Characteristics and Functional Properties of Maillard Reaction Products from α-Lactalbumin and Polydextrose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of α-LA-PD MRPs
2.3. Measurement of Absorbance and Fluorescence Intensity
2.4. Measurement of Free Amino Groups
2.5. SDS-PAGE
2.6. CD and Tryptophan Fluorescence Spectroscopy
2.7. Surface Hydrophobicity (H0)
2.8. Measurement of the Antioxidant Activity
2.8.1. DPPH Radical-Scavenging Activity
2.8.2. Ferric Reducing Antioxidant Power (FRAP)
2.9. Statistical Analysis
3. Results and Discussion
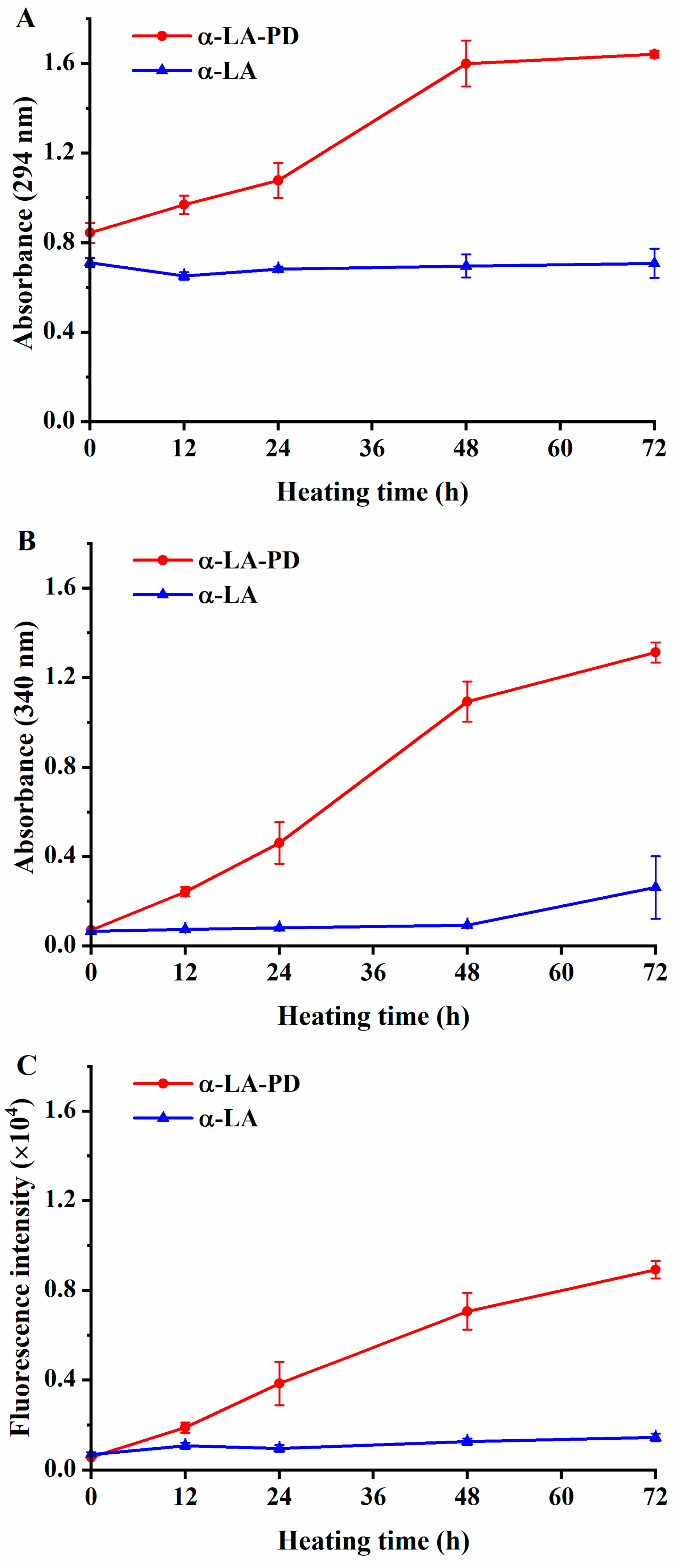
3.1. Absorbance and Fluorescence Intensity
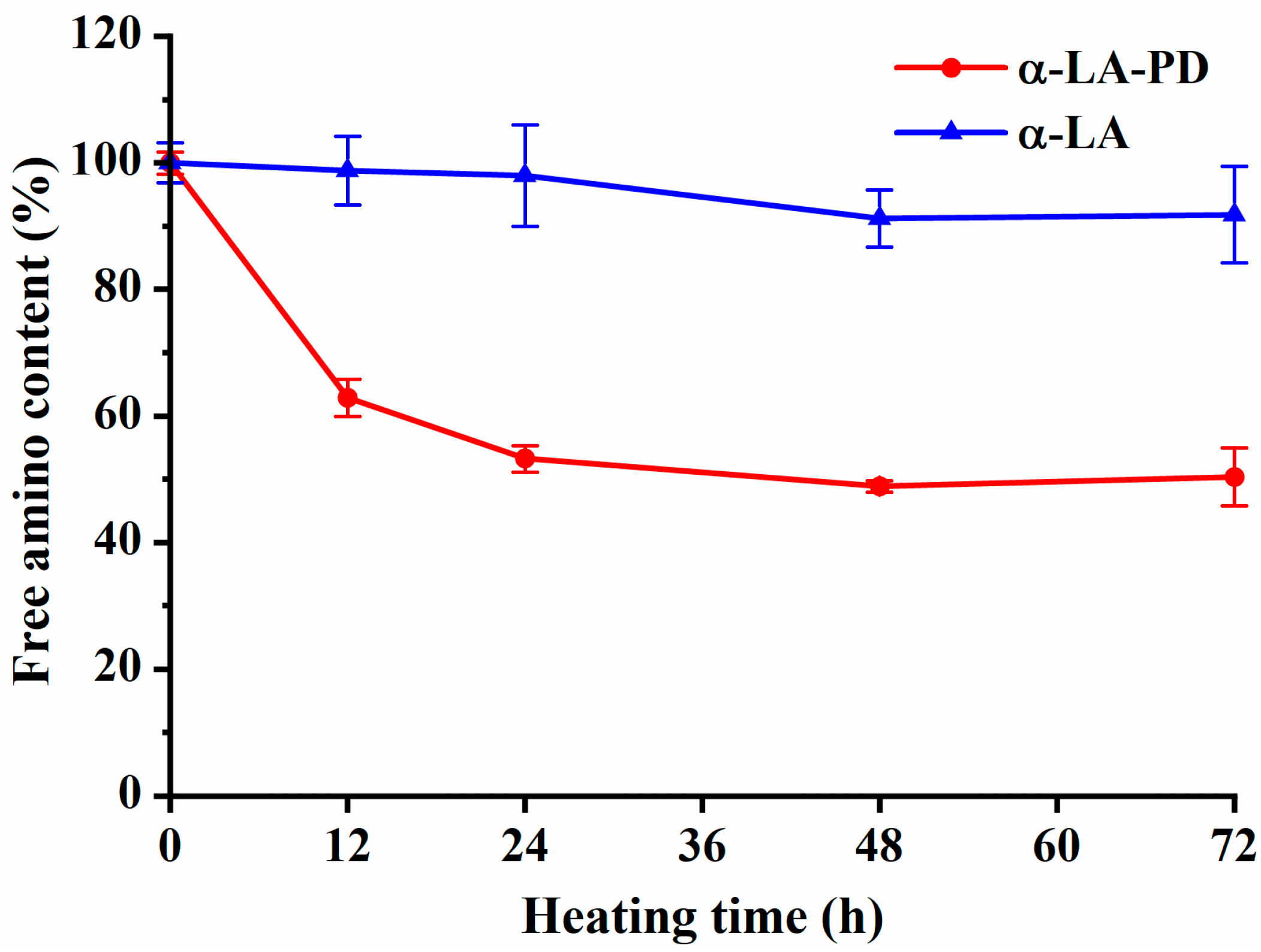
3.2. Variations in Free Amino Group Contents
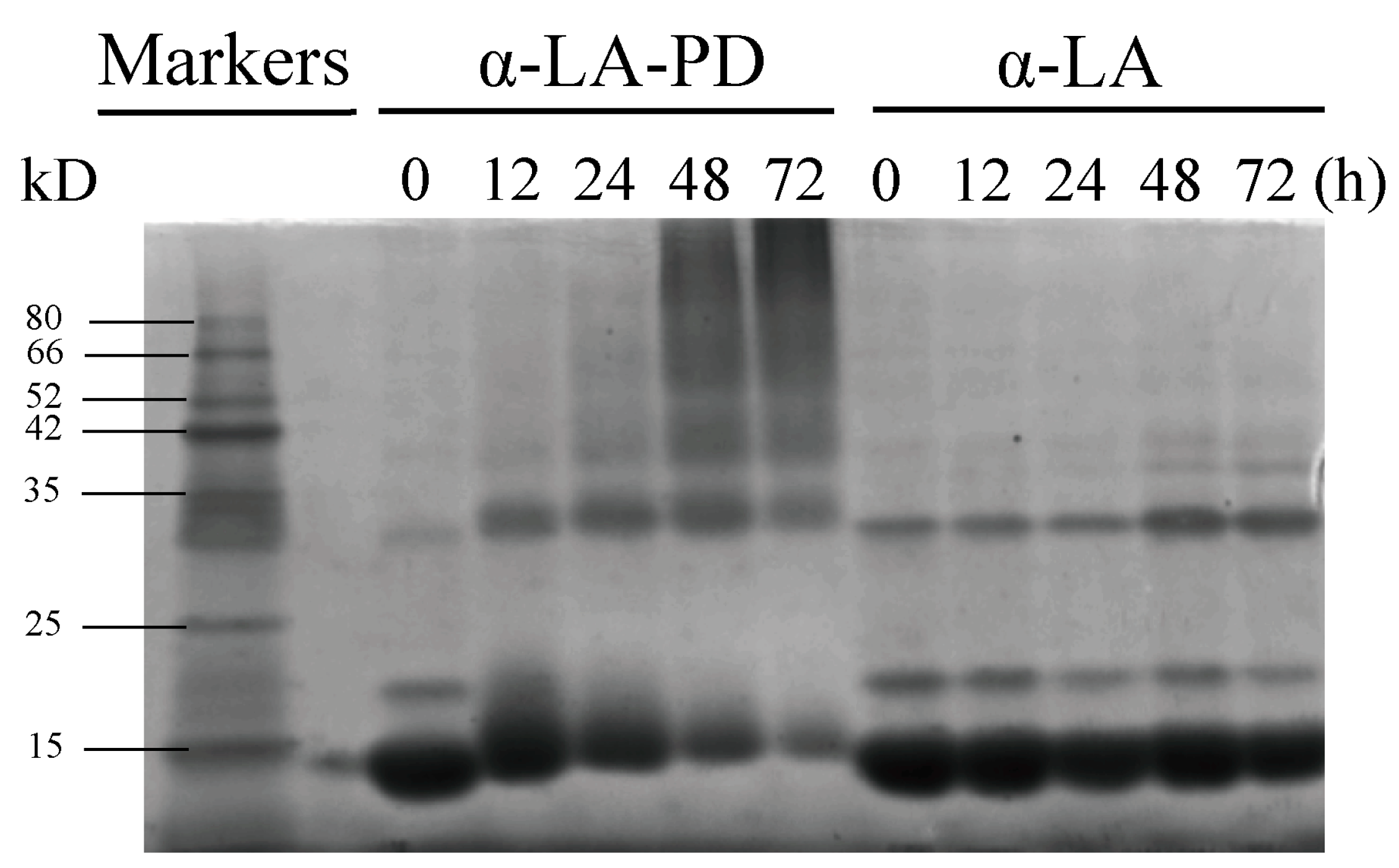
3.3. SDS-PAGE
3.4. CD and Tryptophan Fluorescence Spectroscopy
3.5. Surface Hydrophobicity (H0)
3.6. Antioxidant Activity
3.6.1. DPPH Radical-Scavenging Activity
3.6.2. FRAP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Van Boekel, M.A.J.S. Effect of heating on Maillard Reactions in milk. Food Chem. 1998, 62, 403–414. [Google Scholar] [CrossRef]
- Manley, D.; Clark, H. Manley’s Technology of Biscuits, Crackers and Cookies, 4th ed.; Manley, D., Ed.; Woodhead Publishing: Cambridgeshire, UK, 2011; pp. 576–588. [Google Scholar]
- Joubran, Y.; Moscovici, A.; Portmann, R.; Lesmes, U. Implications of the Maillard Reaction on bovine alpha-lactalbumin and its proteolysis during in vitro infant digestion. Food Funct. 2017, 8, 2295–2308. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, X.; Wang, Z.; He, Z.; Zeng, M.; Chen, J. Characterizing changes in Maillard Reaction indicators in whole milk powder and reconstituted low-temperature pasteurized milk under different preheating conditions. J. Food Sci. 2021, 87, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Engholm-Keller, K.; Fang, Y.; Nielsen, C.F.; Jordà, A.; Lund, M.N.; Chatterton, D.E.W. UHT treatment and storage of liquid infant formula affects protein digestion and release of bioactive peptides. Food Funct. 2022, 13, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Kamau, S.M.; Cheison, S.C.; Chen, W.; Liu, X.-M.; Lu, R.-R. Alpha-lactalbumin: Its production technologies and bioactive peptides. Compr. Rev. Food Sci. Food Saf. 2010, 9, 197–212. [Google Scholar] [CrossRef]
- Cardoso, H.B.; Wierenga, P.A.; Gruppen, H.; Schols, H.A. Maillard induced glycation behaviour of individual milk proteins. Food Chem. 2018, 252, 311–317. [Google Scholar] [CrossRef]
- Ibarra, A.; Astbury, N.M.; Olli, K.; Alhoniemi, E.; Tiihonen, K. Effects of polydextrose on different levels of energy intake. A systematic review and meta-analysis. Appetite 2015, 87, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Nunes, L.; Martins, E.; Tuler Perrone, Í.; Fernandes de Carvalho, A. The Maillard reaction in powdered infant formula. J. Food Nutr. Res. 2019, 7, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Joubran, Y.; Moscovici, A.; Lesmes, U. Antioxidant activity of bovine alpha lactalbumin Maillard products and evaluation of their in vitro gastro-duodenal digestive proteolysis. Food Funct. 2015, 6, 1229–1240. [Google Scholar] [CrossRef]
- Zhang, M.; Zheng, J.; Ge, K.; Zhang, H.; Fang, B.; Jiang, L.; Guo, H.; Ding, Q.; Ren, F. Glycation of α-lactalbumin with different size saccharides: Effect on protein structure and antigenicity. Int. Dairy J. 2014, 34, 220–228. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Ajandouz, E.H.; Tchiakpe, L.S.; Ore, F.D.; Benajiba, A.; Puigserver, A. Effects of pH on caramelization and Maillard Reaction kinetics in fructose-lysine model systems. J. Food Sci. 2001, 66, 926–931. [Google Scholar] [CrossRef]
- Jing, H.; Kitts, D.D. Chemical characterization of different sugar-casein Maillard Reaction products and protective effects on chemical-induced cytotoxicity of Caco-2 cells. Food Chem. Toxicol. 2004, 42, 1833–1844. [Google Scholar] [CrossRef]
- Seifert, A.; Freilich, S.; Kashi, Y.; Livney, Y.D. Protein-oligosaccharide conjugates as novel prebiotics. Polym. Adv. Technol. 2019, 30, 2577–2585. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Kong, B.; Li, P.; Xia, X. Physicochemical and antioxidant properties of Maillard Reaction products formed by heating whey protein isolate and reducing sugars. Int. J. Dairy Technol. 2013, 67, 220–228. [Google Scholar] [CrossRef]
- Luo, S.; Lu, X.; Liu, C.; Zhong, J.; Zhou, L.; Chen, T. Site specific PEGylation of β-lactoglobulin at glutamine residues and its influence on conformation and antigenicity. Food Res. Int. 2019, 123, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Tu, Y.; Liu, W.; Luo, S.; Liu, C. Comparative study on the effects of nystose and fructofuranosyl nystose in the glycation reaction on the antigenicity and conformation of β-lactoglobulin. Food Chem. 2015, 188, 658–663. [Google Scholar] [CrossRef]
- Tu, Y.; Xu, Y.; Ren, F.; Zhang, H. Characteristics and antioxidant activity of Maillard Reaction products from α-lactalbumin and 2′-fucosyllactose. Food Chem. 2020, 316, 126341. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Pyo, M.C.; Yang, S.-Y.; Chun, S.-H.; Oh, N.S.; Lee, K.-W. Protective effects of Maillard reaction products of whey protein concentrate against oxidative stress through an Nrf2-dependent pathway in HepG2 cells. Biol. Pharm. Bull. 2016, 39, 1437–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, J.; Song, X.; Shi, Y.; Gao, F.Z.; Li, B.L.; Li, N.B.; Luo, H.Q. A potential fluorescent probe: Maillard reaction product from glutathione and ascorbic acid for rapid and label-free dual detection of Hg2+ and biothiols. Biosens. Bioelectron. 2016, 81, 473–479. [Google Scholar] [CrossRef]
- Aalaei, K.; Rayner, M.; Sjöholm, I. Chemical methods and techniques to monitor early Maillard reaction in milk products; a review. Crit. Rev. Food Sci. Nutr. 2018, 59, 1829–1839. [Google Scholar] [CrossRef] [Green Version]
- Jongberg, S.; Rasmussen, M.; Skibsted, L.H.; Olsen, K. Detection of advanced glycation end-products (AGEs) during dry-state storage of β-lactoglobulin/lactose. Aust. J. Chem. 2012, 65, 1620. [Google Scholar] [CrossRef]
- Luo, Y.; Tu, Y.; Ren, F.; Zhang, H. Characterization and functional properties of Maillard reaction Products of β-lactoglobulin and polydextrose. Food Chem. 2022, 377, 131749. [Google Scholar] [CrossRef] [PubMed]
- Obando, M.; Soto, E.; De Meulenaer, B. Influence of Oxidized Oils on Digestibility of Caseins in O/W Emulsions. Eur. J. Lipid Sci. Technol. 2018, 120, 1700331. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Xiong, Y.L. Characteristics and functional properties of buckwheat protein–sugar schiff base complexes. LWT 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Wang, W.; Bao, Y.; Chen, Y. Characteristics and antioxidant activity of water-soluble Maillard reaction products from interactions in a whey protein isolate and sugars system. Food Chem. 2013, 139, 355–361. [Google Scholar] [CrossRef]
- Cioni, P.; Strambini, G.B. Tryptophan phosphorescence and pressure effects on protein structure. Biochim. Biophys. Acta 2002, 1595, 116–130. [Google Scholar] [CrossRef]
- Boggione Santos, I.J.; Hernandez Hernandez, H.L.; Cardoso Costa, M.H.; de Queiroz Lafetá, J.A.; dos Reis Coimbra, J.S. Conjugates of α-lactalbumin, β-lactoglobulin, and lysozyme with polysaccharides: Characterization and techno-functional properties. Food Res. Int. 2019, 116, 492–498. [Google Scholar] [CrossRef]
- Zhang, H.; Li, L.; Mittal, G.S. Effects of high pressure processing on soybean beta-conglycinin. J. Food Process Eng. 2009, 33, 568–583. [Google Scholar] [CrossRef]
- Heldt, C.L.; Zahid, A.; Vijayaragavan, K.S.; Mi, X. Experimental and computational surface hydrophobicity analysis of a non-enveloped virus and proteins. Colloids Surf. 2017, 153, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kroes-Nijboer, A.; Venema, P.; Bouman, J.; Van der Linden, E. Influence of protein hydrolysis on the growth kinetics of β-lg fibrils. Langmuir 2011, 27, 5753–5761. [Google Scholar] [CrossRef]
- Jiang, Z.; Brodkorb, A. Structure and antioxidant activity of Maillard reaction products from α-lactalbumin and β-lactoglobulin with ribose in an aqueous model system. Food Chem. 2012, 133, 960–968. [Google Scholar] [CrossRef] [Green Version]
- Nooshkam, M.; Varidi, M.; Bashash, M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chem. 2019, 275, 644–660. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, F.; Chobert, J.-M.; Genot, C.; Haertlé, T. Scavenging of free radicals, antimicrobial, and cytotoxic activities of the Maillard reaction products of β-lactoglobulin glycated with several sugars. J. Agric. Food Chem. 2001, 49, 5031–5038. [Google Scholar] [CrossRef]
- Dong, S.; Panya, A.; Zeng, M.; Chen, B.; McClements, D.J.; Decker, E.A. Characteristics and antioxidant activity of hydrolyzed β-lactoglobulin–glucose Maillard reaction products. Food Res. Int. 2012, 46, 55–61. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, B.; Han, J.; Sun, C.; Li, P. Structure and antioxidant activity of whey protein isolate conjugated with glucose via the Maillard reaction under dry-heating conditions. Food Struct. 2014, 1, 145–154. [Google Scholar] [CrossRef]



| Samples | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| α-LA-PD, 0 h | 31.6 ± 0.7 | 21.7 ± 0.9 | 18.0 ± 0.1 | 28.7 ± 0.5 |
| α-LA-PD, 12 h | 30.5 ± 1.2 | 22.4 ± 1.7 | 18.1 ± 0.2 | 29.0 ± 0.9 |
| α-LA-PD, 24 h | 30.7 ± 0.9 | 22.5 ± 1.1 | 18.1 ± 0.2 | 28.7 ± 0.9 |
| α-LA-PD, 48 h | 32.6 ± 0.5 | 20.9 ± 0.5 | 18.2 ± 0.1 | 28.3 ± 0.5 |
| α-LA-PD, 72 h | 31.6 ± 0.8 | 21.7 ± 1.0 | 18.1 ± 0.2 | 28.6 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, K.; Wang, J.; Luo, Y.; Tu, Y.; Ren, F.; Zhang, H. Characteristics and Functional Properties of Maillard Reaction Products from α-Lactalbumin and Polydextrose. Foods 2023, 12, 2866. https://doi.org/10.3390/foods12152866
Dai K, Wang J, Luo Y, Tu Y, Ren F, Zhang H. Characteristics and Functional Properties of Maillard Reaction Products from α-Lactalbumin and Polydextrose. Foods. 2023; 12(15):2866. https://doi.org/10.3390/foods12152866
Chicago/Turabian StyleDai, Kexin, Jiangpeng Wang, Yingting Luo, Yaqi Tu, Fazheng Ren, and Hao Zhang. 2023. "Characteristics and Functional Properties of Maillard Reaction Products from α-Lactalbumin and Polydextrose" Foods 12, no. 15: 2866. https://doi.org/10.3390/foods12152866





