Bioactive Substance
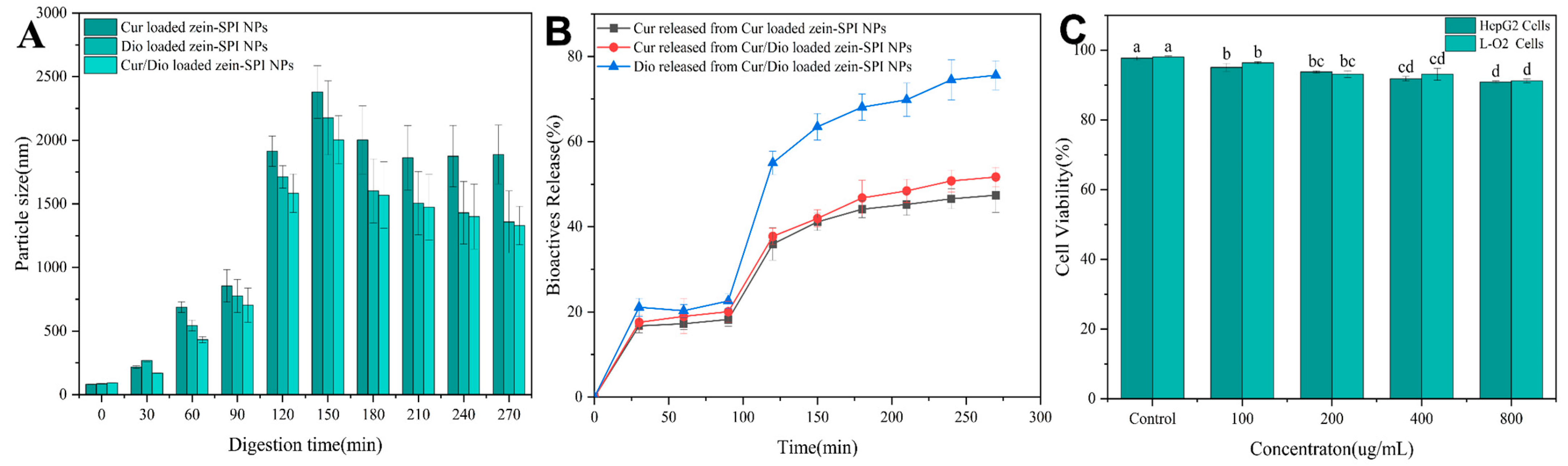
3.9. Bioactive Substance Release
3.10. MTT
3.11. Re-Dispersibility of zein-SPI NPs
4. Conclusions
- zein-SPI NPs were constructed by the pH-driven method with the optimum mass ratio (2:3) of zein to soy protein isolate to form a new plant–food–protein-based nanocarrier with the Z-average of 81.73 nm and Zeta-potential of −39.3 mV.
- The co-encapsulated Cur and Dio changed the structure of zein-SPI NPs, increased the binding sites of Dio in NPs, increased the EE% of Dio from 43.07% to 73.41%, and increased the overall LE% of NPs from 4.61% and 9.03% to 16.54%.
- The co-encapsulated Cur and Dio improved the retention rate of Dio in four weeks from 61.96% to 82.41%, making some progress in the field of co-encapsulated bioactive substances.
- In MTT experiment, HepG2 and L-O2 cells in different concentration groups (0–800 µg/mL) had more than 90% cell vitalities, indicating that zein-SPI NPs have strong biocompatibility and potential medical value. zein-SPI NPs were prepared using the pH-driven method and used for the first time for the co-encapsulation of Cur and Dio.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPI | Soy protein isolate |
| NPs | Nanoparticles |
| Cur | Curcumin |
| Dio | Diosmetin |
| EE | Encapsulation efficiency |
| LE | Loading efficiency |
References
- Bao, W.; Li, K.; Rong, S.; Yao, P.; Hao, L.; Ying, C.; Zhang, X.; Nussler, A.; Liu, L. Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. J. Ethnopharmacol. 2010, 128, 549–553. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Chen, J.; Jiang, B.; Zhang, T. Fabrication, characterization, physicochemical stability and simulated gastrointestinal digestion of pterostilbene loaded zein-sodium caseinate-fucoidan nanoparticles using pH-driven method. Food Hydrocoll. 2021, 119, 106851. [Google Scholar] [CrossRef]
- Gomez-Estaca, J.; Balaguer, M.P.; Gavara, R.; Hernandez-Munoz, P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Eren, T.; Baysal, G.; Dogan, F. Biocidal Activity of Bone Cements Containing Curcumin and Pegylated Quaternary Polyethylenimine. J. Polym. Environ. 2020, 28, 2469–2480. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Pharmacology of Diosmin, a Citrus Flavone Glycoside: An Updated Review. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Wei, Y.Y.; Li, X.h.; Zhang, S.S.; Zhang, R.T.; Li, J.H.; Ma, B.W.; Shao, S.B.; Lv, Z.W.; Ruan, H.; et al. Diosmetin has therapeutic efficacy in colitis regulating gut microbiota, inflammation, and oxidative stress via the circ-Sirt1/Sirt1 axis. Acta Pharmacol. Sin. 2022, 43, 919–932. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Lee, M.G. Dose-independent pharmacokinetics of clindamycin after intravenous and oral administration to rats: Contribution of gastric first-pass effect to low bioavailability. Int. J. Pharmaceut. 2007, 332, 17–23. [Google Scholar] [CrossRef]
- MaHam, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-Based Nanomedicine Platforms for Drug Delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Liu, Z.; Chu, W.; Sun, Q.; Zhao, L.; Tan, X.; Zhang, Y.; Yin, T.; He, H.; Gou, J.; Tang, X. Micelle-contained and PEGylated hybrid liposomes of combined gemcitabine and cisplatin delivery for enhancing antitumor activity. Int. J. Pharmaceut. 2021, 602, 120619. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Cui, Y.; Zhai, G.; Li, L. The construction and characterization of hybrid paclitaxel-in-micelle-in-liposome systems for enhanced oral drug delivery. Colloids Surf. B 2017, 160, 572–580. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Gahruie, H.H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Hong, S.; Dia, V.P.; Zhong, Q. Synergistic anti-inflammatory activity of apigenin and curcumin co-encapsulated in caseins assessed with lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Biol. Macromol. 2021, 193, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; McClements, D.J.; Jian, L.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Core-Shell Biopolymer Nanoparticles for Co-Delivery of Curcumin and Piperine: Sequential Electrostatic Deposition of Hyaluronic Acid and Chitosan Shells on the zein Core. ACS Appl. Mater. Interfaces 2019, 11, 38103–38115. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Lee, J.; Chen, Y.; Hoshino, K. Studies of nanoparticle delivery with in vitro bio-engineered microtissues. Bioact. Mater. 2020, 5, 924–937. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; Joye, I.J.; Espinal-Ruiz, M.; McClements, D.J. Effect of Maillard Conjugates on the Physical Stability of zein Nanoparticles Prepared by Liquid Antisolvent Coprecipitation. J. Agric. Food Chem. 2015, 63, 8510–8518. [Google Scholar] [CrossRef]
- Paliwal, R.; Palakurthi, S. zein in controlled drug delivery and tissue engineering. J. Control Release 2014, 189, 108–122. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Velikov, K.P. zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein. Bba-Proteins Proteom. 2006, 1764, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Zhou, Y.; Jiang, L.; Lyu, Q.; Liu, G.; Wang, X.; Chen, X.; Chen, L. zein-whey protein isolate-carboxymethyl cellulose complex as carrier of apigenin via pH-driven method: Fabrication, characterization, stability, and in vitro release property. Food Chem. 2022, 387, 132926. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Z.; Xia, G.; Xue, F.; Chen, C.; Zhang, Y. Fabrication and characterization of zein/lactoferrin composite nanoparticles for encapsulating 7,8-dihydroxyflavone: Enhancement of stability, water solubility and bioaccessibility. Int. J. Biol. Macromol. 2020, 146, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, J.; Qin, Y.; Jiang, B.; Zhang, T. zein/fucoidan-based composite nanoparticles for the encapsulation of pterostilbene: Preparation, characterization, physicochemical stability, and formation mechanism. Int. J. Biol. Macromol. 2020, 158, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, Y.-C.; Qian, L.H.; Zhang, Y.H.; Gong, P.-X.; Liu, W.; Li, H.-J. Fabrication of foxtail millet prolamin/caseinate/chitosan hydrochloride composite nanoparticles using antisolvent and pH-driven methods for curcumin delivery. Food Chem. 2022, 404, 134604. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Gong, P.-X.; Zhang, Y.H.; Li, H.J. Chondroitin sulfate deposited on foxtail millet prolamin/caseinate nanoparticles to improve physicochemical properties and enhance cancer therapeutic effects. Food Funct. 2022, 13, 5343–5352. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Jin, M.; Xiao, D.; Tian, H.; Zhang, W. Application of supercritical anti-solvent technologies for the synthesis of delivery systems of bioactive food components. Food Biophys. 2008, 3, 186–190. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.-C.; Liu, Y.; Qian, L.-H.; Zhang, Y.-H.; Li, H.-J. Single/co-encapsulation capacity and physicochemical stability of zein and foxtail millet prolamin nanoparticles. Colloid Surf. B 2022, 217, 112685. [Google Scholar] [CrossRef] [PubMed]
- Pan, K.; Zhong, Q. Low energy, organic solvent-free co-assembly of zein and caseinate to prepare stable dispersions. Food Hydrocoll. 2016, 52, 600–606. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Ma, M.; Zhang, S.; Wang, S.; Li, H.; Xu, Y.; Wang, D. Self-assembled composite nanoparticles based on zein as delivery vehicles of curcumin: Role of chondroitin sulfate. Food Funct. 2020, 11, 5377–5388. [Google Scholar] [CrossRef]
- Fan, L.; Lu, Y.; Ouyang, X.K.; Ling, J. Development and characterization of soybean protein isolate and fucoidan nanoparticles for curcumin encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef]
- Chen, X.; Yu, C.; Zhang, Y.; Wu, Y.C.; Ma, Y.; Li, H.J. Co-encapsulation of curcumin and resveratrol in zein-bovine serum albumin nanoparticles using a pH-driven method. Food Funct. 2023, 14, 3169–3178. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Xu, Y.; Wang, D. Construction of biopolymer-based nanoencapsulation of functional food ingredients using the pH-driven method: A review. Crit. Rev. Food Sci. 2021. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Ye, G.; Wu, T.; Li, Z.; Teng, M.; Ma, L.; Qin, M.; Zhao, P.; Fu, Q. Preparation and characterization of novel composite nanoparticles using zein and hyaluronic acid for efficient delivery of naringenin. Food Chem. 2023, 417, 135890. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, H.; Liu, C.; Zhu, J.; Xu, Y.; Zhang, S.; Fan, M.; Zhang, D.; Zhang, Y.; Zhang, Z.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef]
- Dai, L.; Li, R.; Wei, Y.; Sun, C.; Mao, L.; Gao, Y. Fabrication of zein and rhamnolipid complex nanoparticles to enhance the stability and in vitro release of curcumin. Food Hydrocoll. 2018, 77, 617–628. [Google Scholar] [CrossRef]
- Sun, C.; Gao, Y.; Zhong, Q. Effects of acidification by glucono-delta-lactone or hydrochloric acid on structures of zein-caseinate nanocomplexes self-assembled during a pH cycle. Food Hydrocoll. 2018, 82, 173–185. [Google Scholar] [CrossRef]
- Baysal, G.; Olcay, H.S.; Gnnnec, C. Encapsulation and antibacterial studies of goji berry and garlic extract in the biodegradable chitosan. J. Bioact. Compat. Polym. 2023, 38, 209–219. [Google Scholar] [CrossRef]
- Samindra, K.M.S.; Kottegoda, N. Encapsulation of curcumin into layered double hydroxides. Nanotechnol. Rev. 2014, 3, 579–589. [Google Scholar] [CrossRef]
- Yang, Z.; McClements, D.J.; Peng, X.; Qiu, C.; Long, J.; Zhao, J.; Xu, Z.; Meng, M.; Chen, L.; Jin, Z. Co-encapsulation of quercetin and resveratrol in zein/carboxymethyl cellulose nanoparticles: Characterization, stability and in vitro digestion. Food Funct. 2022, 13, 11652–11663. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Z.; Lin, K.; Sun, C.; Dai, L.; Yang, S.; Mao, L.; Yuan, F.; Gao, Y. Fabrication and characterization of resveratrol loaded zein-propylene glycol alginate-rhamnolipid composite nanoparticles: Physicochemical stability, formation mechanism and in vitro digestion. Food Hydrocoll. 2019, 95, 336–348. [Google Scholar] [CrossRef]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J. Food Eng. 2019, 258, 45–53. [Google Scholar] [CrossRef]
- Liu, C.; Xu, B.; McClements, D.J.; Xu, X.; Cui, S.; Gao, L.; Zhou, L.; Xiong, L.; Sun, Q.; Dai, L. Properties of curcumin-loaded zein-tea saponin nanoparticles prepared by antisolvent co-precipitation and precipitation. Food Chem. 2022, 391, 133224. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Sun, Y.; Wang, D.; Sun, P.; Shao, P. Effect of adjusting pH and chondroitin sulfate on the formation of curcumin-zein nanoparticles: Synthesis, characterization and morphology. Carbohyd. Polym. 2020, 250, 116970. [Google Scholar] [CrossRef]
- Bian, Y.; Guo, D. Targeted Therapy for Hepatocellular Carcinoma: Co-Delivery of Sorafenib and Curcumin Using Lactosylated pH-Responsive Nanoparticles. Drug Des. Dev. Ther. 2020, 14, 647–659. [Google Scholar] [CrossRef] [Green Version]
- Neel, A.J.; Hilton, M.J.; Sigman, M.S.; Toste, F.D. Exploiting non-covalent pi interactions for catalyst design. Nature 2017, 543, 637–646. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin Exacerbates Mitophagy and Synergizes with Doxorubicin to Induce Immunogenic Cell Death in Hepatocellular Carcinoma. ACS Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef]
- Hu, J.; Wang, J.; Wang, G.; Yao, Z.; Dang, X. Pharmacokinetics and antitumor efficacy of DSPE-PEG2000 polymeric liposomes loaded with quercetin and temozolomide: Analysis of their effectiveness in enhancing the chemosensitization of drug-resistant glioma cells. Int. J. Mol. Med. 2016, 37, 690–702. [Google Scholar] [CrossRef] [Green Version]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of Chitosan Film Incorporated with Curcumin Extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Tang, F.; Xie, Y.; Cao, H.; Yang, H.; Chen, X.; Xiao, J. Fetal bovine serum influences the stability and bioactivity of resveratrol analogues: A polyphenol-protein interaction approach. Food Chem. 2017, 219, 321–328. [Google Scholar] [CrossRef]
- Zhang, F.; Khan, M.A.; Cheng, H.; Liang, L. Co-encapsulation of alpha-tocopherol and resveratrol within zein nanoparticles: Impact on antioxidant activity and stability. J. Food Eng. 2019, 247, 9–18. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, L.; Yu, Z.; Lin, K.; Yang, S.; Dai, L.; Liu, J.; Mao, L.; Yuan, F.; Gao, Y. Enhanced stability, structural characterization and simulated gastrointestinal digestion of coenzyme Q10 loaded ternary nanoparticles. Food Hydrocoll. 2019, 94, 333–344. [Google Scholar] [CrossRef]
- Yao, K.; Chen, W.; Song, F.; McClements, D.J.; Hu, K. Tailoring zein nanoparticle functionality using biopolymer coatings: Impact on curcumin bioaccessibility and antioxidant capacity under simulated gastrointestinal conditions. Food Hydrocoll. 2018, 79, 262–272. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Meng, D.; Shi, L.; Zhu, L.; Wang, Q.; Liu, J.; Kong, Y.; Hou, M.; Yang, R.; Zhou, Z. Coencapsulation and Stability Evaluation of Hydrophilic and Hydrophobic Bioactive Compounds in a Cagelike Phytoferritin. J. Agric. Food Chem. 2020, 68, 3238–3249. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Dai, L.; Zhang, L.; Gao, Y. Entrapment of curcumin in whey protein isolate and zein composite nanoparticles using pH-driven method. Food Hydrocoll. 2020, 106, 105839. [Google Scholar] [CrossRef]
- Silva, G.S.; Jange, C.G.; Rocha, J.S.S.; Chaves, M.A.; Pinho, S.C. Characterisation of curcumin-loaded proliposomes produced by coating of micronised sucrose and hydration of phospholipid powders to obtain multilamellar liposomes. Int. J. Food Sci. Tech. 2017, 52, 772–780. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Processes improving the dispersibility of spray-dried zein nanoparticles using sodium caseinate. Food Hydrocoll. 2014, 35, 358–366. [Google Scholar] [CrossRef]


| Sample | Group | NPs Size (nm) | PDI | Zeta-Potential (mV) | EE (%) | LE (%) |
|---|---|---|---|---|---|---|
| Cur-loaded | Freshly | 81.89 ± 0.38c | 0.22 ± 0.01ab | −39.37 ± 0.22ab | 84.29 ± 0.51a | 9.03 ± 0.05b |
| Re-dispersed | 84.60 ± 0.78b | 0.26 ± 0.01a | −38.37 ± 0.35a | 78.91 ± 0.76b | ||
| Dio-loaded | Freshly | 86.21 ± 0.98b | 0.20 ± 0.02b | −39.07 ± 0.48ab | 43.07 ± 1.55d | 4.61 ± 0.17c |
| Re-dispersed | 86.24 ± 0.70b | 0.22 ± 0.02ab | −39.60 ± 0.23ab | 37.46 ± 1.38e | ||
| Cur/Dio-loaded | Freshly | 91.45 ± 0.64a | 0.19 ± 0.01b | −40.10 ± 0.55b | 80.95 ± 1.36ab (Cur)/73.41 ± 1.72c (Dio) | 16.54 ± 0.61a |
| Re-dispersed | 93.23 ± 0.43a | 0.20 ± 0.01b | −39.37 ± 0.38ab | 80.48 ± 1.22ab (Cur)/72.40 ± 0.95c (Dio) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Shan, J.; Fu, Z.; Ju, H.; Chen, X.; Xu, G.; Liu, Y.; Li, H.; Wu, Y. Co-Encapsulation of Curcumin and Diosmetin in Nanoparticles Formed by Plant-Food-Protein Interaction Using a pH-Driven Method. Foods 2023, 12, 2861. https://doi.org/10.3390/foods12152861
Yu C, Shan J, Fu Z, Ju H, Chen X, Xu G, Liu Y, Li H, Wu Y. Co-Encapsulation of Curcumin and Diosmetin in Nanoparticles Formed by Plant-Food-Protein Interaction Using a pH-Driven Method. Foods. 2023; 12(15):2861. https://doi.org/10.3390/foods12152861
Chicago/Turabian StyleYu, Chong, Jingyu Shan, Ze Fu, Hao Ju, Xiao Chen, Guangsen Xu, Yang Liu, Huijing Li, and Yanchao Wu. 2023. "Co-Encapsulation of Curcumin and Diosmetin in Nanoparticles Formed by Plant-Food-Protein Interaction Using a pH-Driven Method" Foods 12, no. 15: 2861. https://doi.org/10.3390/foods12152861






